Cost-Effective Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) and Based on Imprinted Receptors for Fluvoxamine Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus
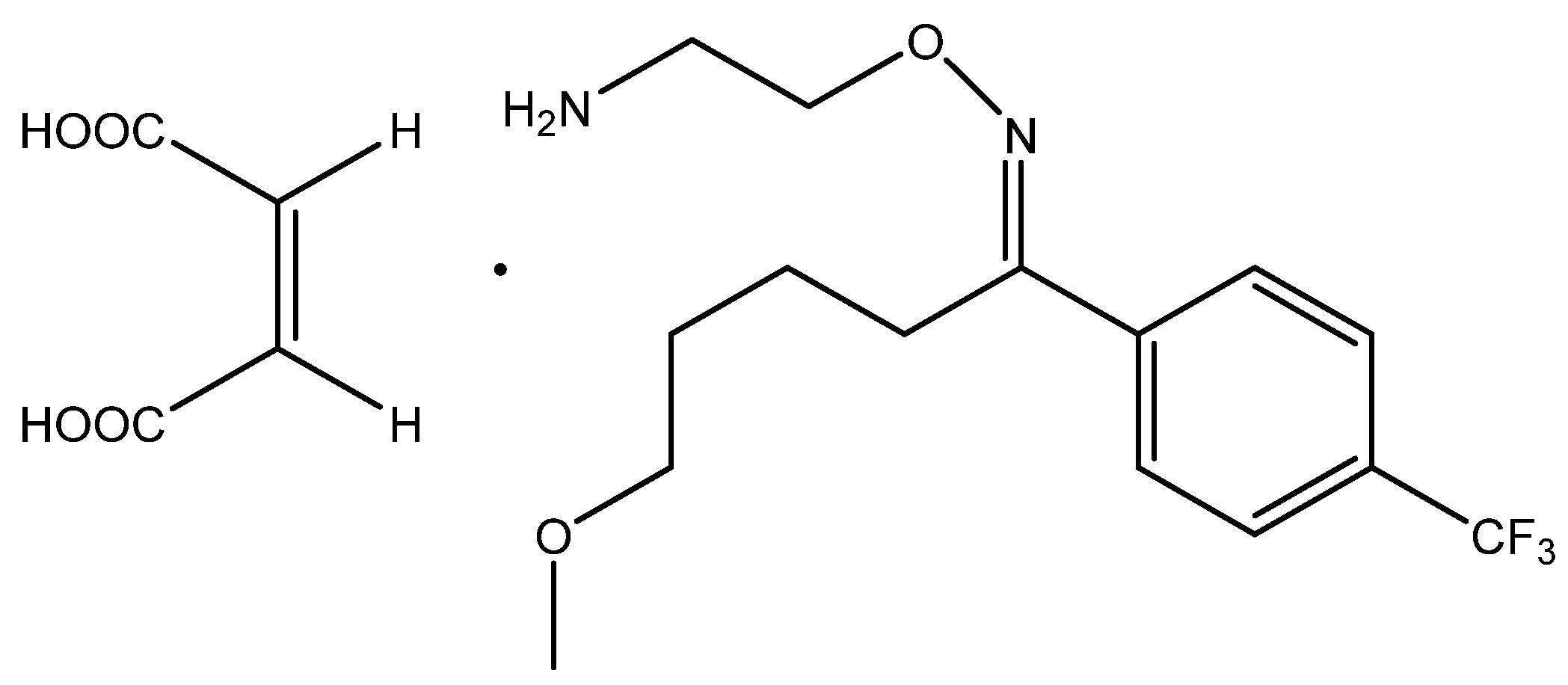
2.3. Synthesis of Host-Tailored Receptors
2.4. Fabrication of the Sensors and EMF Measurements
2.5. Analytical Applications
3. Results and Discussions
3.1. Characterization of the MIP Particles
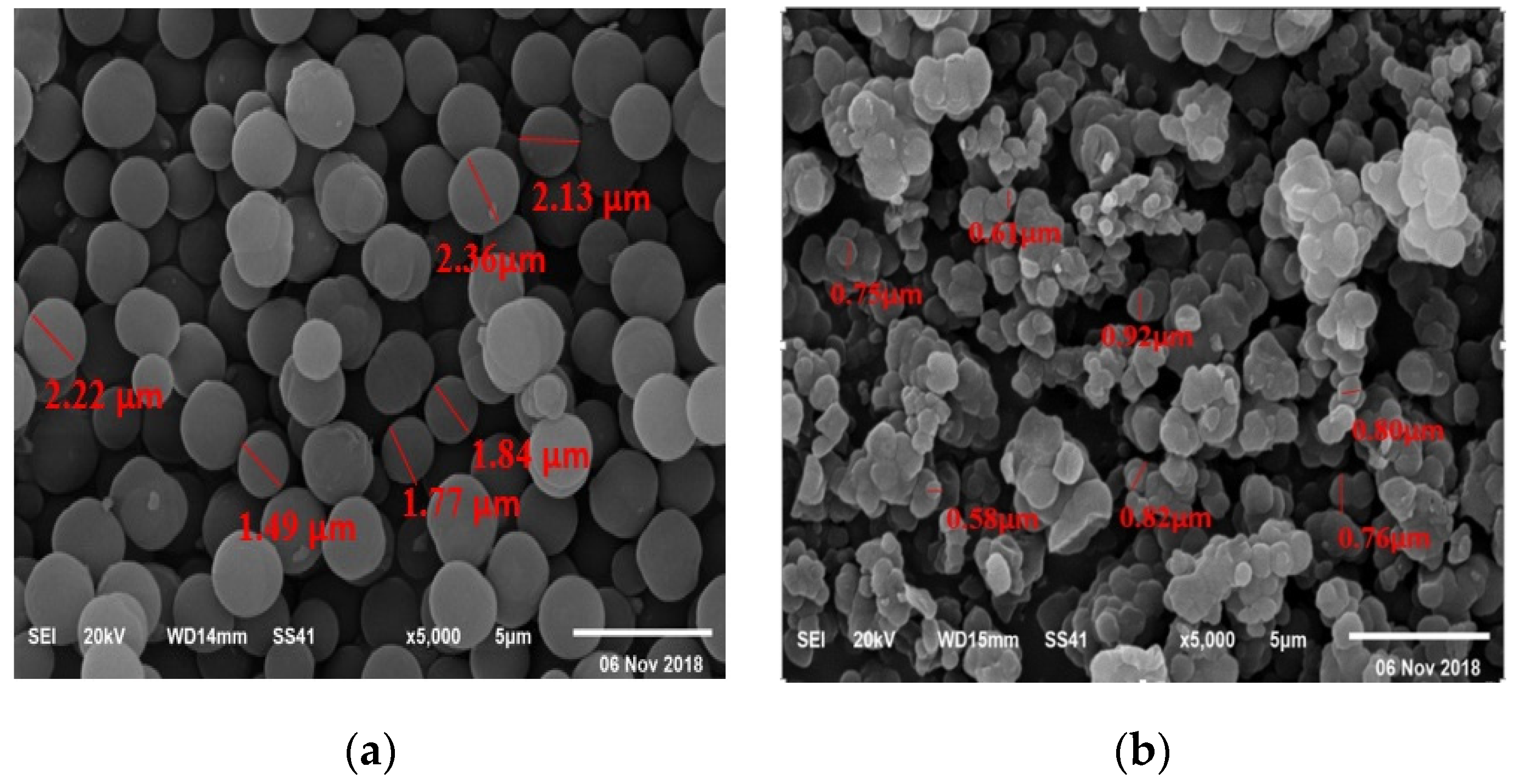
3.1.1. FT-IR Analysis
3.1.2. SEM Analysis
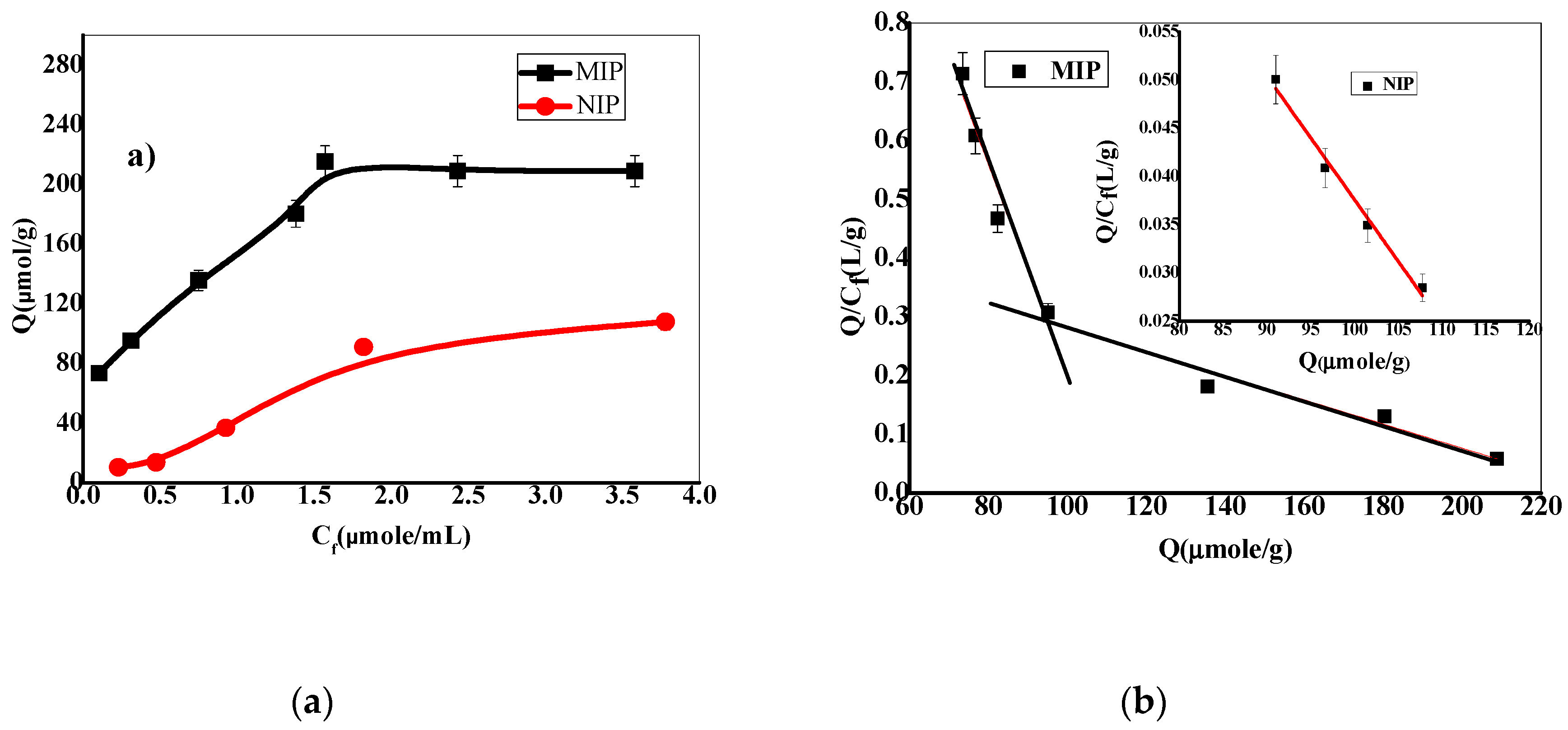
3.1.3. Binding Isotherms and Scatchard Analysis
3.2. Analytical Features of the Proposed Sensors
3.2.1. Membrane Optimization
3.2.2. Method Robustness
3.2.3. Selectivity Studies
3.3. Chronopotentiometry (CP) and Electrochemical Impedance (EI) Measurements
3.4. Analytical Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, R.F.; Abdel-Rahman, R.F.; Farid, O.A.; El-Marasy, S.A.; Hessin, A.F. Combined hepatoprotective and antidepressant effects of resveratrol in an acute model of depression. Bull. Fac. Pharm. Cairo Uni. 2014, 52, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Serafini, G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J. Psychiatry 2012, 2, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Dsm-Iv-tr, A. Diagnostic and Statistical Manual of Mental Disorders, Text Revision; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- El Tanbouly, N.; El Sayed, A.M.; Ali, Z.Y.; Abdel Wahab, S.; El Gayed, S.H.; Ezzat, S.M.; Senousy, E.; Safwat, A.; Choucry, M.A.; Abdel-Sattar, E. Antidepressant-Like Effect of Selected Egyptian Cultivars of Flaxseed Oil on a Rodent Model of Postpartum Depression. Evid-Based Compl Alt. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, S. Characteristics and use of new antidepressant drugs. Japan Med. Assoc. J. 2004, 47, 265–269. [Google Scholar]
- Katzung, B. Drugs of Abuse, Basic and Clinical Pharmacology, 10th ed.; McGraw-Hill Companies: New York, NY, USA, 2007. [Google Scholar]
- Wilde, M.I.; Plosker, G.L.; Benfield, P. Fluvoxamine. Drugs 1993, 46, 895–924. [Google Scholar] [CrossRef] [PubMed]
