Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
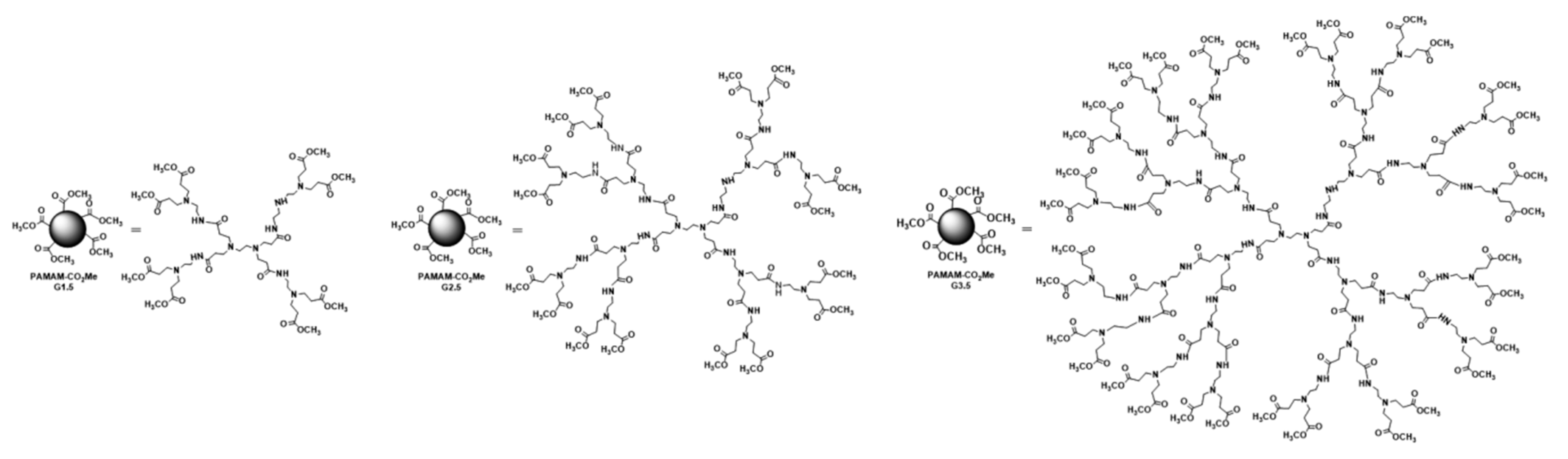
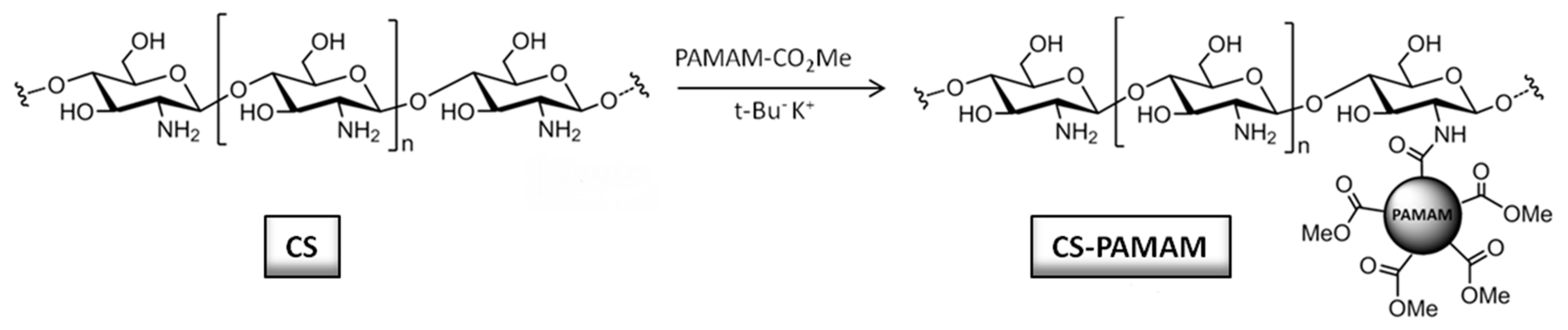
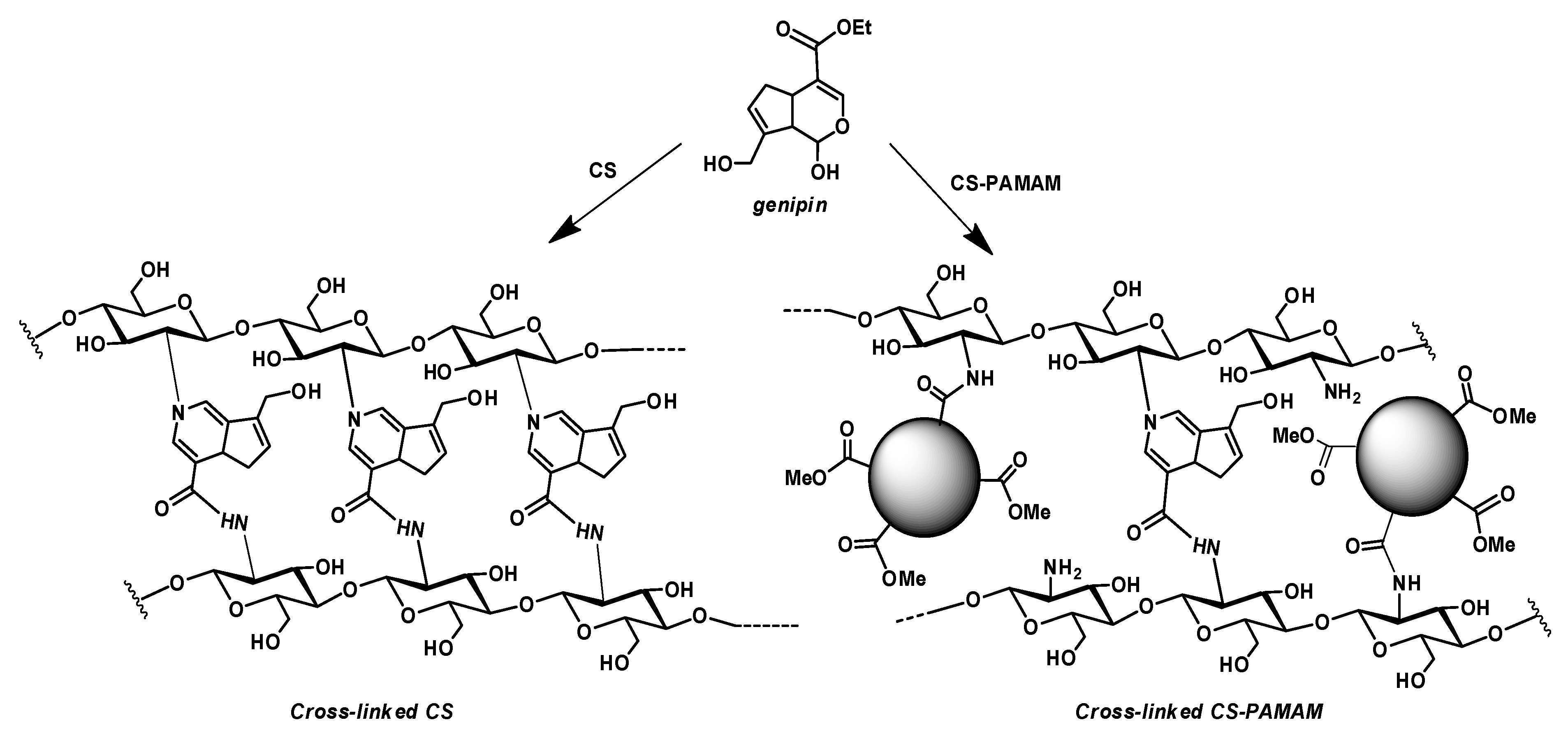
2.2.1. Synthesis of PAMAM Dendrimers and Chitosan–PAMAM Chains
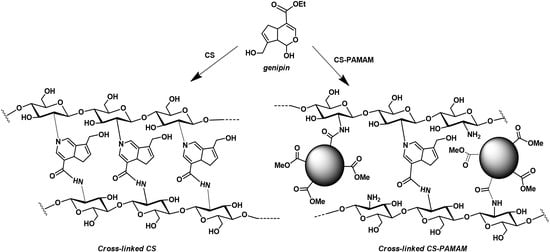
2.2.2. Synthesis of Chitosan Hydrogel (CS Sample)
2.2.3. Synthesis of Chitosan-Hydroxyapatite Hydrogel (CS-HA Sample)
2.2.4. Synthesis of CS-PAMAM-HA Hydrogel (CS-D1.5-HA, CS-D2.5-HA, CS-D3.5-HA Samples)
2.2.5. Synthesis of Ketoprofen-Doped Hydrogel (CS-HA-Keto and CS-D1.5-HA-Keto, CS-D2.5-HA-Keto, CS-D3.5-HA-Keto Samples)
2.2.6. Drug Release Studies
2.2.7. Rheological Studies
3. Results and Discussion
3.1. Synthesis of Chitosan-Based Hydrogels
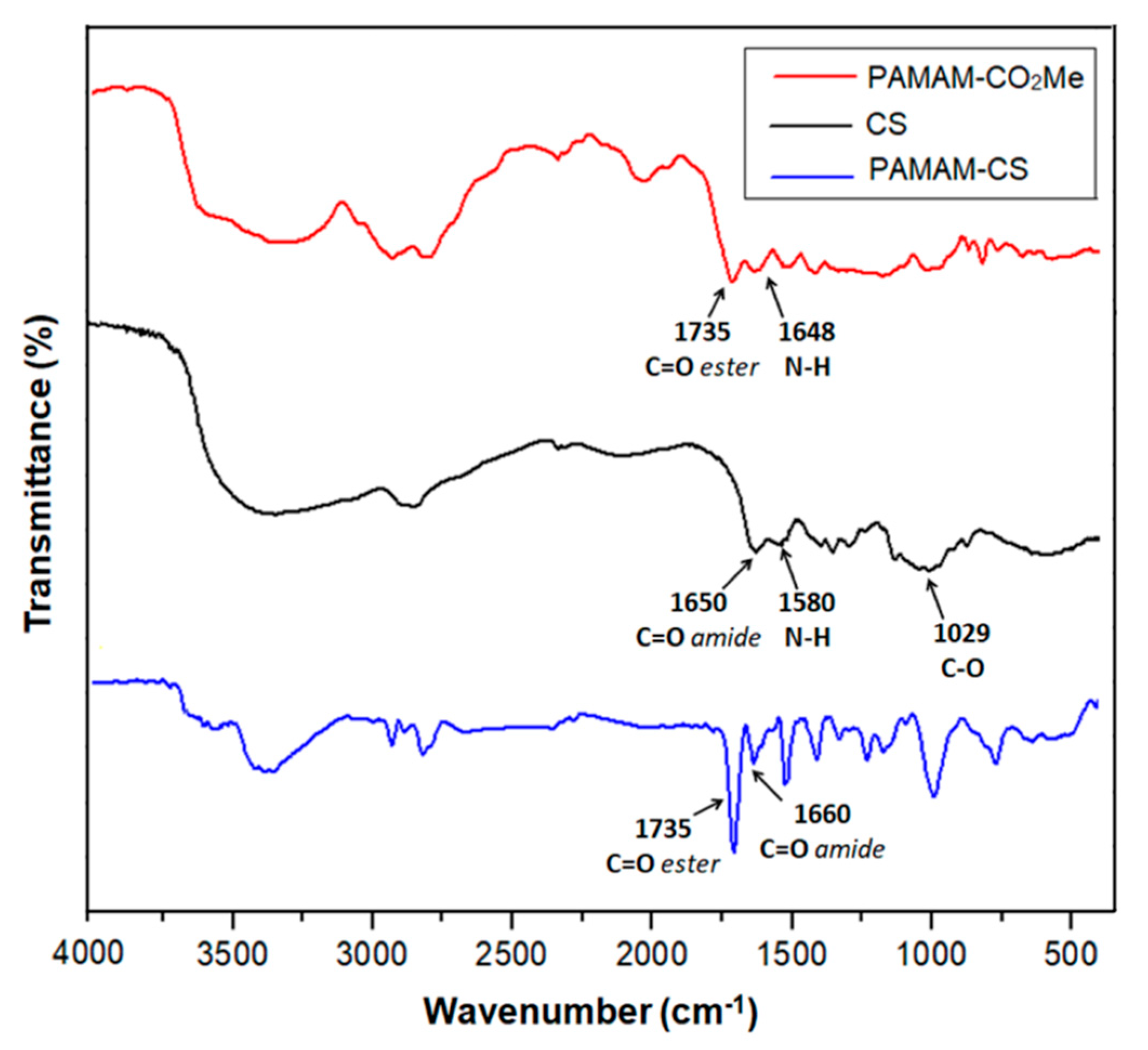
3.1.1. Synthesis of Chitosan–PAMAM Chains
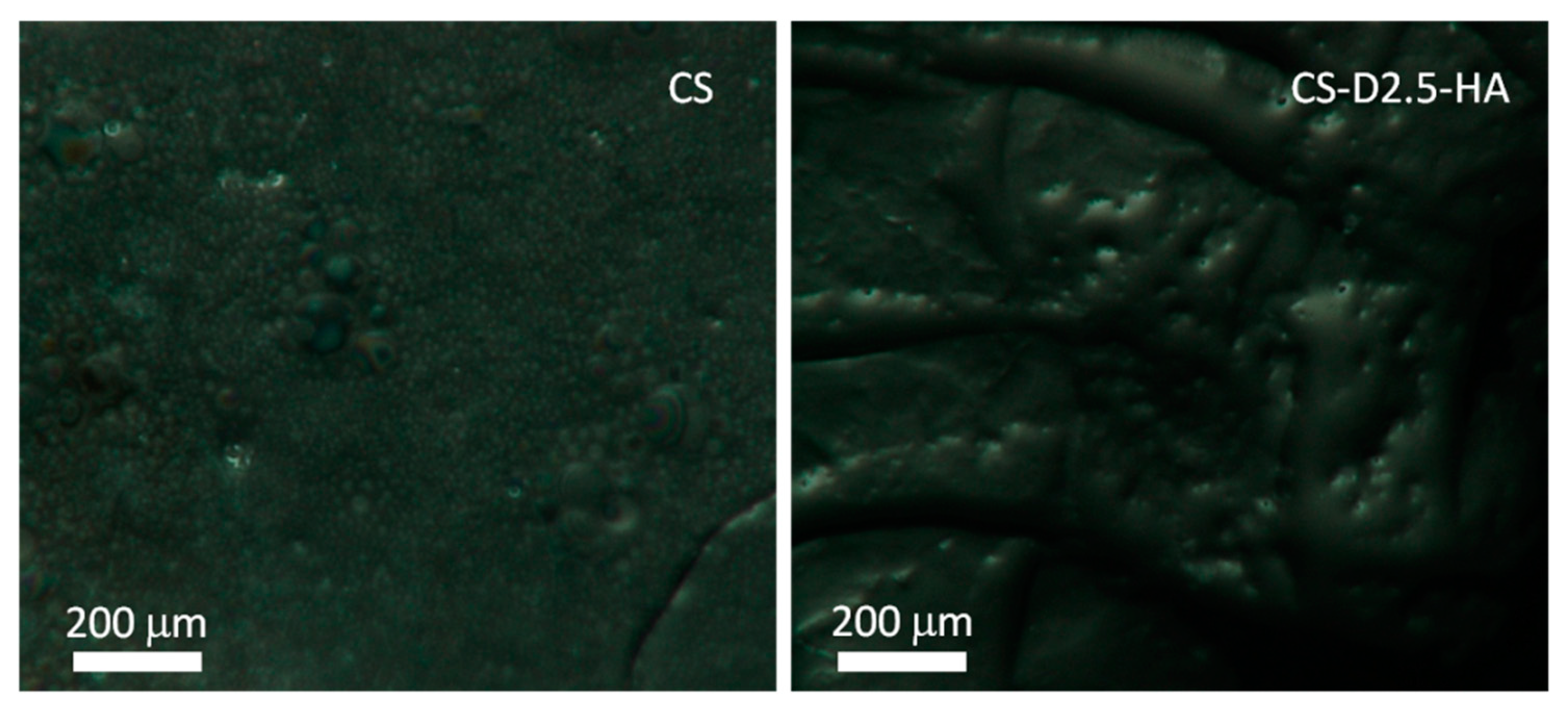
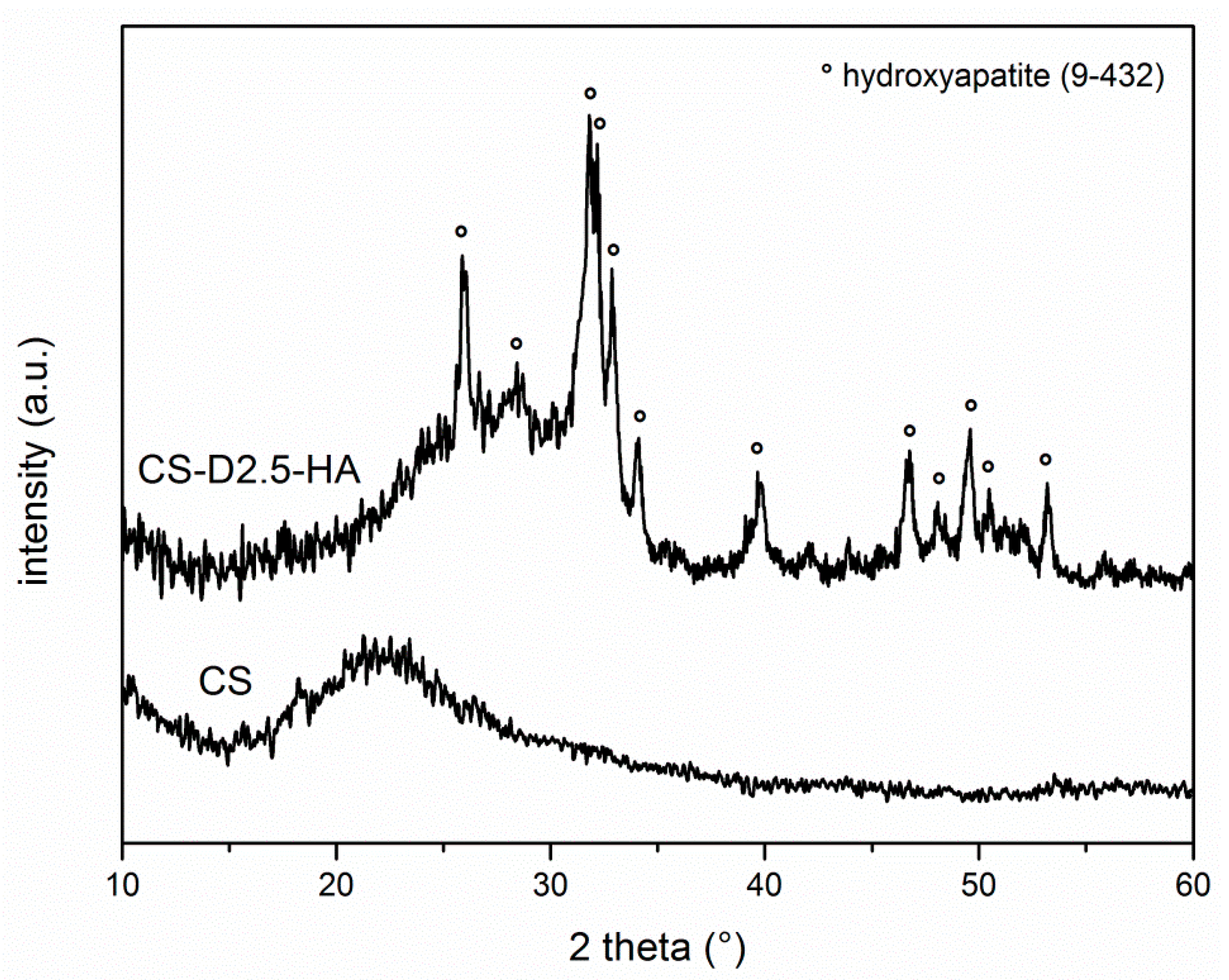
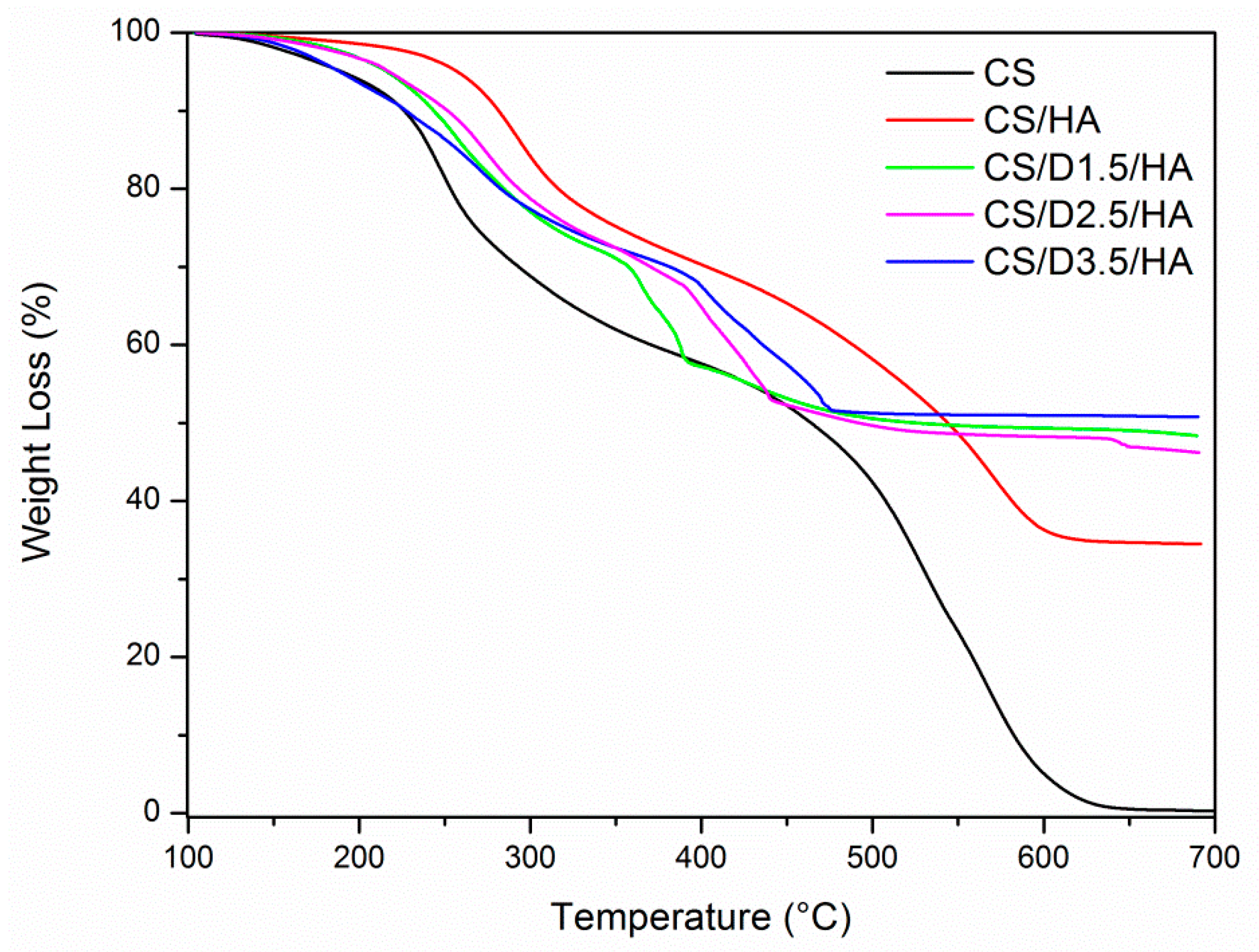
3.1.2. Synthesis of CS-D-HA Hydrogels
3.2. Drug Release Studies
