Study of the Effect of Methyldiethanolamine Initiator on the Recording Properties of Acrylamide Based Photopolymer
Abstract
1. Introduction
2. Theoretical Background
2.1. Diffraction Efficiency of Thick Volume Gratings
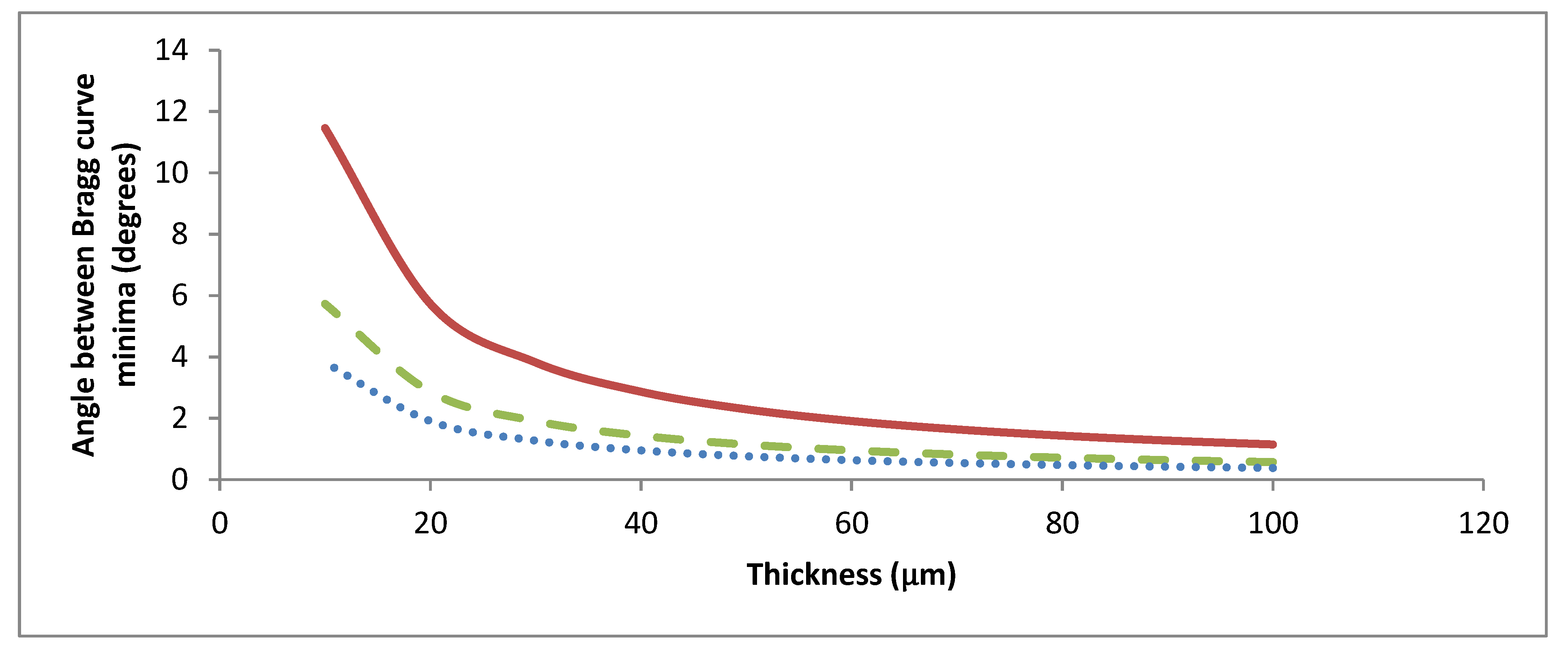
2.2. Dependence of the Angular and Wavelength Selectivity on the Thickness of Gratings
2.3. Expected Effect of the Size of the Initiator
3. Materials and Methods
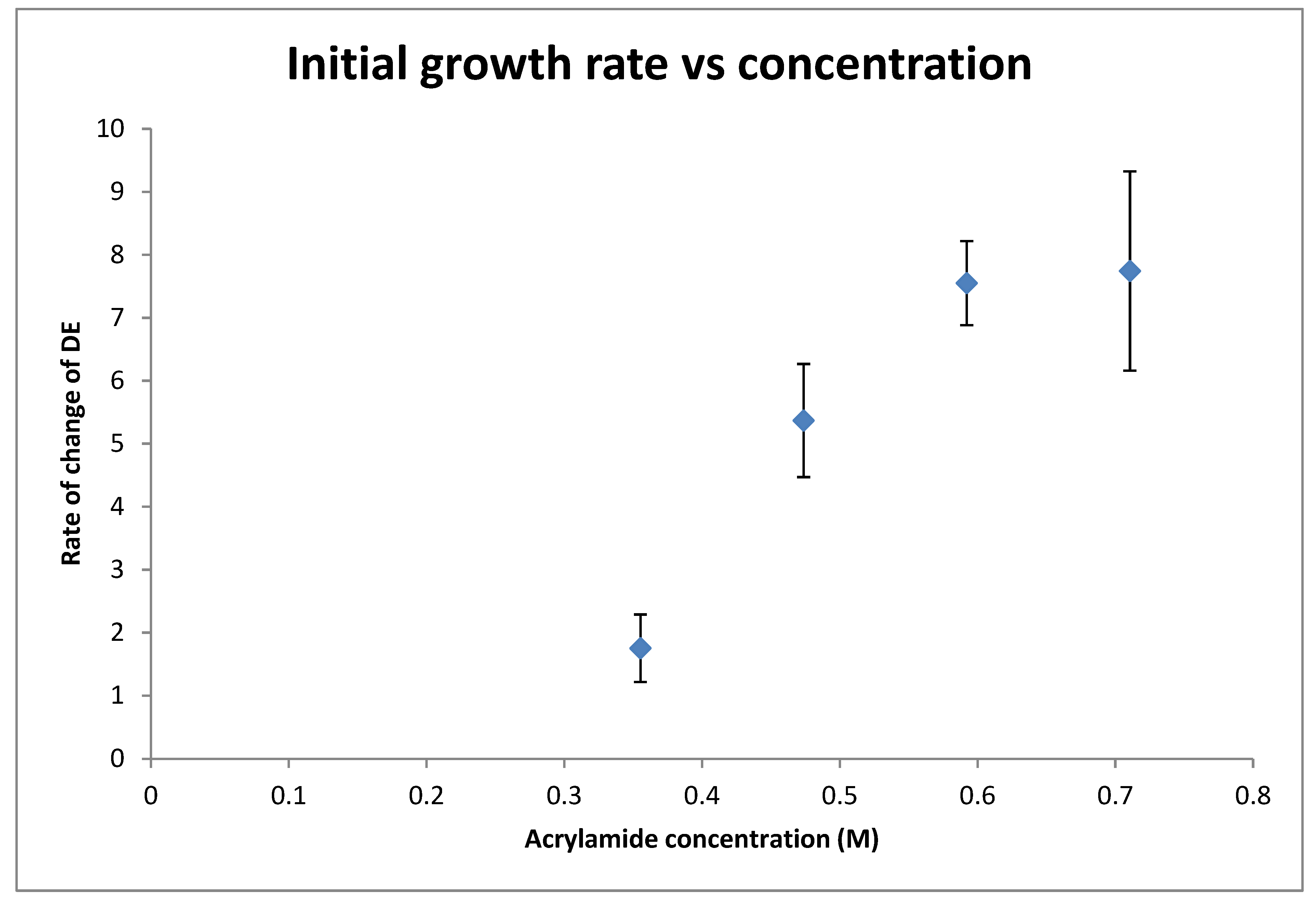
3.1. Effect of Acrylamide Concentration on Growth Rate
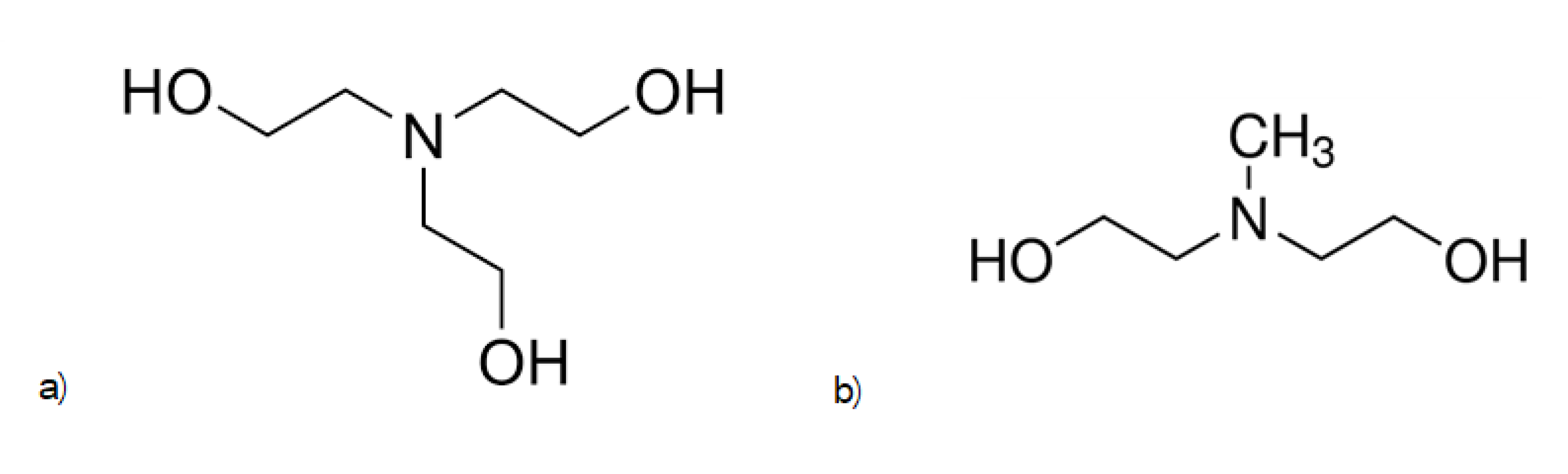
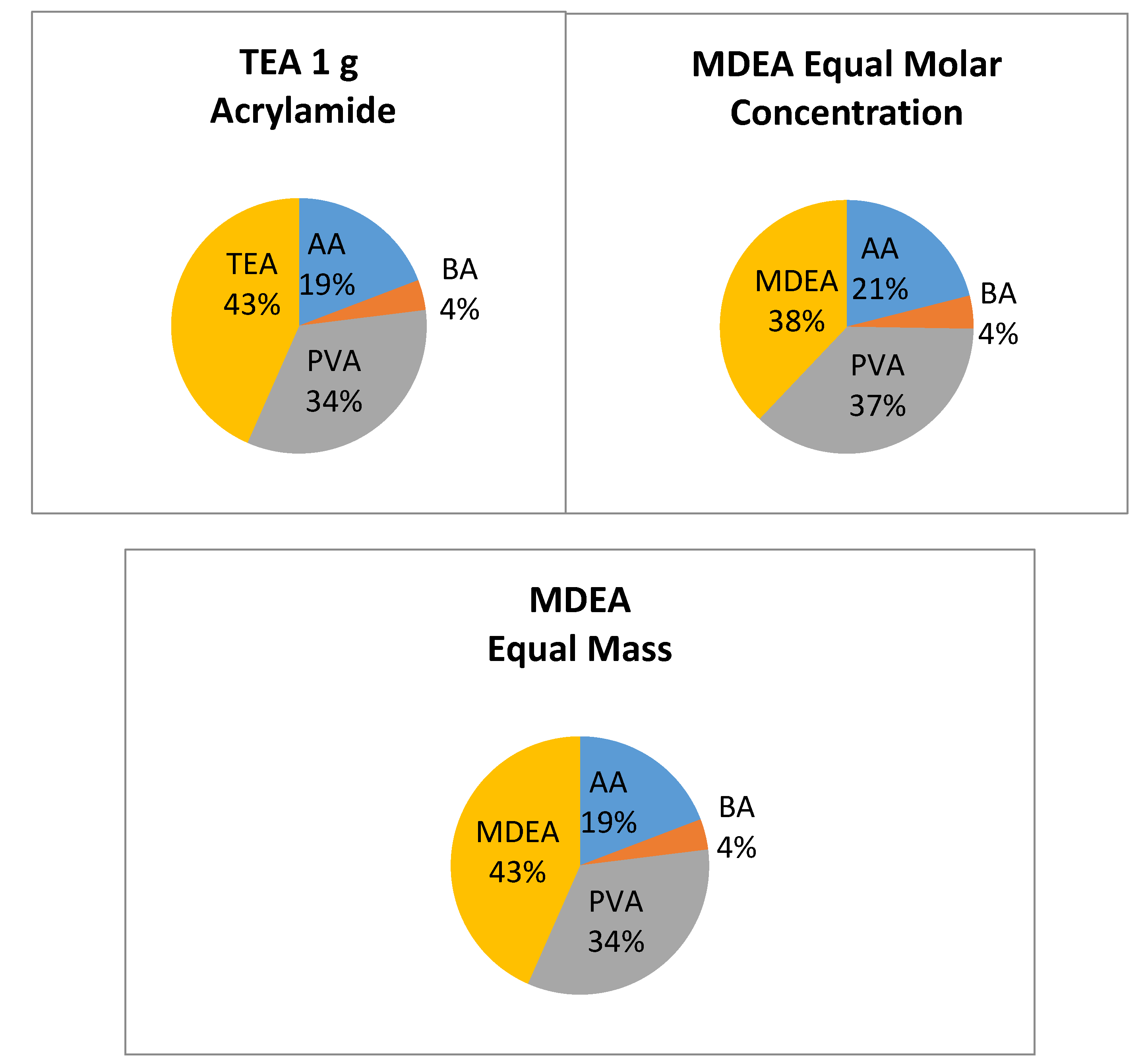
3.2. Preparation of Samples with Different Initiators
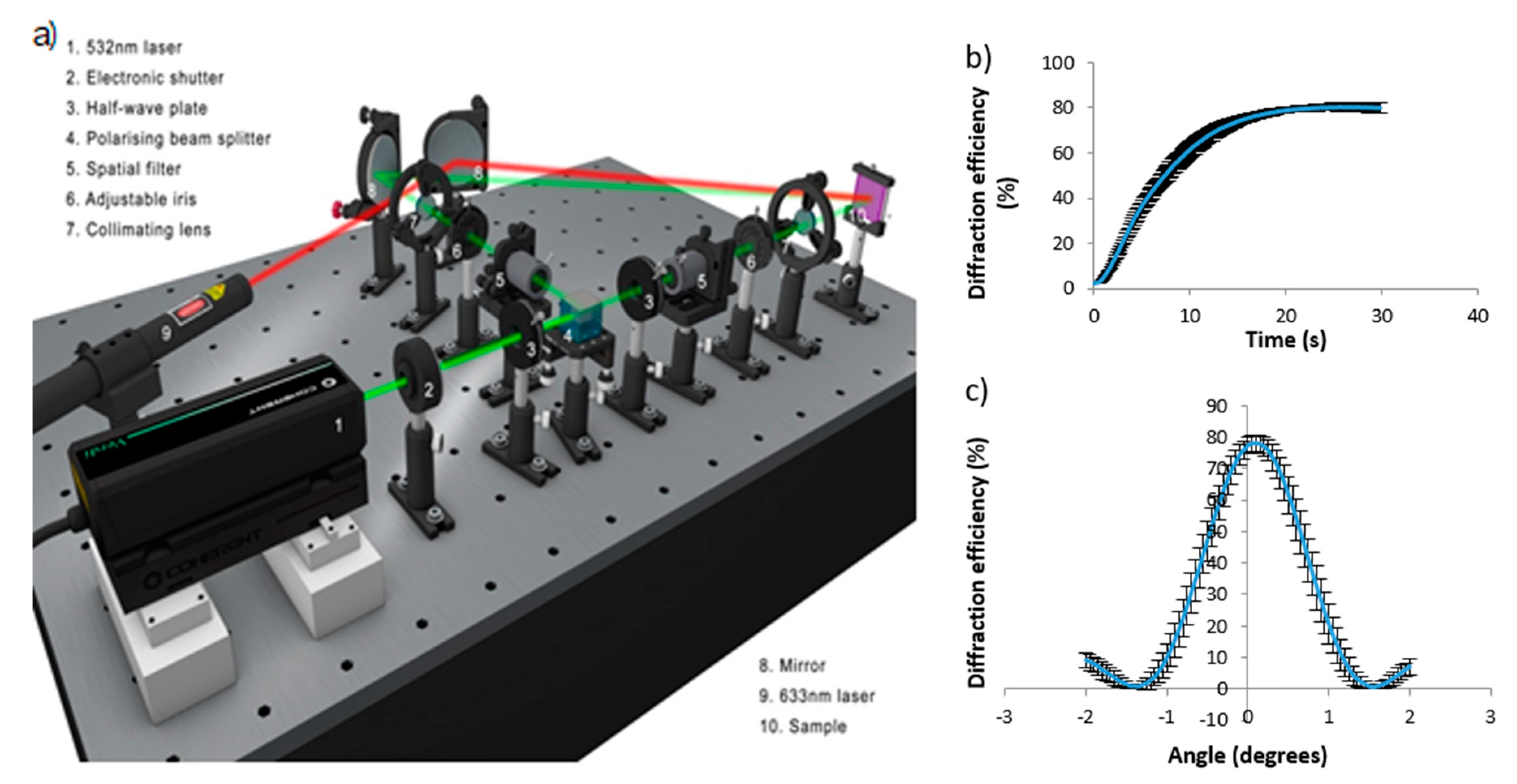
3.3. Recording
3.4. UV Stability Tests
4. Results
4.1. Effect of Monomer Concentration on Initial Rate of Recording
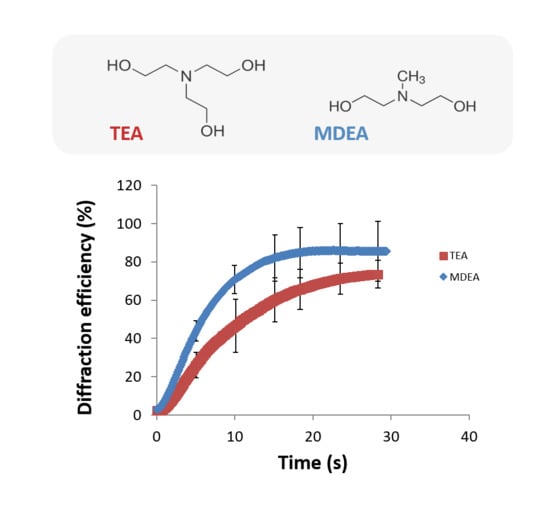