- Pullen, R.H.; Fatmi, A.A. Determination of fluvoxamine in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. 1992, 574, 101–107. [Google Scholar] [CrossRef]
- De Jong, G. The use of a pre-column for the direct high-performance liquid chromatographic determination of the anti-depressants clovoxamine and fluvoxamine in plasma. J. Chromatogr. B: Biomed. Sci. Appl. 1980, 183, 203–211. [Google Scholar] [CrossRef]
- Wong, S.H.; Kranzler, H.R.; Dellafera, S.; Fernandes, R. Determination of fluvoxamine concentration in plasma by reversed-phase liquid chromatography. Biomed. Chromatogr. 1994, 8, 278–282. [Google Scholar] [CrossRef]
- Ulu, S.T. Determination and validation of an LC method for fluvoxamine in tablets. Chromatographia 2006, 64, 169–173. [Google Scholar] [CrossRef]
- Comission, B.P. British Pharmacopoeia; The Stationery Office: London, UK, 2009. [Google Scholar]
- U.P. USP30-NF25; US Pharmacopoeial Convention, Inc.: Rockville, MD, USA, 2007. [Google Scholar]
- Härtter, S.; Wetzel, H.; Hiemke, C. Automated determination of fluvoxamine in plasma by column-switching high-performance liquid chromatography. Clin. Chem. 1992, 38, 2082–2086. [Google Scholar] [CrossRef]
- Ohkubo, T.; Shimoyama, R.; Otani, K.; Yoshid, K.; Higuchi, H.; Shimizu, T. High-performance liquid chromatographic determination of fluvoxamine and fluvoxamino acid in human plasma. Anal. Sci. 2003, 19, 859–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foglia, J.P.; Birder, L.A.; Perel, J.M. Determination of fluvoxamine in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Biomed. 1989, 495, 295–302. [Google Scholar] [CrossRef]
- Wille, S.M.; Van Hee, P.; Neels, H.M.; Van Peteghem, C.H.; Lambert, W.E. Comparison of electron and chemical ionization modes by validation of a quantitative gas chromatographic–mass spectrometric assay of new generation antidepressants and their active metabolites in plasma. J. Chromatogr. A 2007, 1176, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Berzas Nevado, J.; Villasenor Llerena, M.; Contento Salcedo, A.; Nuevo, E.A. Determination of fluoxetine, fluvoxamine, and