3.3. Rheological Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Albertsson, A.-C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. In Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002; Volume 157, pp. 1–40. ISBN 978-3-540-45734-3. [Google Scholar]
- Visco, A.; Scolaro, C.; Giamporcaro, A.; De Caro, S.; Tranquillo, E.; Catauro, M. Threads made with blended biopolymers: Mechanical, physical and biological features. Polymers 2019, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Piskin, E. Biodegradable polymers as biomaterials. J. Biomater. Sci. Polym. Ed. 1995, 6, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Jianghua, L.; Chao, C.; Jiarui, L.; Jun, L.; Jia, L.; Tiantian, S.; Lihao, W.; Haotian, W.; Guangli, Y. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide based scaffolds for soft tissue engineering applications. Polymers 2019, 11, 1. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D. Biomaterials and their potential applications for dental tissue engineering. J. Mater. Chem. 2010, 20, 8730–8746. [Google Scholar] [CrossRef]
- Rodríguez Vázquez, M.; Vega Ruiz, B.; Ramos Zúñiga, R.; Saldaña Koppel, D.A.; Quiñones Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- Argüelles Monal, W.M.; Lizardi Mendoza, J.; Fernández Quiroz, D.; Recillas Mota, M.T.; Montiel Herrera, M. Chitosan derivatives: Introducing new functionalities with a controlled molecular architecture for innovative materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Kong, Y.; Zheng, H.; Ke, W.; Chen, X.; Yin, Y.; Yi, Y. Preparation and properties of poly(amidoamine) dendrimer/quaternary ammonium chitosan hydrogels. J. Wuhan Univ. Technol. 2018, 33, 736–743. [Google Scholar] [CrossRef]
- Sashiwa, H.; Yajima, H.; Aiba, S. Synthesis of a chitosan dendrimer hybrid and its biodegradation. Biomacromolecules 2003, 4, 1244–1249. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, C.H.; Wayne Pack, D. Polymeric carriers for gene delivery: Chitosan and poly(amidoamine) dendrimers. Curr. Pharm. Des. 2010, 16, 2350–2368. [Google Scholar] [CrossRef]