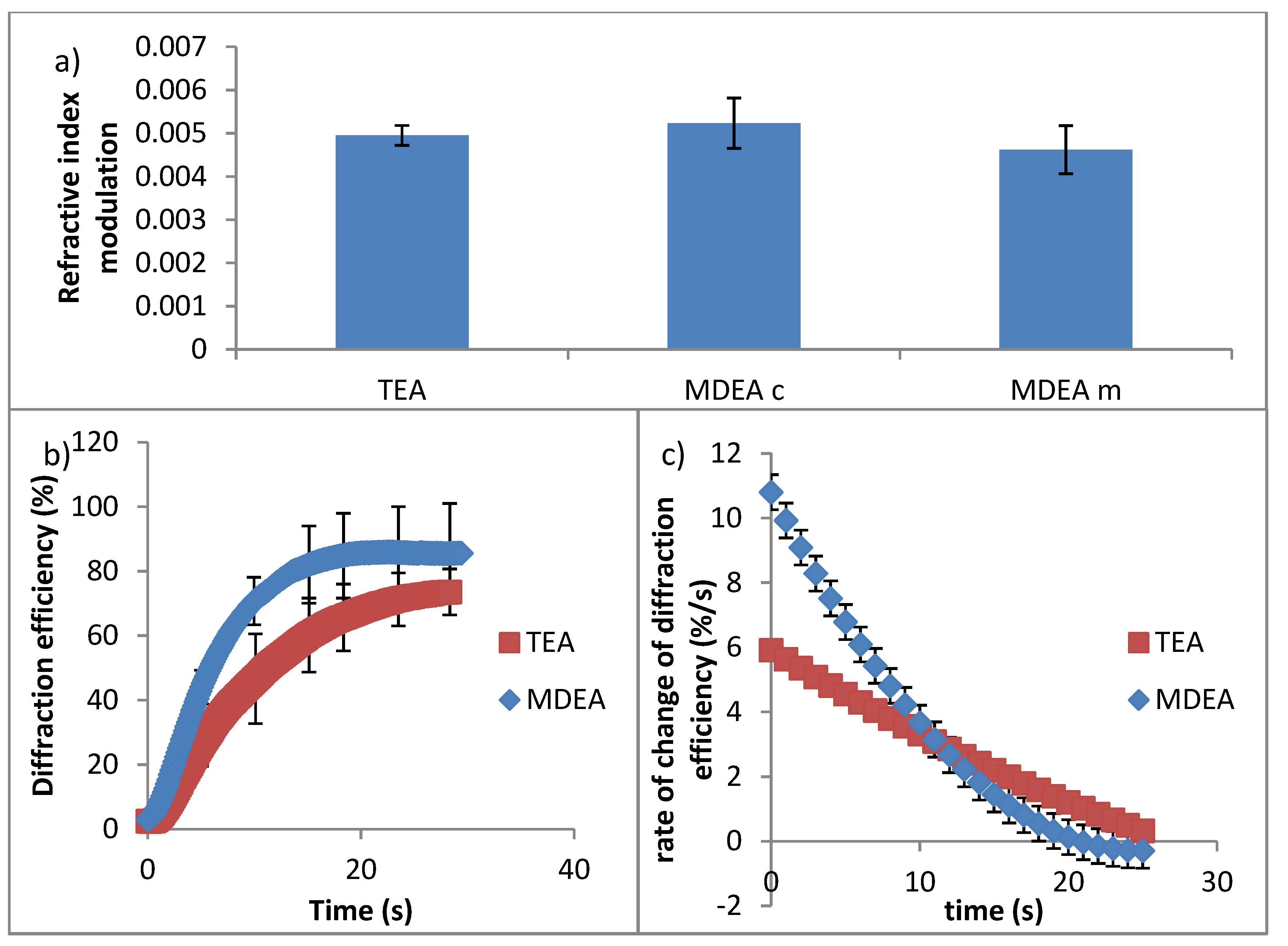
4.2. Study of the Effect of the Initiator on the Recording Properties of the Photopolymer
4.3. Investigation of the Effect of Exposure to UV Light on the Diffraction Efficiency of the Diffraction Gratings
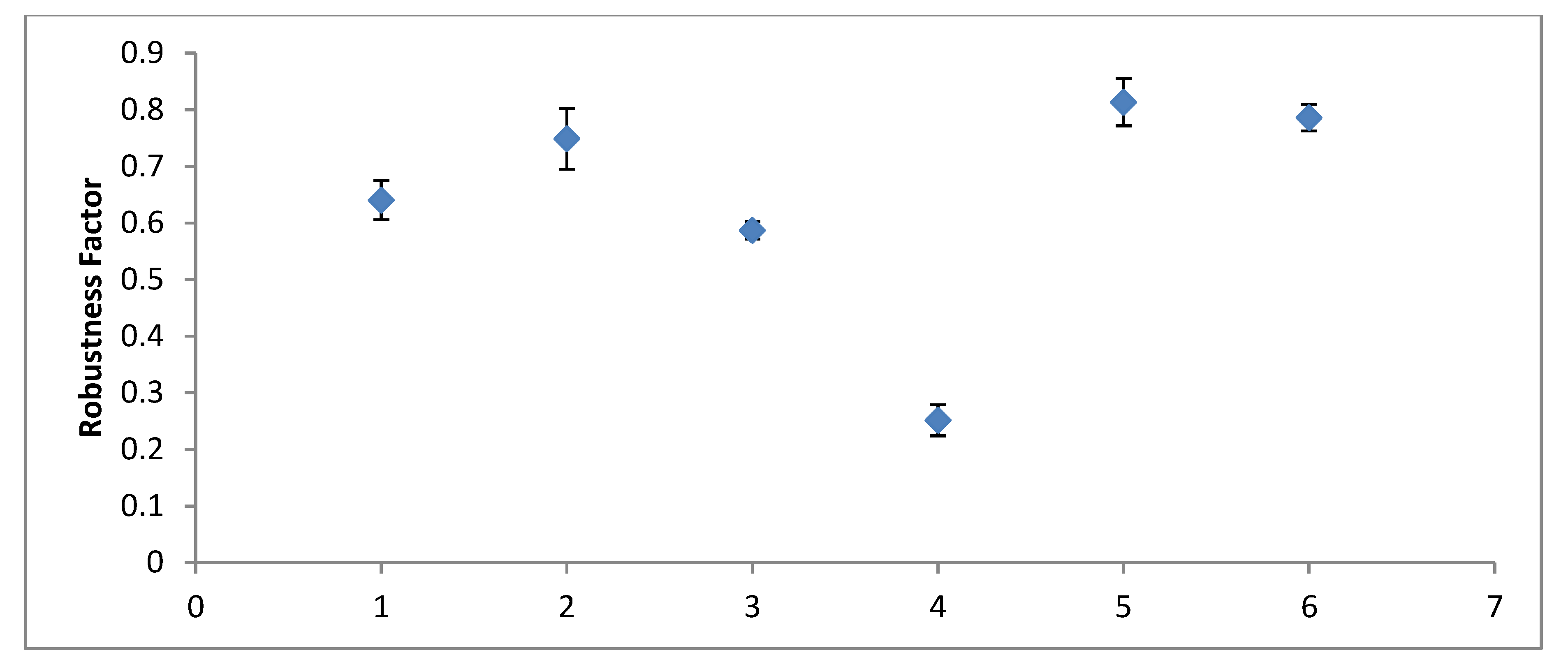
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jurbergs, D.; Bruder, F.K.; Deuber, F.; Fäcke, T.; Hagen, R.; Hönel, D.; Rölle, T.; Weiser, M.S.; Volkov, A. New recording materials for the holographic industry. In Proceedings of the SPIE 7233, Practical Holography XXIII: Materials and Applications, San Jose, CA, USA, 3 February 2009; p. 72330K. [Google Scholar]
- Collados, M.V.; Chemisana, D.; Atencia, J. Holographic solar energy systems: The role of optical elements. Renew. Sustain. Energy Rev. 2016, 59, 130–140. [Google Scholar] [CrossRef]