clomipramine in pharmaceutical formulations by capillary gas chromatography. J. Chromatogr. Sci. 2000, 38, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Nevado, J.B.; Salcedo, A.C.; Llerena, M.V.; Nuevo, E.A. Method development and validation for the simultaneous determination of fluoxetine and fluvoxamine in pharmaceutical preparations by capillary electrophoresis. Anal. Chim. 2000, 417, 169–176. [Google Scholar] [CrossRef]
- Flores, J.R.; Nevado, J.J.B.; Salcedo, A.M.C.; Díaz, M.P.C. Development of a capillary zone electrophoretic method to determine six antidepressants in their pharmaceutical preparations. Experimental design for evaluating the ruggedness of method. J. Sep. Sci. 2004, 27, 33–40. [Google Scholar] [CrossRef]
- Lin, E.P.; Chiu, T.C.; Hsieh, V. Dispersive liquid–liquid microextraction combined with acetonitrile stacking through capillary electrophoresis for the determination of three selective serotonin reuptake inhibitor drugs in body fluids. J. Sep. Sci. 2016, 39, 4841–4850. [Google Scholar] [CrossRef]
- Darwish, I.A.; Amer, S.M.; Abdine, H.H.; Al-Rayes, L.I. Spectrofluorimetric determination of fluvoxamine in dosage forms and plasma via derivatization with 4-chloro-7-nitrobenzo-2-oxa-1, 3-diazole. J. Fluoresc. 2009, 19, 463–471. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Chen, F. Determination of fluvoxamine maleate in human urine and human serum using alkaline KMnO4–rhodamine B chemiluminescence. Luminescence 2017, 32, 1077–1083. [Google Scholar] [CrossRef]
- Hassanzadeh, J.; Amjadi, M. Sensitive and selective determination of fluvoxamine maleate using a sensitive chemiluminescence system based on the alkaline permanganate–Rhodamine B–gold nanoparticles reaction. Luminescence 2015, 30, 439–443. [Google Scholar] [CrossRef]
- Ajayi, R.F.; Nxusani, E.; Douman, S.F.; Jonnas, A.; Baker, P.G.L.; Iwuoha, E.I. An amperometric cytochrome P450-2D6 biosensor system for the detection of the selective serotonin reuptake inhibitors (SSRIs) paroxetine and fluvoxamine. J. Nano Res.-Sw. 2016, 44, 208–228. [Google Scholar] [CrossRef]