- Abedi Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Uswatta, S.P.; Okeke, I.U.; Jayasuriya, A.C. Injectable porous nano hydroxyapatite/chitosan/tripolyphosphate scaffolds with improved compressive strength for bone regeneration. Mater. Sci. Eng. C 2016, 69, 505–512. [Google Scholar] [CrossRef]
- Reza Mahdavinia, G.; Karimi, M.H.; Soltaniniya, M.; Massoumi, B. In vitro evaluation of sustained ciprofloxacin release from κ-carrageenan-crosslinked chitosan/hydroxyapatite hydrogel nanocomposites. Int. J. Biol. Macromol. 2019, 126, 443–453. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinkedwith genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Piperopoulos, E.; Ashok, D.; Cembran, A.; Tricoli, A.; Nisbet, D. Engineering of chitosan-hydroxyapatite-magnetite hierarchical scaffolds for guided bone growth. Materials 2019, 12, 2321. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R.D.K. Controlled and extended drug release behaviorof chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Espro, C.; Galvagno, S.; Tampieri, A.; Montesi, M.; Panseri, S.; Sandri, M. Tethering of gly-arg-gly-asp-ser-pro-lyspeptides on mg-doped hydroxyapatite. Engineering 2017, 3, 55–59. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Espro, C.; Galvagno, S. Drug delivery strategies for bone tissue regeneration. In Biomimetic Approaches for Tissue Healing; Panseri, S., Taraballi, F., Cunha, C., Eds.; OMICS Group eBooks: Foster City, CA, USA, 2015; pp. 1–39. [Google Scholar]
- Tsai, C.C.; Huang, R.N.; Sung, H.W.; Liang, H.C. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J. Biomed. Mater. Res. 2000, 52, 58–65. [Google Scholar] [CrossRef]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; et al. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B 2014, 117, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Tan, Z.; Sun, F.; Sheng, L.; Zhang, X.; Yao, F. Synthesis and characterization of quaternizedcarboxymethyl chitosan/poly(amidoamine) dendrimer core–shell nanoparticles. Mater. Sci. Eng. C 2012, 32, 2026–2036. [Google Scholar] [CrossRef]
