Shape Tuning and Size Prediction of Millimeter-Scale Calcium-Alginate Capsules with Aqueous Core
Abstract
1. Introduction
2. Experimental
2.1. Materials
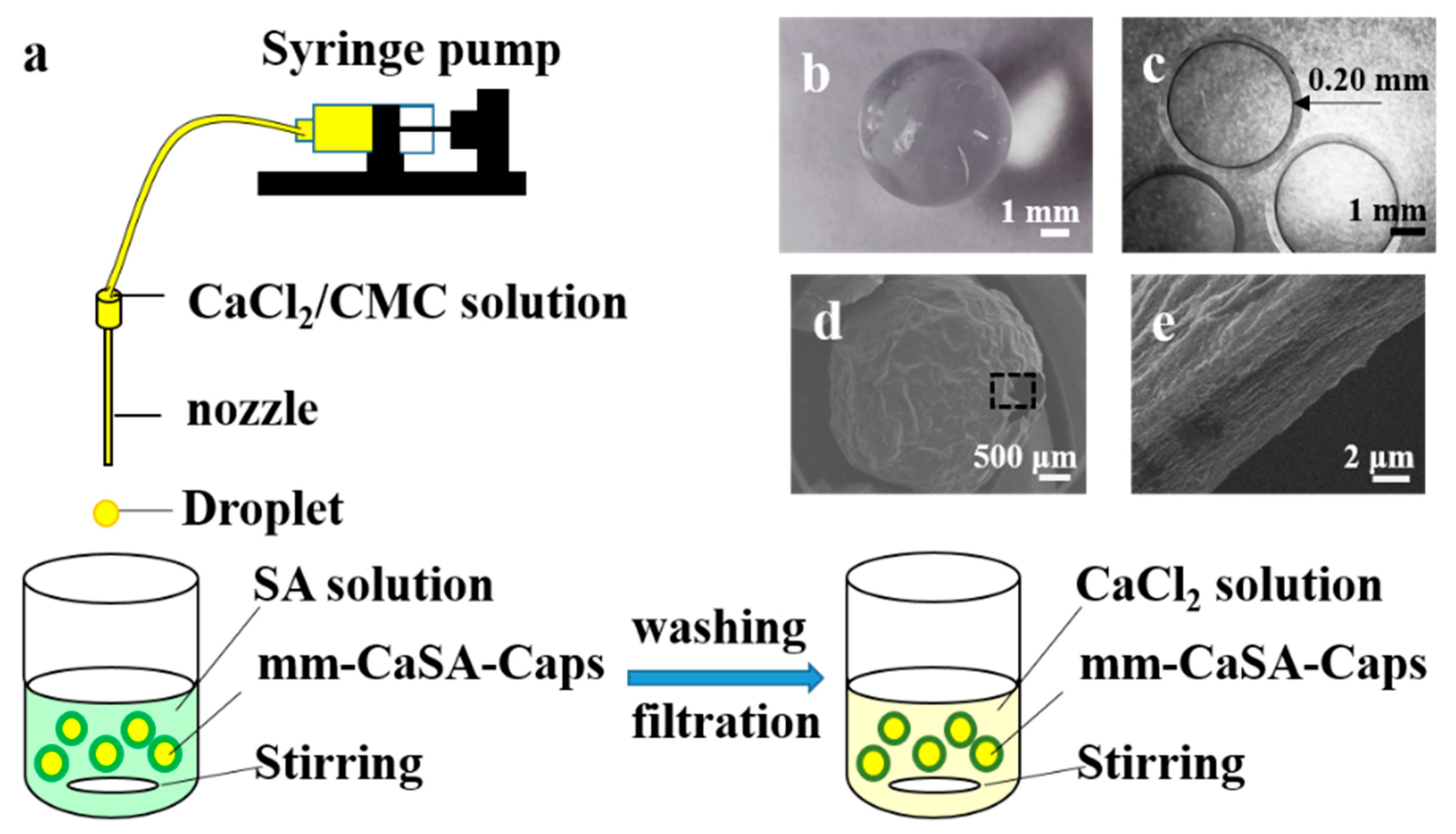
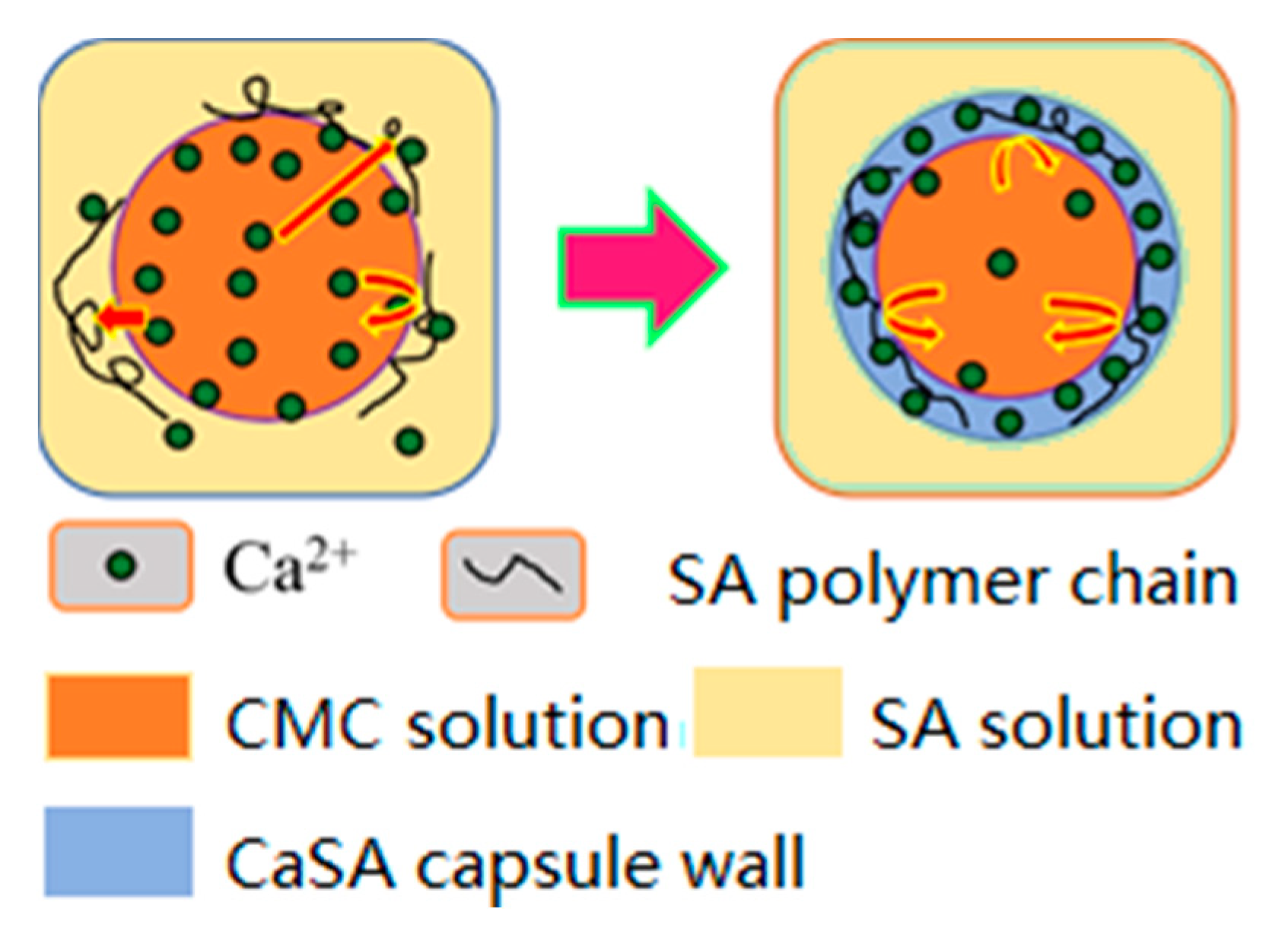
2.2. Preparation of mm-CaSA-Caps
2.3. Experimentation
2.4. Measurement of Solution Properties
2.5. Capsule Characterization
2.6. Prediction of the Diameter of mm-CaSA-Caps
3. Results and Discussion
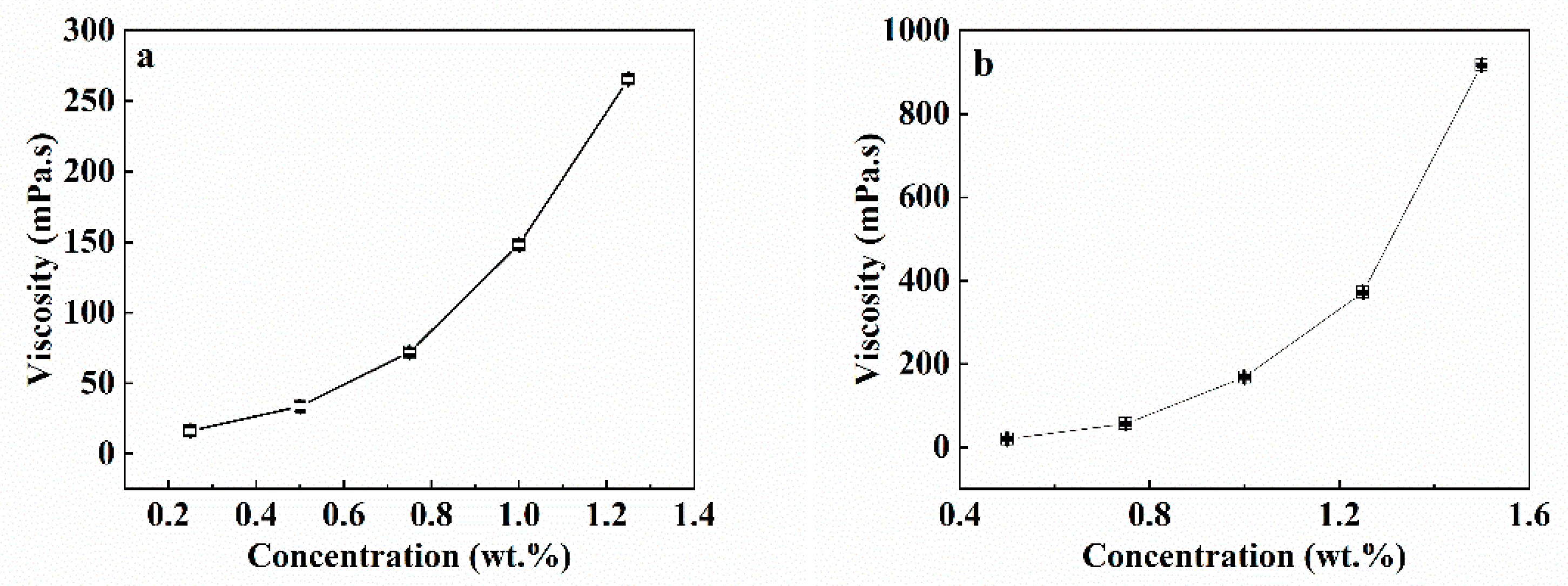
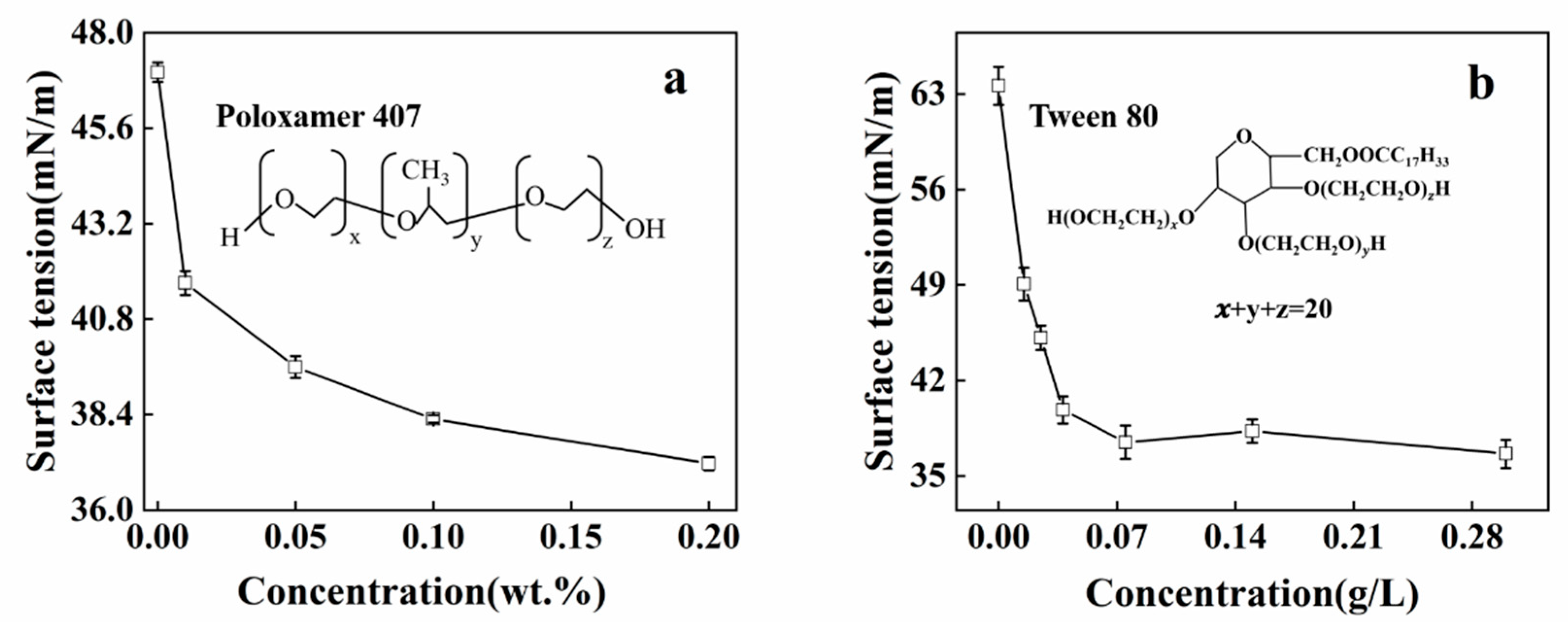
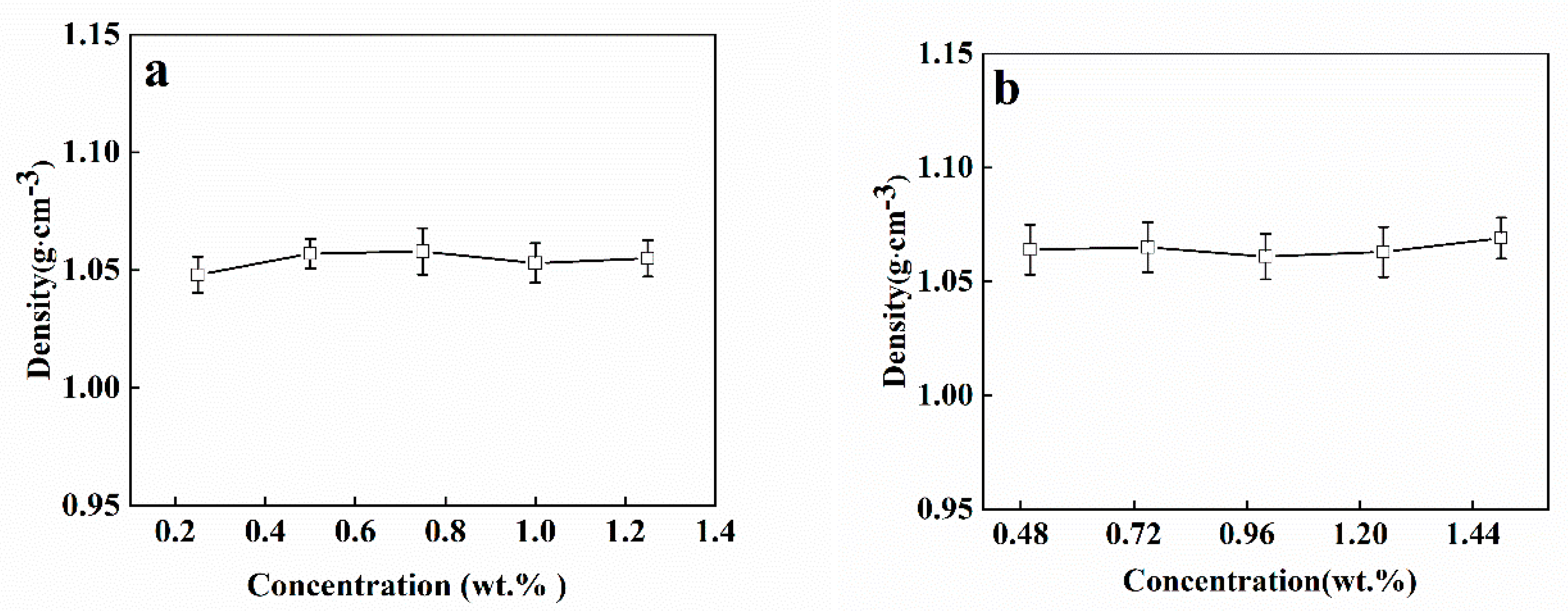
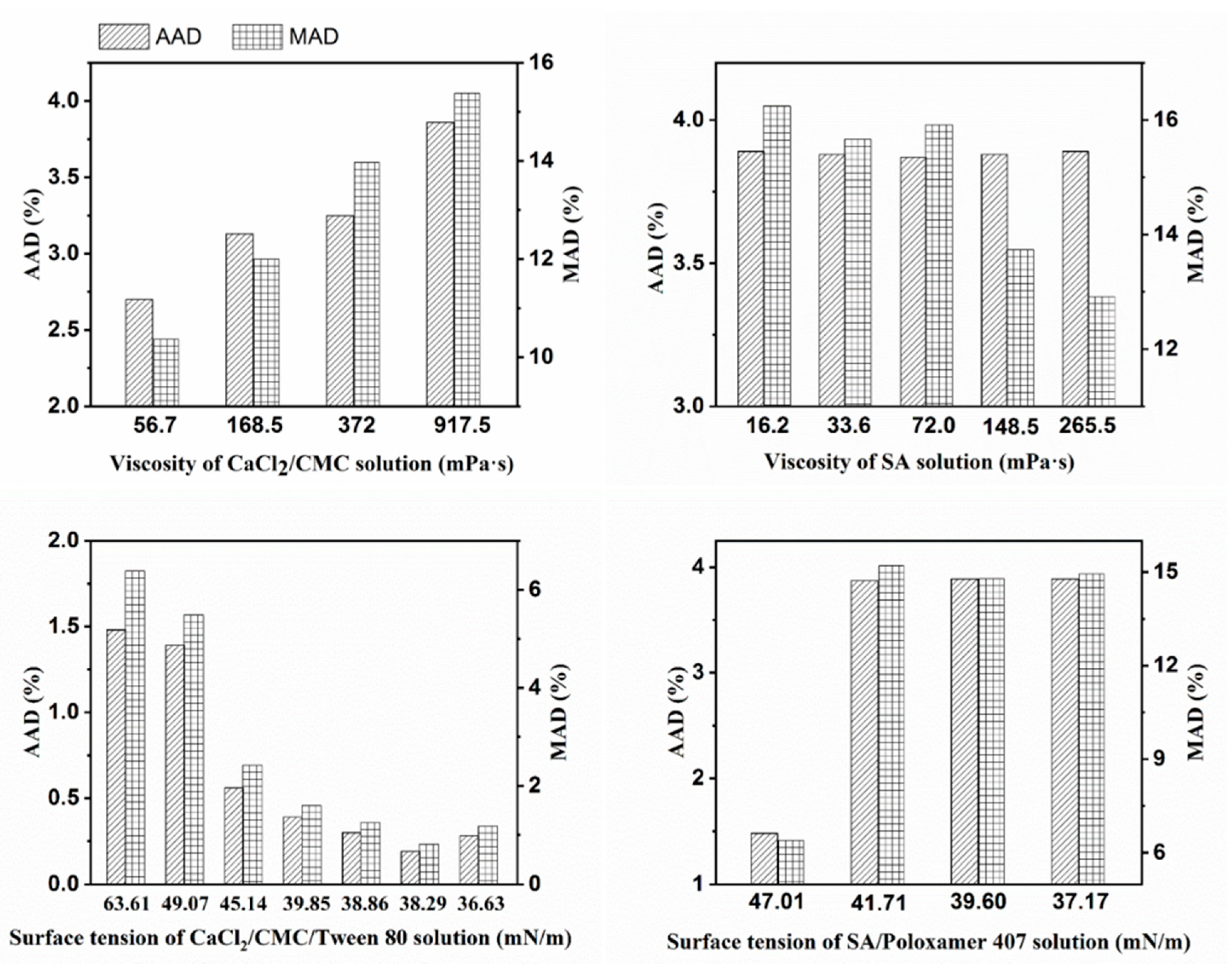
3.1. Solution Properties
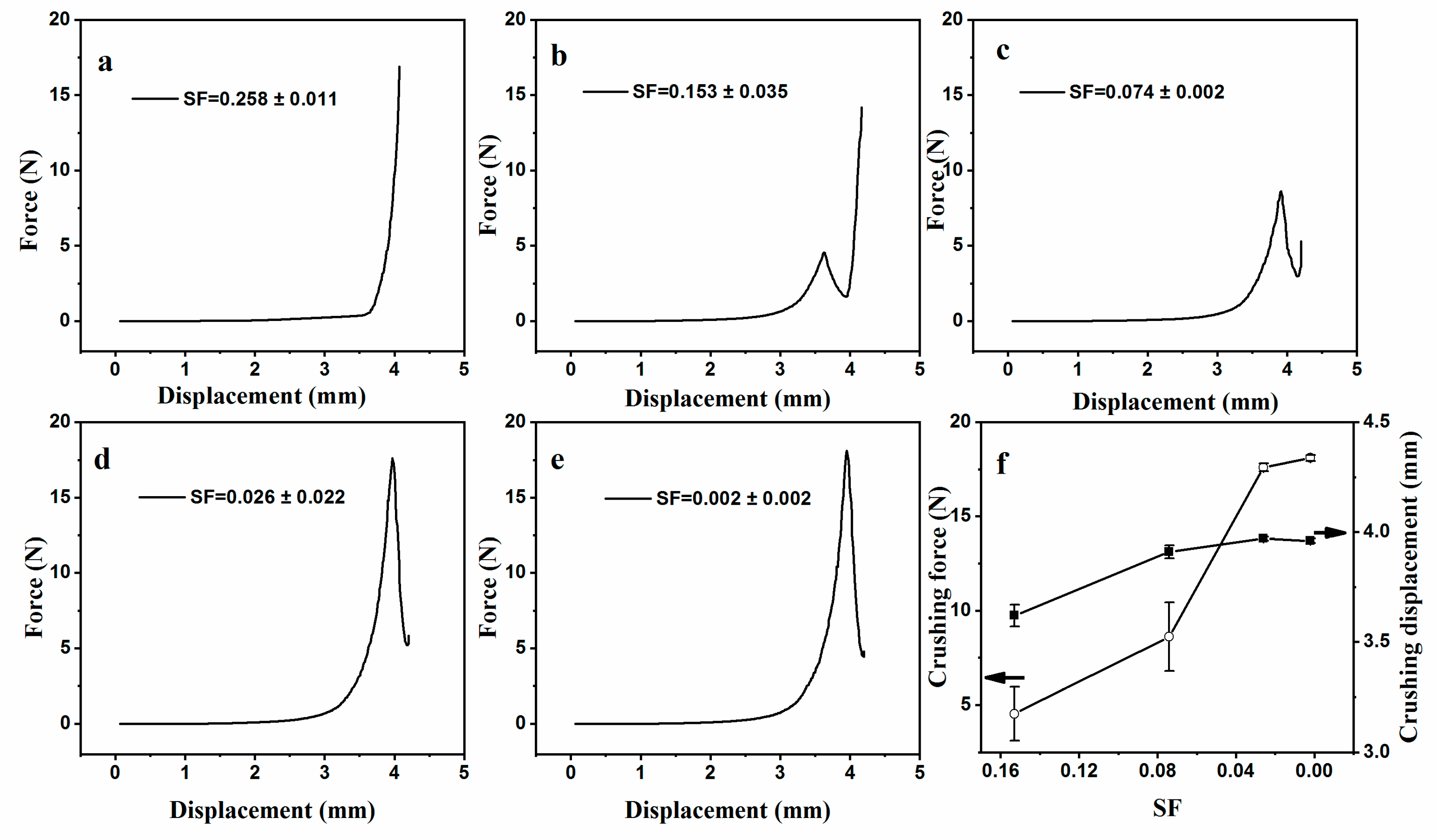
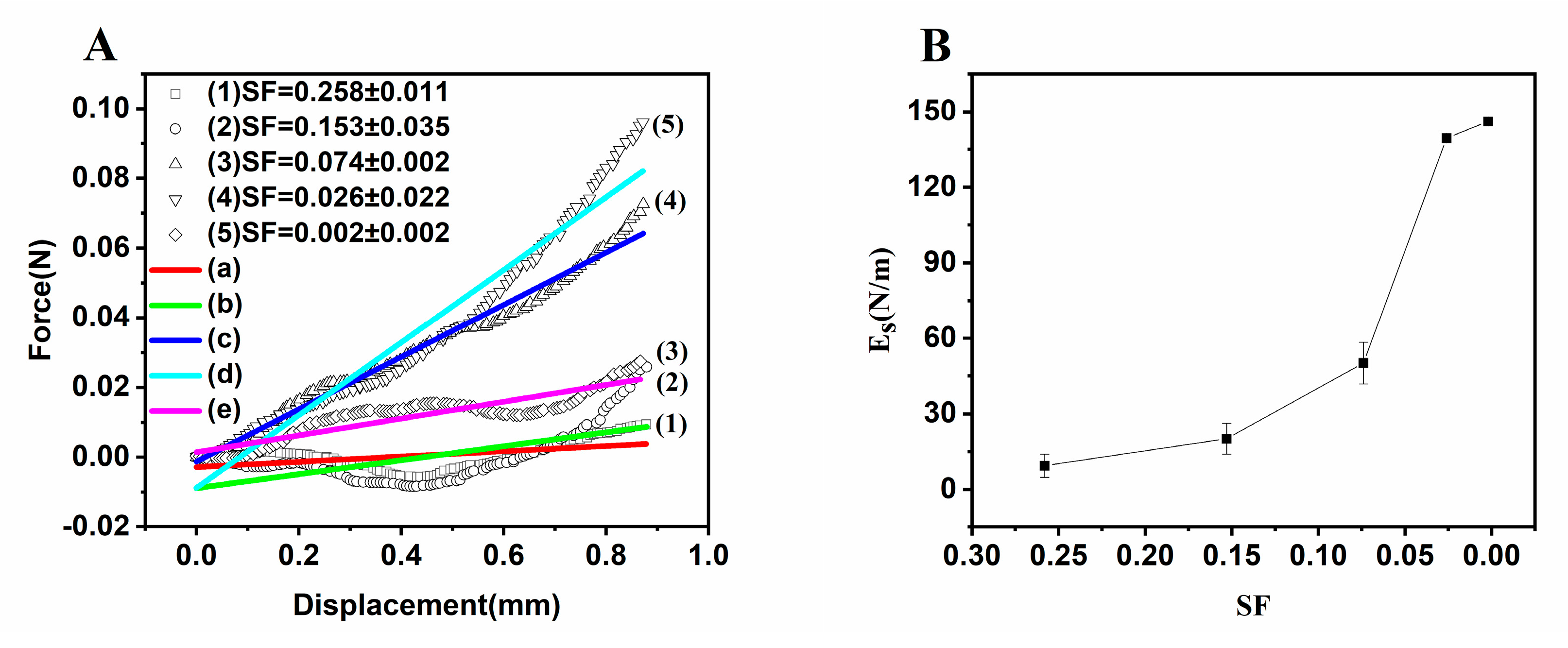
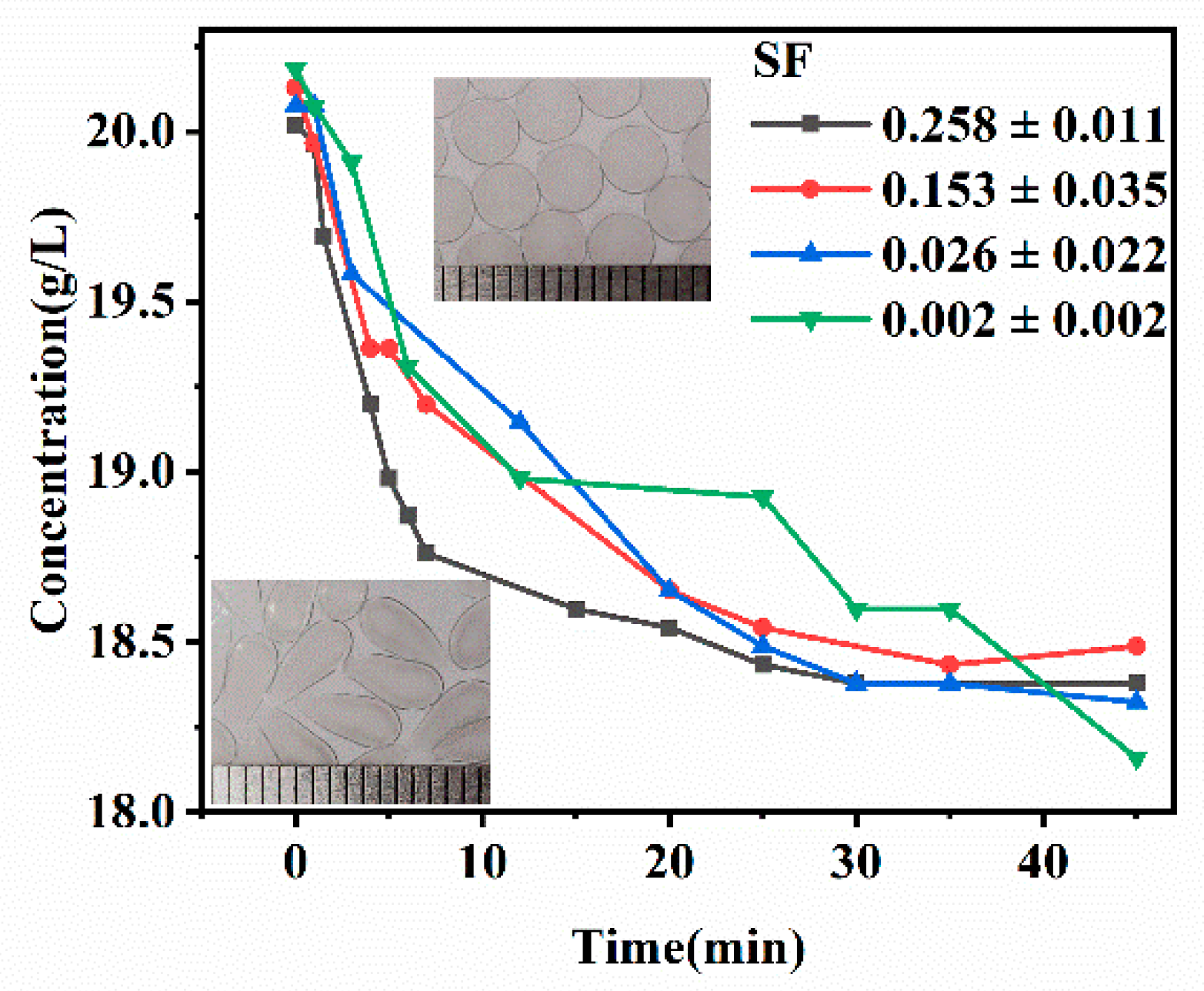
3.2. Tuning Mechanical and Permeation Properties by Controlling SF
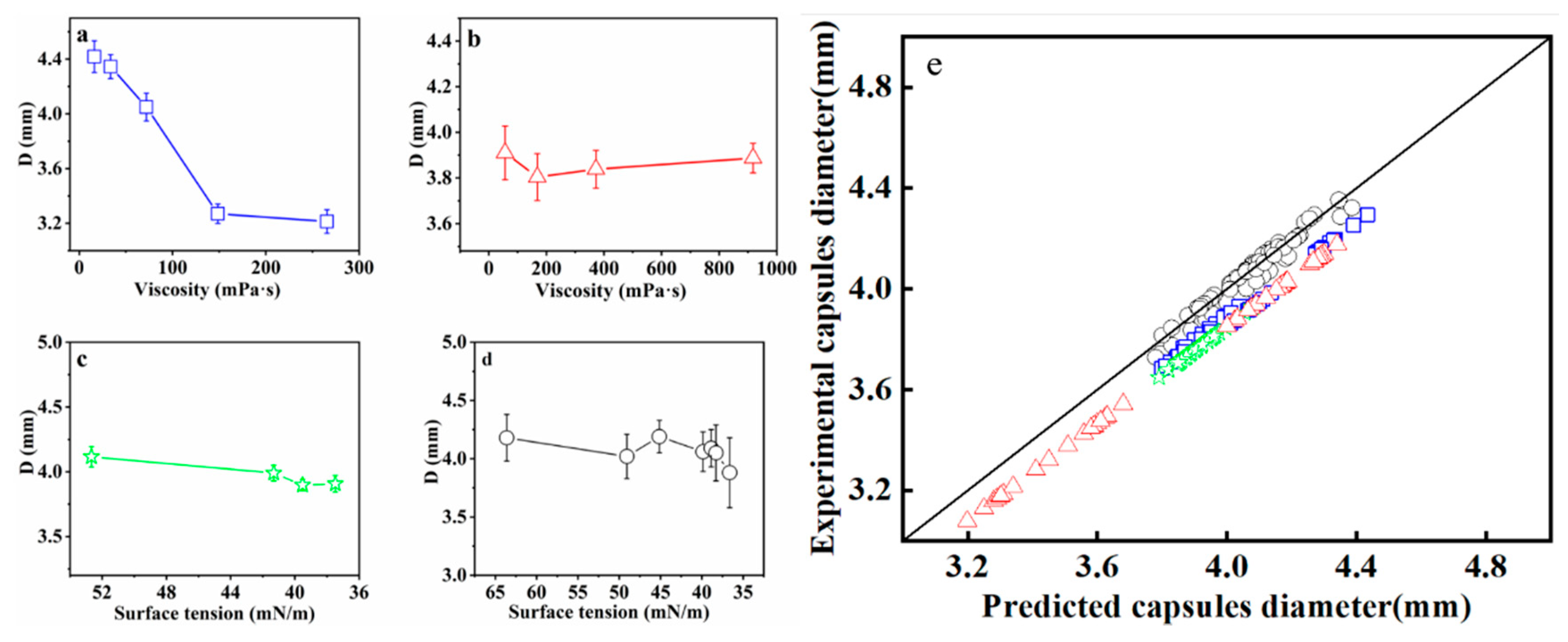
3.3. Size Control and Prediction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davarcı, F.; Turan, D.; Ozcelik, B.; Poncelet, D. The influence of solution viscosities and surface tension on calcium-alginate microbead formation using dripping technique. Food Hydrocolloid 2017, 62, 119–127. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Prasertsan, P. Enhanced thermal stability of cyclodextrin glycosyltransferase in alginate–gelatin mixed gel beads and the application for β-cyclodextrin production. Biocatal. Agric. Biotechnol. 2015, 4, 717–726. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Park, J.K.; Chang, H.N. Microencapsulation of microbial cells. Biotechnol. Adv. 2000, 18, 303–319. [Google Scholar] [CrossRef]
- Bremond, N.; Santanach-Carreras, E.; Chu, L.Y.; Bibette, J. Formation of liquid-core capsules having a thin hydrogel membrane: Liquid pearls. Soft Matter 2010, 6, 2484–2488. [Google Scholar] [CrossRef]
- Blandino, A.; Macı́as, M.; Cantero, D. Immobilization of glucose oxidase within calcium alginate gel capsules. Process. Biochem. 2001, 36, 601–606. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Y.; Xie, R.; Liu, J.; Ju, X.; Meng, T.; Chu, L. Novel calcium-alginate capsules with aqueous core and thermo-responsive membrane. J. Colloid. Interface Sci. 2011, 353, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.C.; Tsao, I.F.; Sakoda, A.; Wang, H. Techniques for preparing hydrogel membrane capsules. Biotechnol. Tech. 1988, 2, 271–276. [Google Scholar] [CrossRef]
- Wischnewski, C.; Zwar, E.; Rehage, H.; Kierfeld, J. Strong deformation of ferrofluid-filled elastic alginate capsules in inhomogenous magnetic fields. Langmuir 2018, 34, 13534–13543. [Google Scholar] [CrossRef]