- Nevado, J.B.; Rodriguez Flores, J.; Castañeda Peñalvo, G. Voltammetric Behavior of Fluvoxamine Using Square-Wave and Adsorptive Stripping Square-Wave Techniques. Determination in Pharmaceutical Products. Electroanalysis 2000, 12, 1059–1063. [Google Scholar] [CrossRef]
- Elmali, F.; Alpdogan, G.; Sungur, S.; Aycan, Ş. Polarographic determination of fluvoxamine maleate in tablets. Turk. J. Chem. 2000, 24, 299–302. [Google Scholar]
- Alhaider, A.A.; Hagga, M.E.M.; Alawady, M.E.; Gad-kariem, E.A. Spectrophotometric Determination of Fluvoxamine in Tablets Based on Charge-Transfer Complex with Chloranil. Anal. Lett. 1993, 26, 887–901. [Google Scholar] [CrossRef]
- Kishore, M.; Koteswarao, A.; Janardhan, M. Validation of new spectrophotometric methods for the determination of Fluvoxamine as maleate in pharmaceutical formulations. Res. J. Pharm. Tech. 2011, 4, 450–453. [Google Scholar]
- Devarajan, S.; Manavalan, G.; Balasubramanian, K.; Annamalai, J.; Madduri, N. A new spectrophotometric method for the determination of fluvoxamine maleate in pure form and in pharmaceutical formulation. J. Appl. Pharm. Sci. 2015, 5, 081–086. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.M.; Amr, A.E.G.E.; El-Naby, H.A.; El-Naggar, M.; Kamel, A.H.; Khalifa, N.M. Novel aminoacridine sensors based on molecularly imprinted hybrid polymeric membranes for static and hydrodynamic drug quality control monitoring. Materials 2019, 12, 3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, A.H.; Soror, T.Y.; Al-Romian, F.M. Flow through potentiometric sensors based on molecularly imprinted polymers for selective monitoring of mepiquat residue, a quaternary ammonium herbicide. Anal. Methods 2012, 4, 3007–3012. [Google Scholar] [CrossRef]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2, 4-dichlorophenol as a food Taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mat. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef]