- Arguelles Monal, W.; Goycoolea, F.M.; Peniche, C.; Higuera Ciapara, I. Polymer gels and networks. Rheological study of the chitosan/glutaraldehyde chemical gel system. Polym. Gels Netw. 1998, 6, 429–440. [Google Scholar] [CrossRef]
- Kim, B.R.; Lee, H.G.; Kang, S.B.; Sung, G.H.; Kim, J.J.; Park, J.K.; Lee, S.G.; Yoon, Y.J. tert-Butoxide-Assisted amidation of esters under green conditions. Synthesis 2012, 44, 42–50. [Google Scholar]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Nasiri, N.; Mukherjee, S.; Panneerselvan, A.; Nisbet, D.R.; Tricoli, A. Optimally hierarchical nanostructured hydroxyapatite coatings for superior prosthesis biointegration. ACS Appl. Mater. Interfaces 2018, 10, 24840–24849. [Google Scholar] [CrossRef]
- Dong, Q.X.; Chen, Q.J.; Yang, W.; Zheng, Y.L.; Liu, X.; Li, Y.L.; Yang, M.B. Thermal properties and flame retardancy of polycarbonate/hydroxyapatite nanocomposite. J. Appl. Polym. Sci. 2008, 109, 659–663. [Google Scholar] [CrossRef]