- Sherif, H.; Naydenova, I.; Martin, S.; McGinn, C.; Toal, V. Characterisation of an acrylamide-based photopolymer for data storage utilizing holographic angular multiplexing. J. Opt. A 2005, 7, 255–260. [Google Scholar] [CrossRef]
- Mikulchyk, T.; Martin, S.; Naydenova, I. Investigation of the sensitivity to humidity of an acrylamide-based photopolymer containing N-phenylglycine as a photoinitiator. Opt. Mater. 2014, 37, 810–815. [Google Scholar] [CrossRef][Green Version]
- Rajesh, C.S.; Aswathy, G.; Pillai, J.J.; Sreekumar, K.; Joseph, R.; Kartha, C.S. Stretching the sensitivity and stability of an acrylamide based photopolymer material with mixture of dyes. Opt Laser Technol. 2018, 104, 183–189. [Google Scholar] [CrossRef]
- Martin, S.; Leclere, P.; Renotte, Y.; Toal, V.; Lion, Y. Characterization of an acrylamide-based dry photopolymer holographic recording material. Opt. Eng. 1994, 33, 3942–3946. [Google Scholar]
- Hirabayashi, K.; Kanbara, H.; Mori, Y. Multilayer holographic recording using a two-color-absorption photopolymer. Appl. Opt. 2007, 46, 8402–8410. [Google Scholar] [CrossRef] [PubMed]
- Meka, C.; Jallapuram, R.; Naydenova, I.; Martin, S.; Toal, V. Development of a panchromatic acrylamide-based photopolymer for multicolor reaction holography. Appl. Opt. 2010, 49, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, W.; Feng, J.; Jiang, G.; Wang, X.; Liu, H. The effect of three electron donors on the initiator system efficiency of photopolymer film photosensitized by methylene blue. Mater. Des. 2017, 120, 186–192. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, S.; Huang, M.; Dong, Y.; Gan, F. Effects of dyes and initiators on the holographic data storage properties of photopolymer. In Proceedings of the SPIE Volume 5966, Seventh International Symposium on Optical Storage (ISOS 2005), Zhanjiang, China, 15 September 2005; p. 59660P. [Google Scholar]
- Ortuno, M.; Márquez, A.; Gallego, S.; Neipp, C.; Fernandez, E. Pyrromethene dye and non-redox initiator system in a hydro-philic binder photopolymer. Opt. Mater. 2007, 30, 227–230. [Google Scholar] [CrossRef]
- Ibrahim, A.; Di Stefano, L.; Tarzi, O. High-performance photoinitiating systems for free radical photopolymerization. Application to holographic recording. Photochem. Photobiol. 2013, 89, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.B.; Fioletov, V.E. Surface ultraviolet radiation. Atmos. Ocean 2008, 46, 159–184. [Google Scholar] [CrossRef]
- Martin, S. A New Photopolymer Recording Material for Holographic Applications: Photochemical and Holographic Studies towards an Optimized System. Ph.D. Thesis, Dublin Institute of Technology, Dublin, Ireland, 1995; p. 123. [Google Scholar]
- Rogers, B.; Martin, S.; Naydenova, I. Development of photopolymer material with improved dynamic range and sensitivity. In Proceedings of the SPIE 11081, Active Photonic Platforms XI, San Diego, CA, USA, 5 September 2019; p. 110812G. [Google Scholar]
- Mackey, D.; O’Reilly, P.; Naydenova, I. Theoretical modeling of the effect of polymer chain immobilization rates on holographic recording in photopolymers. JOSA A 2016, 33, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Cody, D.; Mihaylova, E.; O’Neill, L.; Babeva, T.; Awala, H.; Retoux, R.; Mintova, S.; Naydenova, I. Effect of zeolite nanoparticles on the optical properties of diacetone acrylamide-based photopolymer. Opt. Mater. 2014, 37, 181–187. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Li, Z.; Sun, X. Improvement of ultrafast holographic performance in silver nanoprisms dispersed photopolymer. Opt. Express 2018, 26, 6993–7004. [Google Scholar] [CrossRef] [PubMed]
- Kogelnik, H. Coupled wave theory for thick hologram gratings. Bell Syst. Technol. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Ghosh, A.; Nirala, A.K.; Yadav, H.L. Dependence of wavelength selectivity of holographic PV concentrator on processing parameters. Optik 2015, 126, 622–625. [Google Scholar] [CrossRef]
- Atkins, P.; De Paula, J. The Mobilities of Ions in “Physical Chemistry”, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2006; p. 765. [Google Scholar]
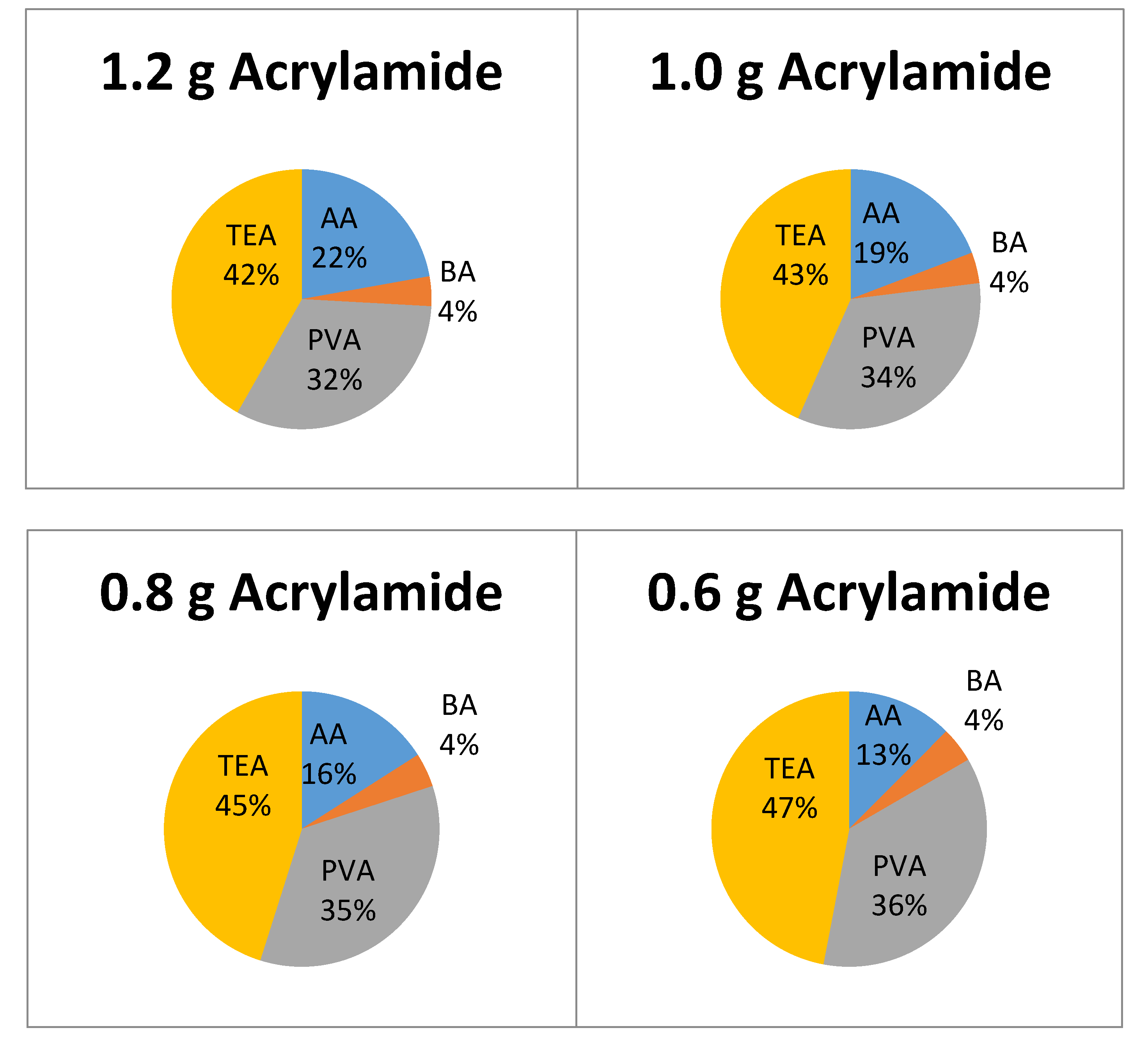
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, B.; Martin, S.; Naydenova, I. Study of the Effect of Methyldiethanolamine Initiator on the Recording Properties of Acrylamide Based Photopolymer. Polymers 2020, 12, 734. https://doi.org/10.3390/polym12040734
Rogers B, Martin S, Naydenova I. Study of the Effect of Methyldiethanolamine Initiator on the Recording Properties of Acrylamide Based Photopolymer. Polymers. 2020; 12(4):734. https://doi.org/10.3390/polym12040734
Chicago/Turabian StyleRogers, Brian, Suzanne Martin, and Izabela Naydenova. 2020. "Study of the Effect of Methyldiethanolamine Initiator on the Recording Properties of Acrylamide Based Photopolymer" Polymers 12, no. 4: 734. https://doi.org/10.3390/polym12040734
APA StyleRogers, B., Martin, S., & Naydenova, I. (2020). Study of the Effect of Methyldiethanolamine Initiator on the Recording Properties of Acrylamide Based Photopolymer. Polymers, 12(4), 734. https://doi.org/10.3390/polym12040734