- Elbehery, N.H.A.; Amr, A.E.G.E.; Kamel, A.H.; Elsayed, E.A.; Hassan, S.S.M. Novel potentiometric 2, 6-Dichlorophenolindophenolate (DCPIP) membrane-based sensors: Assessment of their input in the determination of total phenolics and ascorbic acid in beverages. Sensors 2019, 19, 2058. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, N.S.; Amr, A.E.G.E.; El-Tantawy, A.S.M.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Tailor-made specific recognition of cyromazine pesticide integrated in a potentiometric strip cell for environmental and food analysis. Polymers 2019, 11, 1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, A.H.; Hassan, A.M.E. Solid contact potentiometric sensors based on host-tailored molecularly imprinted polymers for creatine assessment. Int. J. Electrochem. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Mirata, F.; Resmini, M. Molecularly Imprinted Polymers for Catalysis and Synthesis, Molecularly Imprinted Polymers in Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 107–129. [Google Scholar]
- Lok, C.; Son, R. Application of molecularly imprinted polymers in food sample analysis–a perspective. Int. Food Res. J. 2009, 16, 127–140. [Google Scholar]
- Chen, G.; Jin, M.; Du, P.; Zhang, C.; Cui, X.; Zhang, Y.; She, Y.; Shao, H.; Jin, F.; Wang, S. A sensitive chemiluminescence enzyme immunoassay based on molecularly imprinted polymers solid-phase extraction of parathion. Anal. Biochem. 2017, 530, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Azodi-Deilami, S.; Abdouss, M.; Kordestani, D. Synthesis and characterization of the magnetic molecularly imprinted polymer nanoparticles using N, N-bis methacryloyl ethylenediamine as a new cross-linking agent for controlled release of meloxicam. Appl. Biochem. Biotechnol. 2014, 172, 3271–3286. [Google Scholar] [CrossRef]
- Asadi, E.; Azodi-Deilami, S.; Abdouss, M.; Khaghani, S. Cyproterone synthesis, recognition and controlled release by molecularly imprinted nanoparticle. Appl. Biochem. Biotechnol. 2012, 167, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K. Molecularly imprinted polymers in analytical chemistry. Analyst 2001, 126, 747–756. [Google Scholar] [CrossRef]
- Ye, L.; Haupt, K. Molecularly imprinted polymers as antibody and receptor mimics for assays, sensors and drug discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef]