- Branca, C.; Crupi, C.; D’Angelo, G.; Khouzami, K.; Rifici, S.; Visco, A.; Wanderlingh, U. Effect of montmorillonite on the rheological properties of dually crosslinked guar gum-based hydrogels. J. Appl. Polym. Sci. 2015, 132, 41373. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [PubMed]


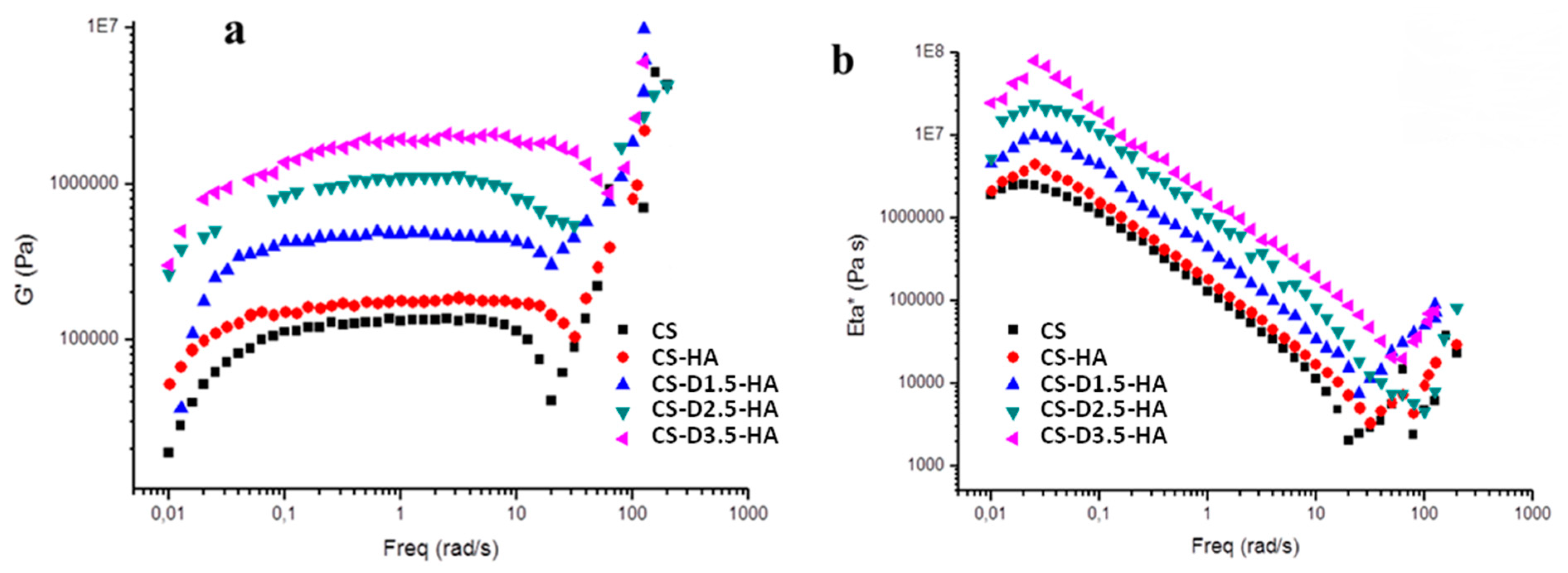
| Sample Code | Sample Composition | Gelling Time (Figure 6) (h:m) | Freq. 0.1 rad/s | Freq. 10 rad/s | ||
|---|---|---|---|---|---|---|
| G’ (Pa) | η* (106 Pa∙s) | G’ (Pa) | η* (106 Pa∙s) | |||
| CS | 100 wt% pure chitosan | 1:05 | 112,752 | 1.128 | 114,072 | 0.011409 |
| CS-HA | 65 wt% pure chitosan + 35 wt% hydroxyapatite | 1:05 | 151,501 | 1.519 | 171,425 | 0.017144 |
| CS-D1.5-HA | 50 wt% dendrimer modified chitosan + 50 wt% hydroxyapatite | 1:35 | 424,121 | 4.401 | 428347.7 | 0.033921 |
| CS-D2.5-HA | 50 wt% dendrimer modified chitosan + 50 wt% hydroxyapatite | 2:05 | 838,935 | 10.665 | 808,642 | 0.081778 |
| CS-D3.5-HA | 50 wt% dendrimer modified chitosan + 50 wt% hydroxyapatite | 2:40 | 1.36962 × 106 | 18.821 | 1.8724 × 106 | 0.191665 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistone, A.; Iannazzo, D.; Celesti, C.; Scolaro, C.; Giofré, S.V.; Romeo, R.; Visco, A. Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties. Polymers 2020, 12, 754. https://doi.org/10.3390/polym12040754
Pistone A, Iannazzo D, Celesti C, Scolaro C, Giofré SV, Romeo R, Visco A. Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties. Polymers. 2020; 12(4):754. https://doi.org/10.3390/polym12040754
Chicago/Turabian StylePistone, Alessandro, Daniela Iannazzo, Consuelo Celesti, Cristina Scolaro, Salvatore V. Giofré, Roberto Romeo, and Annamaria Visco. 2020. "Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties" Polymers 12, no. 4: 754. https://doi.org/10.3390/polym12040754
APA StylePistone, A., Iannazzo, D., Celesti, C., Scolaro, C., Giofré, S. V., Romeo, R., & Visco, A. (2020). Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties. Polymers, 12(4), 754. https://doi.org/10.3390/polym12040754