- Meiser, I.; Müller, S.C.; Zimmermann, H.; Ehrhart, F. Quantitative 3d high speed video analysis of capsule formation during encapsulation processes. Proceedings of World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 204–207. [Google Scholar]
- Lee, B.B.; Ibrahim, R.; Chu, S.Y.; Zulkifli, N.A.; Ravindra, P. Alginate liquid core capsule formation using the simple extrusion dripping method. J. Polym. Eng. 2015, 35, 311–318. [Google Scholar] [CrossRef]
- Messaoud, G.B.; Sánchez-González, L.; Probst, L.; Desobry, S. Influence of internal composition on physicochemical properties of alginate aqueous-core capsules. J. Colloid Interface Sci. 2016, 469, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Carin, M.; Barthes-Biesel, D.; Edwards-Levy, F.; Postel, C.; Andrei, D.C. Compression of biocompatible liquid-filled HSA-alginate capsules: Determination of the membrane mechanical properties. Biotechnol. Bioeng. 2010, 82, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Fery, A.; Weinkamer, R. Mechanical properties of micro- and nanocapsules: Single-capsule measurements. Polymer 2007, 48, 7221–7235. [Google Scholar] [CrossRef]
- Aguero, L.; Zaldivar-Silva, D.; Pena, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Huang, G.; Wang, H.; Xiao, J. Effect of high coacervation temperature on the physicochemical properties of resultant microcapsules through induction of Maillard reaction between soybean protein isolate and chitosan. J. Food. Eng. 2018, 234, 91–97. [Google Scholar] [CrossRef]
- He, F.; Mei, L.; Ju, X.; Xie, R.; Wang, W.; Liu, Z.; Chu, L. pH-responsive controlled release characteristics of solutes with different molecular weights diffusing across membranes of Ca-alginate/protamine/silica hybrid capsules. J. Membr. Sci. 2015, 474, 233–243. [Google Scholar] [CrossRef]
- Mei, L.; Xie, R.; Yang, C.; Ju, X.; Wang, J.; Zhang, Z.; Chu, L. Bio-inspired mini-eggs with pH-responsive membrane for enzyme immobilization. J. Membr. Sci. 2013, 429, 313–322. [Google Scholar] [CrossRef]
- Mou, C.; Wang, W.; Li, Z.; Ju, X.; Xie, R.; Deng, N.; Chu, L. Trojan-Horse-Like Stimuli-Responsive Microcapsules. Adv. Sci. 2018, 5, 1700960. [Google Scholar] [CrossRef]
- Ribeiro, C.; Borges, J.; Costa, A.M.S.; Gaspar, V.M.; Bermudez, V.Z.; Mano, J.F. Preparation of well-dispersed chitosan/alginate hollow multilayered microcapsules for enhanced cellular internalization. Molecules 2018, 23, 625. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Xie, R.; Ju, X.; Yu, Y.; Chu, L.; Zhang, Z. Alginate/protamine/silica hybrid capsules with ultrathin membranes for laccase immobilization. AIChE J. 2013, 59, 380–389. [Google Scholar] [CrossRef]
- Chan, E.S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Balanč, B.; Kalušević, A.; Drvenica, I.; Coelho, M.T.; Djordjević, V.; Alves, V.D.; Sousa, I.; Moldão-Martins, M.; Rakić, V.; Nedović, V.; et al. Calcium–alginate–inulin microbeads as carriers for aqueous carqueja extract. J. Food. Sci. 2016, 81, E65–E75. [Google Scholar] [CrossRef] [PubMed]
- Leick, S.; Kott, M.; Degen, P.; Henning, S.; Päsler, T.; Suter, D.; Rehage, H. Mechanical properties of liquid-filled shellac composite capsules. Phys. Chem. Chem. Phys. 2011, 13, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Degen, P.; Zwar, E.; Schulz, I.; Rehage, H. Magneto-responsive alginate capsules. J. Phys. Condens. Mat. 2015, 27, 194105. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Mei, L.; Lin, D.; Yao, S. Diffusion coefficients in intrahollow calcium alginate microcapsules. J. Chem. Eng. Data 2004, 49, 475–478. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, C.; Xu, G.; Tao, Z.; Zhang, B.; Liu, G. Density measurements of endothermic hydrocarbon fuel at sub- and supercritical conditions. J. Chem. Eng. Data 2011, 56, 2980–2986. [Google Scholar] [CrossRef]
- Watanabe, H.; Matsuyama, T.; Yamamoto, H. Experimental study on electrostatic atomization of highly viscous liquids. J. Electrostat. 2003, 57, 183–197. [Google Scholar] [CrossRef]
- Katakam, M.; Bell, L.N.; Banga, A.K. Effect of surfactants on the physical stability of recombinant human growth hormone. J. Pharm. Sci. 1995, 84, 713–716. [Google Scholar] [CrossRef]
- Bohorquez, M.; Koch, C.; Trygstad, T.; Pandit, N. A study of the temperature-dependent micellization of pluronic f127. J. Colloid Interf. Sci. 1999, 216, 34–40. [Google Scholar] [CrossRef]
- Lee, B.B.; Ravindra, P.; Chan, E.S. Size and shape of calcium alginate beads produced by extrusion dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Deng, Q.; Anilkumar, A.V.; Wang, T. The phenomenon of bubble entrapment during capsule formation. J. Colloid Interf. Sci. 2009, 333, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Poncelet, D.; Adiwijaya, Z.; Karaoglan, E.; Poncelet, D. Oil encapsulation in coreâ shell alginate capsules by inverse gelation. I: Dripping methodology. J. Microencapsul. 2017, 34, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate–carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Mei, L.; Wu, G.; Lin, D.; Yao, S. Gelation conditions and transport properties of hollow calcium alginate capsules. Biotechnol. Bioeng. 2004, 87, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Lee, B.B.; Ravindra, P.; Poncelet, D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion–dripping method. J. Colloid Interf. Sci. 2009, 338, 63–72. [Google Scholar] [CrossRef]
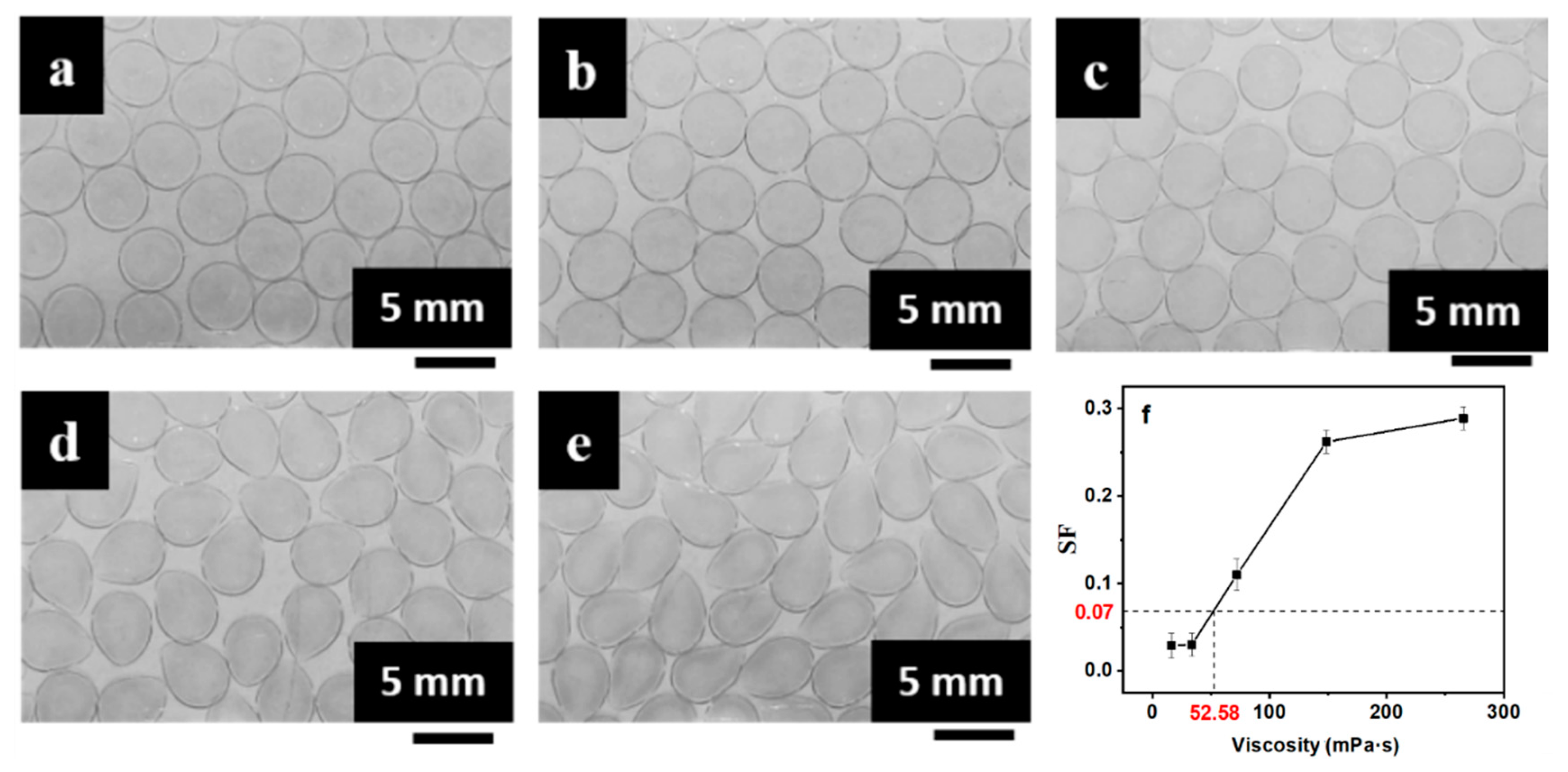
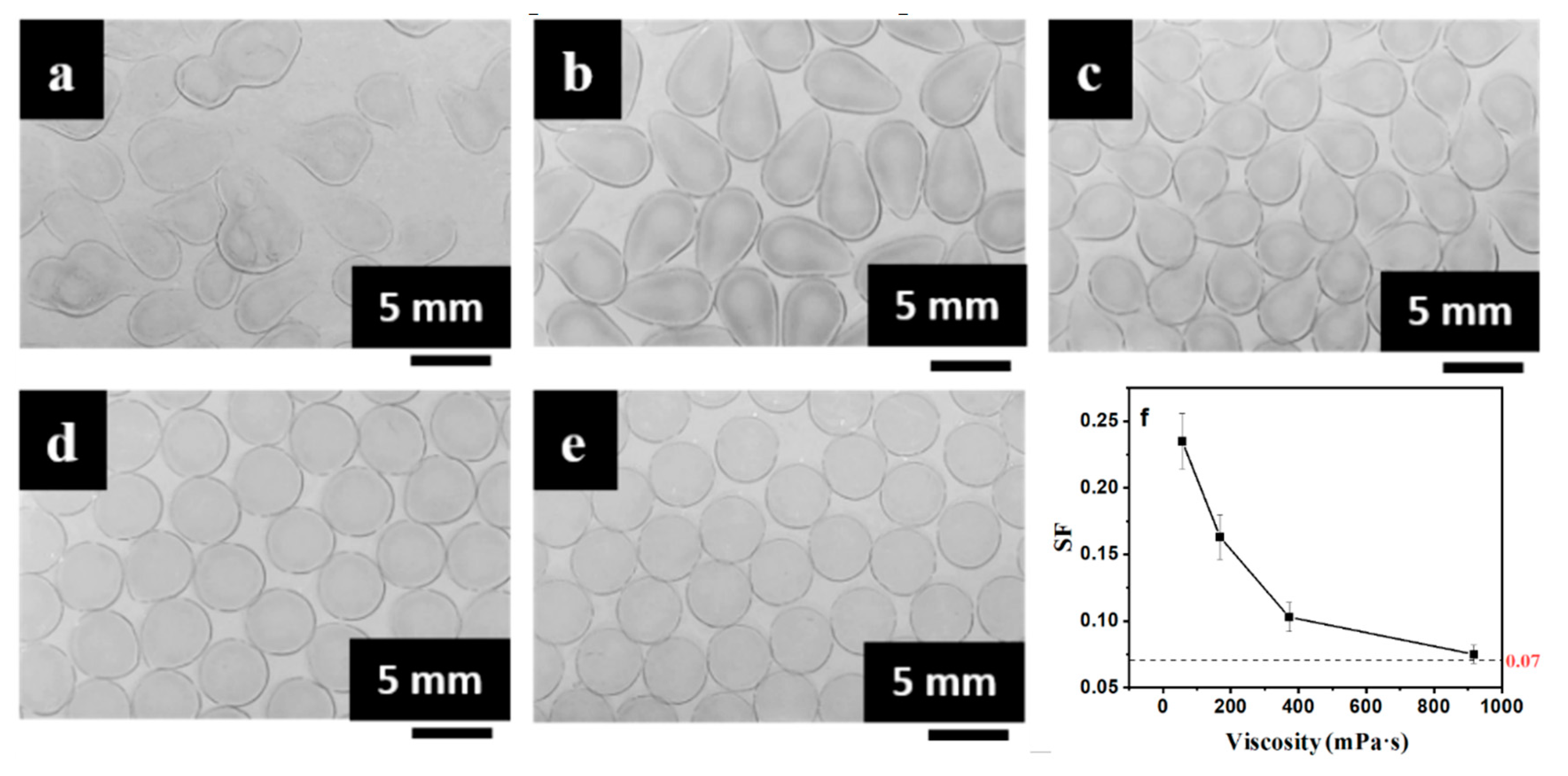
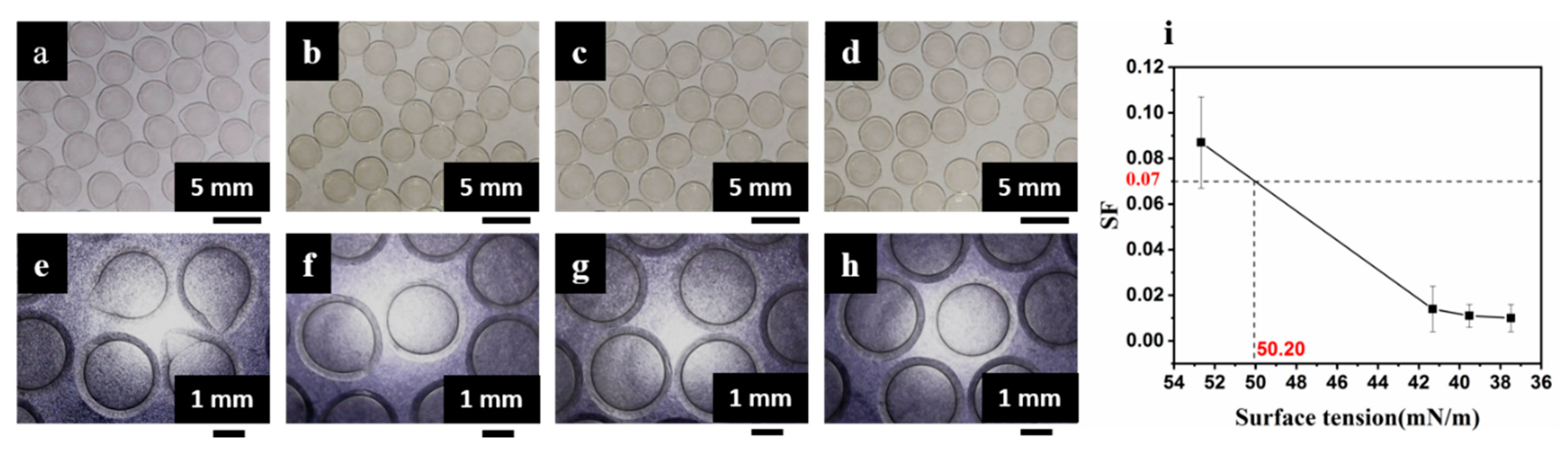
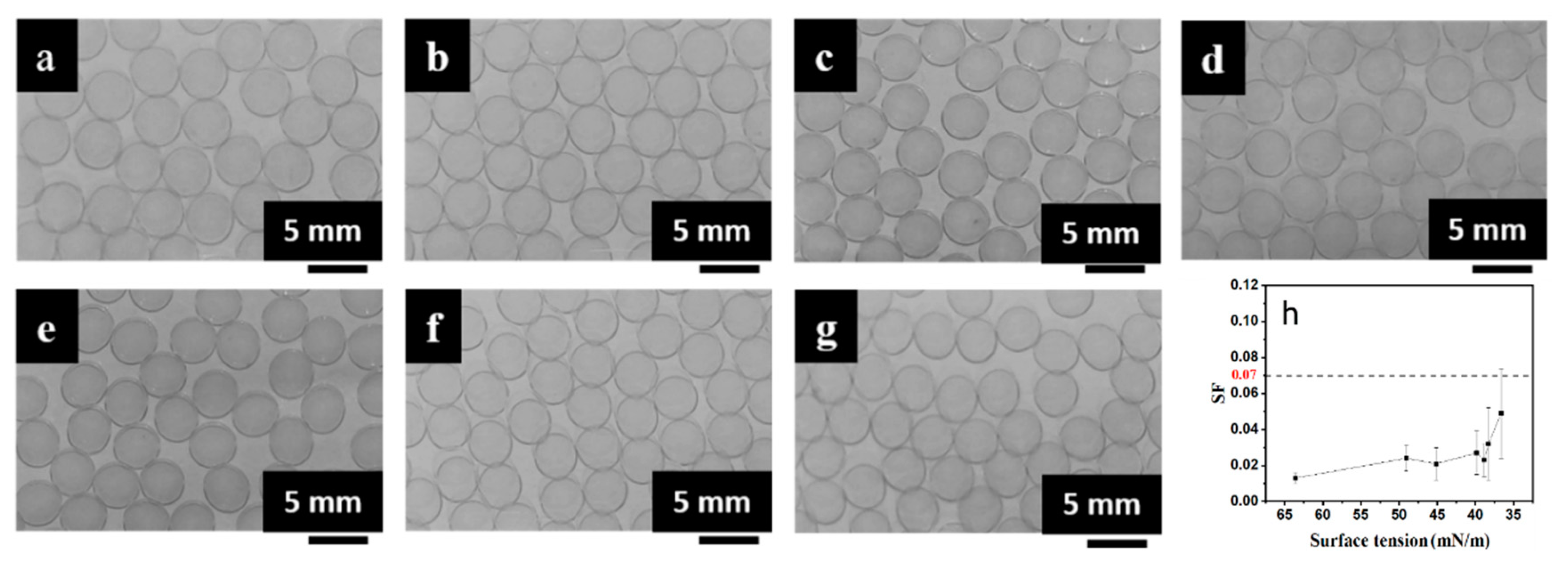
| Viscosity of CaCl2/CMC (mPa·s) | Viscosity of SA (mPa·s) | ||||
|---|---|---|---|---|---|
| 16.2 | 33.6 | 72.0 | 148.5 | 265.5 | |
| 20.0 | / | / | / | / | / |
| 56.7 | 0.067 ± 0.026 | 0.086 ± 0.029 | 0.258 ± 0.011 | 0.394 ± 0.016 | 0.371 ± 0.024 |
| 168.5 | 0.032 ± 0.019 | 0.018 ± 0.011 | 0.153 ± 0.035 | 0.289 ± 0.012 | 0.323 ± 0.007 |
| 372.0 | 0.009 ± 0.006 | 0.006 ± 0.004 | 0.026 ± 0.022 | 0.230 ± 0.014 | 0.242 ± 0.011 |
| 917.5 | 0.009 ± 0.006 | 0.008 ± 0.008 | 0.002 ± 0.002 | 0.136 ± 0.009 | 0.219 ± 0.009 |
| Viscosity of CaCl2/CMC (mPa·s) | Viscosity of SA (mPa·s) | ||||
|---|---|---|---|---|---|
| 16.2 | 33.6 | 72.0 | 148.5 | 265.5 | |
| 20.0 | / | / | / | / | / |
| 56.7 | 4.769 ± 0.137 | 4.757 ± 0.129 | 3.951 ± 0.087 | 2.940 ± 0.087 | 3.131 ± 0.150 |
| 168.5 | 4.355 ± 0.176 | 4.243 ± 0.109 | 3.847 ± 0.115 | 3.336 ± 0.074 | 3.239 ± 0.042 |
| 372.0 | 4.271 ± 0.071 | 4.221 ± 0.037 | 4.323 ± 0.176 | 3.208 ± 0.067 | 3.167 ± 0.065 |
| 917.5 | 4.278 ± 0.081 | 4.170 ± 0.072 | 4.076 ± 0.027 | 3.597 ± 0.054 | 3.313 ± 0.091 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Guo, Q.; Huang, W.; Zhang, T.; Wang, J.; Zhang, Y.; Huang, L.; Tang, Y. Shape Tuning and Size Prediction of Millimeter-Scale Calcium-Alginate Capsules with Aqueous Core. Polymers 2020, 12, 688. https://doi.org/10.3390/polym12030688
Zhao J, Guo Q, Huang W, Zhang T, Wang J, Zhang Y, Huang L, Tang Y. Shape Tuning and Size Prediction of Millimeter-Scale Calcium-Alginate Capsules with Aqueous Core. Polymers. 2020; 12(3):688. https://doi.org/10.3390/polym12030688
Chicago/Turabian StyleZhao, Jinchao, Qing Guo, Wei Huang, Teng Zhang, Jing Wang, Yu Zhang, Leping Huang, and Youhong Tang. 2020. "Shape Tuning and Size Prediction of Millimeter-Scale Calcium-Alginate Capsules with Aqueous Core" Polymers 12, no. 3: 688. https://doi.org/10.3390/polym12030688
APA StyleZhao, J., Guo, Q., Huang, W., Zhang, T., Wang, J., Zhang, Y., Huang, L., & Tang, Y. (2020). Shape Tuning and Size Prediction of Millimeter-Scale Calcium-Alginate Capsules with Aqueous Core. Polymers, 12(3), 688. https://doi.org/10.3390/polym12030688