- Li, H.-H.; Wang, H.-H.; Li, W.-T.; Fang, X.-X.; Guo, X.-C.; Zhou, W.-H.; Cao, X.; Kou, D.-X.; Zhou, Z.-J.; Wu, S.-X. A novel electrochemical sensor for epinephrine based on three dimensional molecularly imprinted polymer arrays. Sens. Actuators B Chem. 2016, 222, 1127–1133. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Yang, N.; Chen, C.; Zhang, M. Chitosan-Based Surface Molecularly Imprinted Polymer Microspheres for Sustained Release of Sinomenine Hydrochloride in Aqueous Media. Appl. Biochem. Biotechnol. 2018, 185, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galal Eldin, A.; Amr, E.; El-Galil, A.; Kamel, A.H.; Hassan, S.S.M. Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer As Solid Transducer for Ultratrace Determination of Azides. Molecules 2019, 24, 1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galichet, L.; Moffat, A.; Osselton, M.; Widdop, B. Clarke’s Analysis of Drugs and Poisons; Pharmaceutical Press: London, UK, 2004. [Google Scholar]
- Zhang, W.; She, X.; Wang, L.; Fan, H.; Zhou, Q.; Huang, X.; Tang, J. Preparation, characterization and application of a molecularly imprinted polymer for selective recognition of sulpiride. Materials 2017, 10, 475. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, T.; Ganjali, M.R.; Akhoundian, M. Synthesis and application of different nano-sized imprinted polymers for the preparation of promethazine membrane electrodes and comparison of their efficiencies. Int. J. Electrochem. Sci. 2012, 7, 7655–7674. [Google Scholar]
- Ratautaite, V.; Plausinaitis, D.; Baleviciute, I.; Mikoliunaite, L.; Ramanaviciene, A.; Ramanavicius, A. Characterization of caffeine-imprinted polypyrrole by a quartz crystal microbalance and electrochemical impedance spectroscopy. Sens. Actuators B 2015, 212, 63–71. [Google Scholar] [CrossRef]
- Baleviciute, I.; Ratautaite, V.; Ramanaviciene, A.; Balevicius, Z.; Broeders, J.; Crouxd, D.; McDonaldd, M.; Vahidpourd, F.; Thoelend, R.; De Ceuninckd, W.; et al. Evaluation of theophylline imprinted polypyrrole film. