Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Light-Colored Lignin
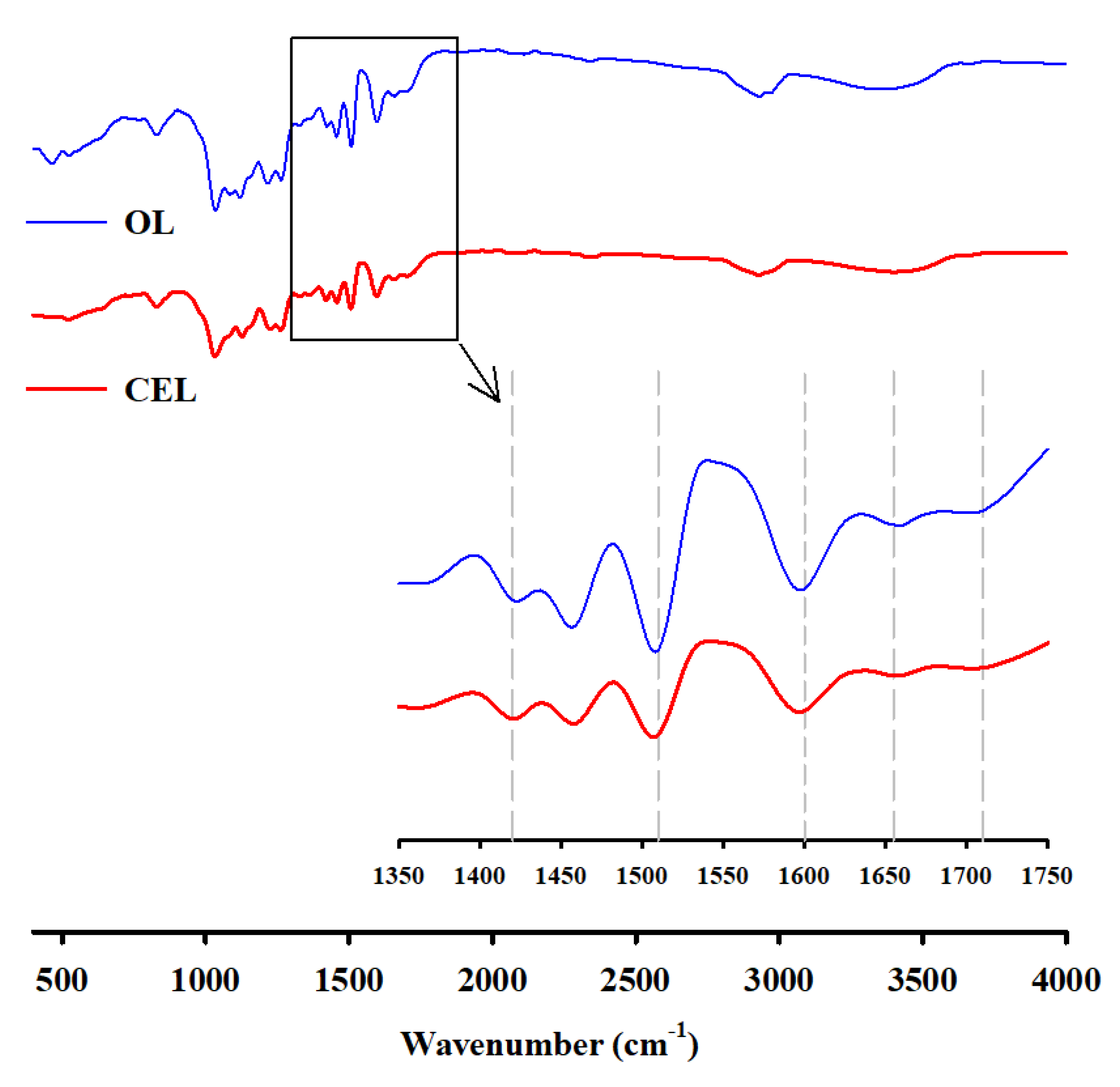
2.3. Color Analysis and FTIR Spectroscopy of Light-Colored Lignin
2.4. Preparation of Lignin Nanoparticles
2.5. Characterization of CEL-NP
2.6. Compositional Analysis of Rice Husks and the Prepared Lignins
2.7. Preparation and UV Transmittance Measurement of CEL-NP-Blended Creams
3. Results and Discussion
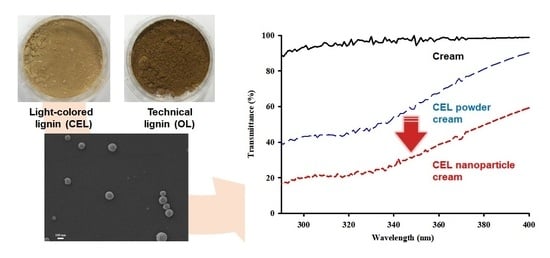
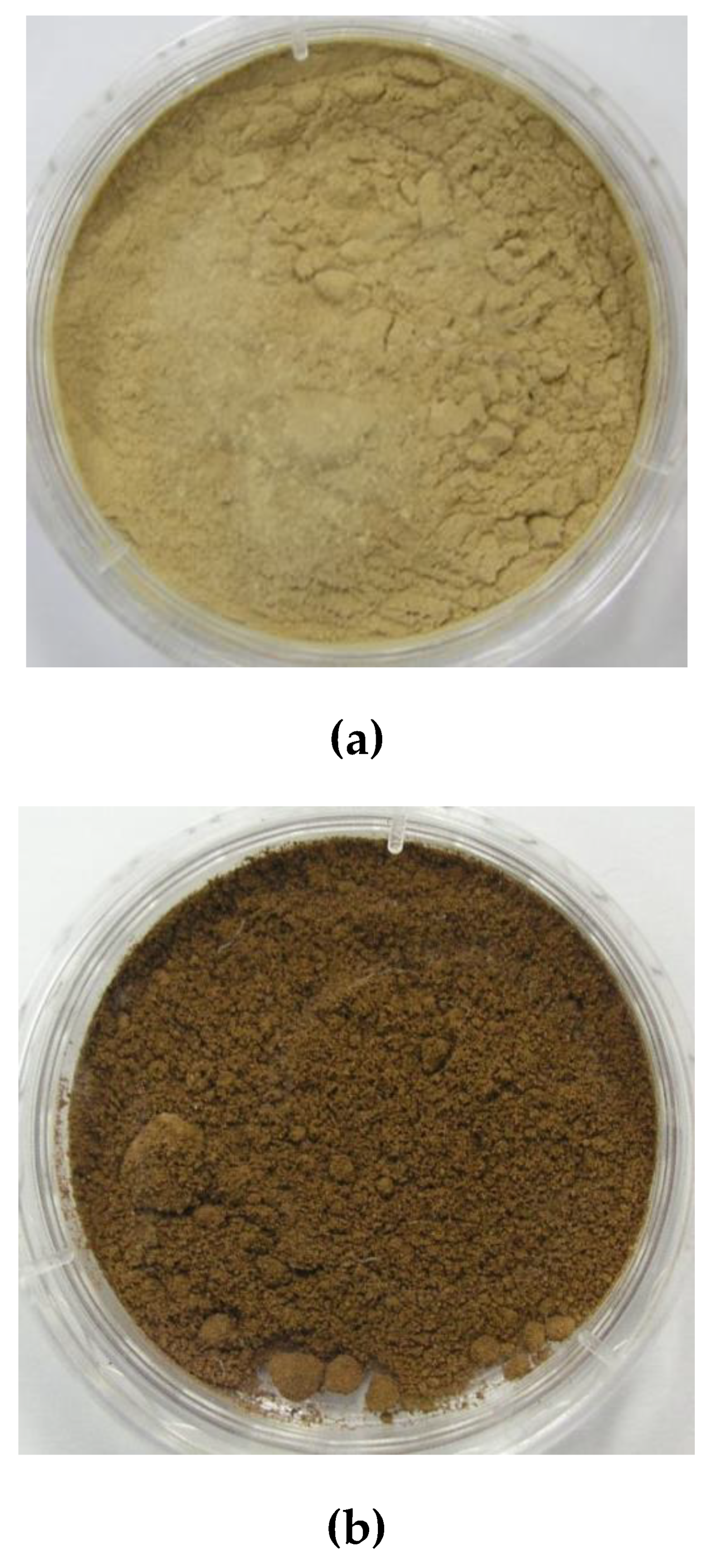
3.1. Preparation and Color Evaluation of Light-Colored Lignin
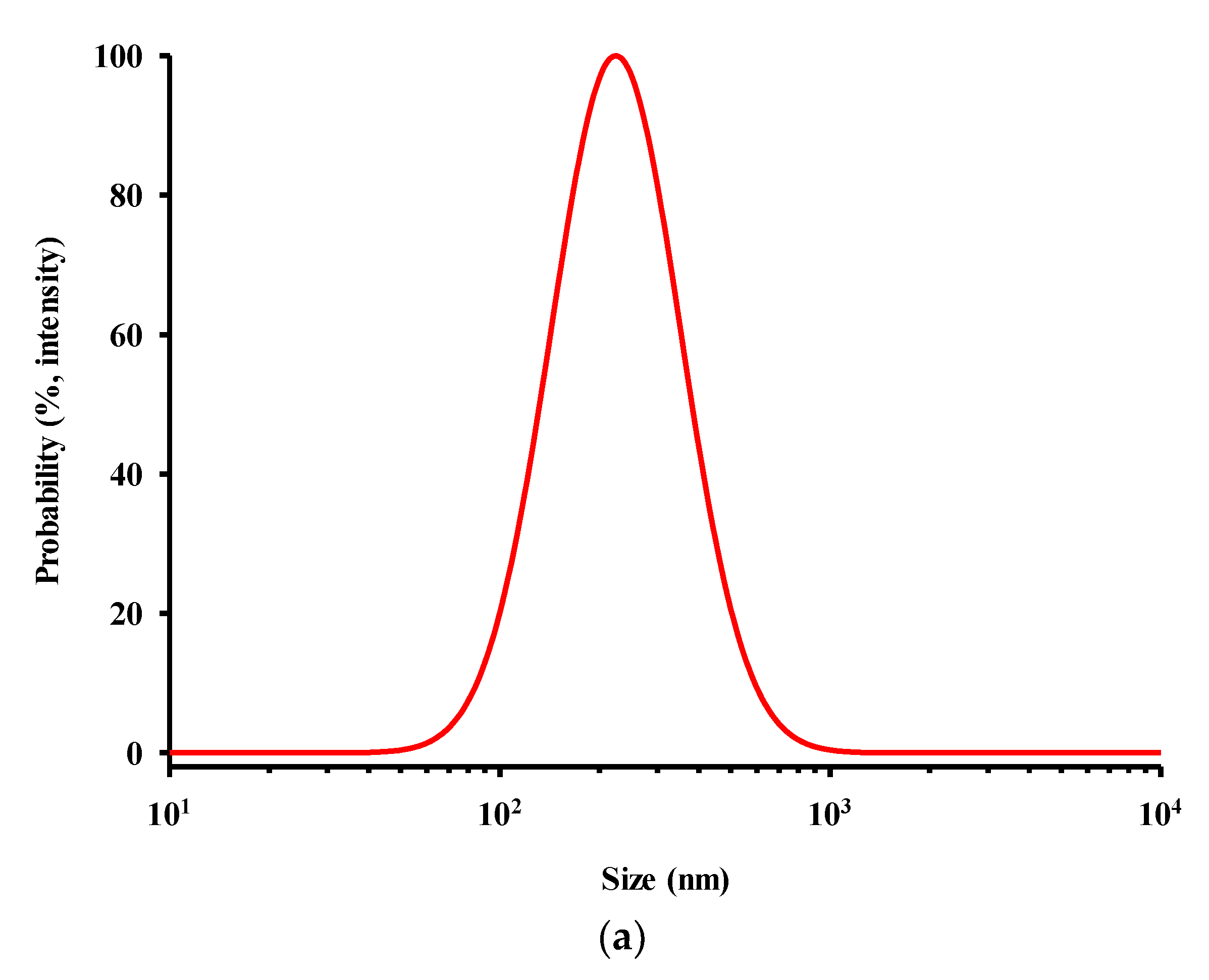
3.2. Preparation and Characterization of CEL-NP
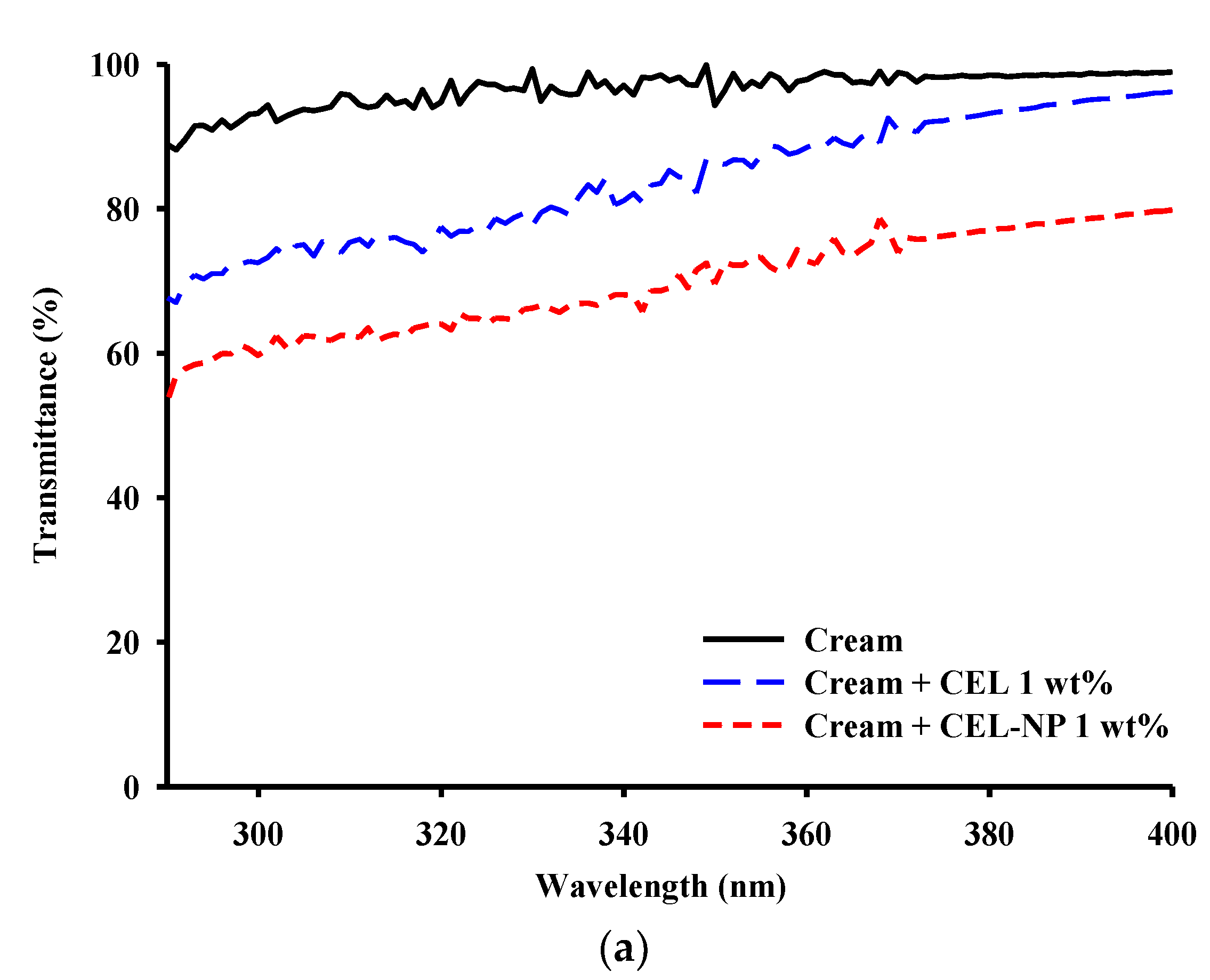
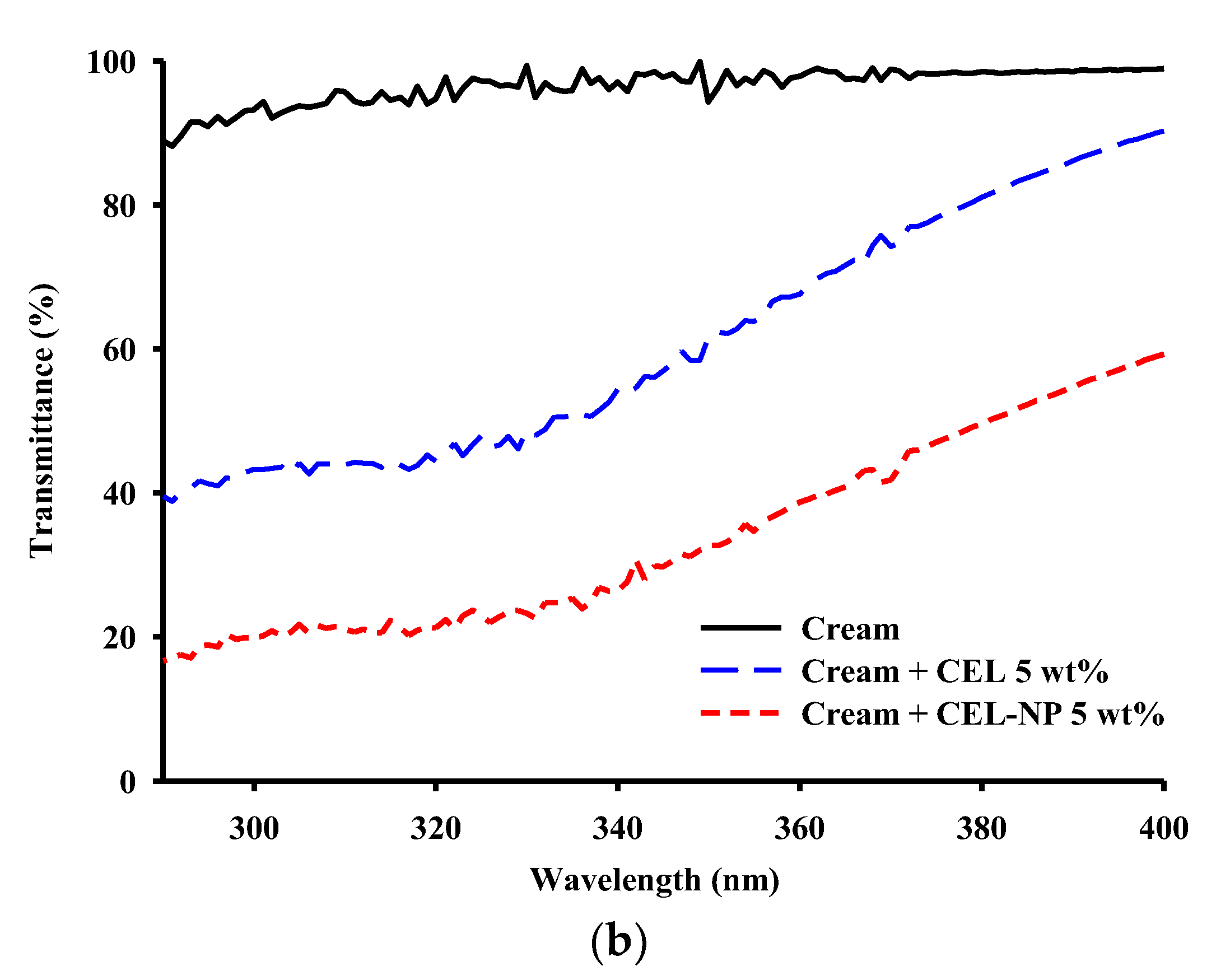
3.3. UV Blocking Performance of CEL-NP-Blended Cream
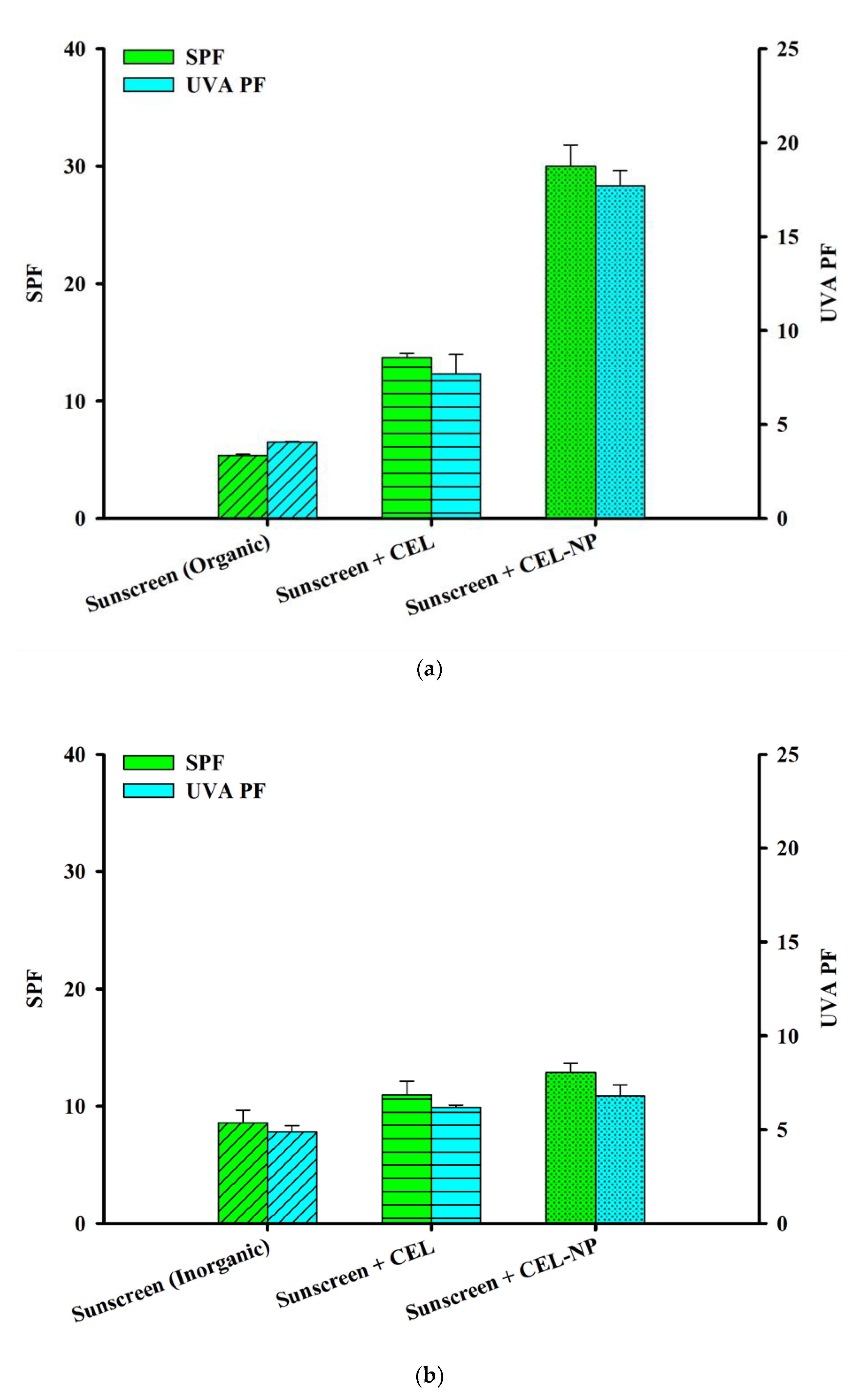
3.4. Synergistic Effects of CEL-NP with Sunscreens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morabito, K.; Shapley, N.C.; Steeley, K.G.; Tripathi, A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int. J. Cosmet. Sci. 2011, 33, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.T.; Christensen, T. Toxicity and phototoxicity of chemical sun filters. Radiat. Prot. Dosim. 2000, 91, 283–286. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J.W.; Won, K. Preparation of poly(lactide)/lignin/silver nanoparticles composite films with UV light barrier and antibacterial properties. Int. J. Biol. Macromol. 2018, 107, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent cellulose/technical lignin composite films for advanced packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Adler, E. Lignin chemistry-past, present and future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Gao, J.; Li, H. Coloring characteristics of in situ lignin during heat treatment. Wood Sci. Technol. 2012, 46, 33–40. [Google Scholar]
- Kim, J.Y.; Hwang, H.; Oh, S.; Kim, Y.S.; Kim, U.J.; Choi, J.W. Investigation of structural modification and thermal characteristics of lignin after heat treatment. Int. J. Biol. Macromol. 2014, 66, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ajao, O.; Jeaidi, J.; Benali, M.; Restrepo, A.M.; El Mehdi, N.; Boumghar, Y. Quantification and variability analysis of lignin optical properties for colour-dependent industrial applications. Molecules 2018, 23, 377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for white natural sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lee, S.H.; Won, K. Wood powder as a new natural sunscreen ingredient. Biotechnol. Bioprocess Eng. 2019, 24, 258–263. [Google Scholar] [CrossRef]
- Chang, H.M.; Cowling, E.B.; Brown, W. Comparative studies on cellulolytic enzyme lignin and milled wood lignin of sweetgum and spruce. Holzforschung 1975, 29, 153–159. [Google Scholar] [CrossRef]
- Wang, K.; Bauer, S.; Sun, R.-C. Structural transformation of Miscanthus × giganteus lignin fractionated under mild formosolv, basic organosolv, and cellulolytic enzyme conditions. J. Agric. Food Chem. 2012, 60, 144–152. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Wang, H.-M.; Yuan, T.-Q.; Sun, R.-C. Green and facile preparation of regular lignin nanoparticles with high yield and their natural broad-spectrum sunscreens. ACS Sustain. Chem. Eng. 2019, 7, 2658–2666. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure. Natl. Renew. Energy Lab. 2008, 1617, 1–16. [Google Scholar]
- Diffey, B.L.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Dimitrovska Cvetkovska, A.; Manfredini, S.; Ziosi, P.; Molesini, S.; Dissette, V.; Magri, I.; Scapoli, C.; Carrieri, A.; Durini, E.; Vertuani, S. Factors affecting SPF in vitro measurement and correlation with in vivo results. Int. J. Cosmet. Sci. 2017, 39, 310–319. [Google Scholar] [CrossRef]
- Ferrero, L.; Pissavini, M.; Marguerie, S.; Zastrow, L. Sunscreen in vitro spectroscopy: Application to UVA protection assessment and correlation with in vivo persistent pigment darkening. Int. J. Cosmet. Sci. 2002, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.J.; Yeh, T.F.; Chang, H.M.; Matsumoto, Y.; Kadla, J.F. Elucidation of the structure of cellulolytic enzyme lignin. Holzforschung 2006, 60, 389–397. [Google Scholar] [CrossRef]
- Salanti, A.; Zoia, L.; Orlandi, M.; Zanini, F.; Elegir, G. Structural characterization and antioxidant activity evaluation of lignins from rice husk. J. Agric. Food Chem. 2010, 58, 10049–10055. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Koch, H.; Fischer, K. Role of hemicelluloses in the formation of chromophores during heat treatment of bleached chemical pulps. Macromol. Symp. 2006, 232, 98–106. [Google Scholar] [CrossRef]
- Salca, E.-A.; Kobori, H.; Inagaki, T.; Yoichi Kojima, Y.; Suzuki, S. Effect of heat treatment on colour changes of black alder and beech veneers. J. Wood Sci. 2016, 62, 297–304. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Fan, Y.; Tshabalala, M.A.; Stark, N.M. Heat-induced chemical and color changes of extractive-free black locust (Robinia pseudoacacia) wood. BioResources 2012, 7, 2236–2248. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Gao, J.; Stark, N.M. The effect of heat treatment on the chemical and color change of black locust (Robinia pseudoacacia) wood flour. BioResources 2012, 7, 1157–1170. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhepe, P.L. Isolation of lignin by organosolv process from different varieties of rice husk: Understanding their physical and chemical properties. Bioresour. Technol. 2016, 221, 310–317. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from micro- to nanosize: Production methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for nano- and microscaled carrier systems: Applications, trends, and challenges. ChemSusChem 2019, 12, 2039–2054. [Google Scholar] [CrossRef]
- Henn, A.; Mattinen, M.-L. Chemo-enzymatically prepared lignin nanoparticles for value-added applications. World J. Microbiol. Biotechnol. 2019, 35, 125. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Wimmer, R. Aerosol assisted self-assembly as a route to synthesize solid and hollow spherical lignin colloids and its utilization in layer by layer deposition. Ultrason. Sonochem. 2017, 35, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of renewable lignin hollow nanospheres with a single hole by self-assembly. ACS Sustain. Chem. Eng. 2017, 5, 2273–2281. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crop. Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Liu, X.; Fu, S. Micromorphology influence on the color performance of lignin and its application in guiding the preparation of light-colored lignin sunscreen. ACS Sustain. Chem. Eng. 2018, 6, 12532–12540. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crop. Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The self-assembly of lignin and its application in nanoparticle synthesis: A short review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, X.; Zhou, M.; Qian, Y.; Yu, H.; Qiu, X. Investigation of aggregation and assembly of alkali lignin using iodine as a probe. Biomacromolecules 2011, 12, 1116–1125. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, C.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Seeking brightness from nature: J-aggregation-induced emission in cellulolytic enzyme lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 3169–3175. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Sunscreen performance of lignin from different technical resources and their general synergistic effect with synthetic sunscreens. ACS Sustain. Chem. Eng. 2016, 4, 4029–4035. [Google Scholar] [CrossRef]



| Sample | Composition (%) | |||
|---|---|---|---|---|
| Glucan | Hemicellulose | Lignin | Ash | |
| RH | 31.7 | 15.8 | 23.3 | 20.0 |
| OL | 1.2 | 6.6 | 84.5 | ND |
| CEL | 2.2 | 8.9 | 85.1 | ND |
| CEL-NP | 1.0 | 6.3 | 89.5 | ND |
| Sample | SCI | SCE | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | L* | a* | b* | ΔE | |
| White reference a | 99.5 | −0.1 | −0.1 | 0.0 | 97.3 | −0.1 | 0.1 | 0.0 |
| RH | 71.6 | 4.6 | 21.3 | 35.5 | 65.3 | 5.2 | 26.5 | 41.8 |
| OL | 55.1 | 7.5 | 15.9 | 47.8 | 44.4 | 10.1 | 27.1 | 60.2 |
| CEL | 66.8 | 5.4 | 15.9 | 36.8 | 59.9 | 6.3 | 20.3 | 42.9 |
| Sample (wt %) | CEL | CEL-NP | ||
|---|---|---|---|---|
| SPF (290–400 nm) | UVA PF (320–400 nm) | SPF (290–400 nm) | UVA PF (320–400 nm) | |
| 1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.0 |
| 5 | 2.2 ± 0.1 | 1.5 ± 0.0 | 4.3 ± 0.4 | 2.6 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens. Polymers 2020, 12, 699. https://doi.org/10.3390/polym12030699
Lee SC, Yoo E, Lee SH, Won K. Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens. Polymers. 2020; 12(3):699. https://doi.org/10.3390/polym12030699
Chicago/Turabian StyleLee, Sang Cheon, Eunjin Yoo, Sang Hyun Lee, and Keehoon Won. 2020. "Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens" Polymers 12, no. 3: 699. https://doi.org/10.3390/polym12030699
APA StyleLee, S. C., Yoo, E., Lee, S. H., & Won, K. (2020). Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens. Polymers, 12(3), 699. https://doi.org/10.3390/polym12030699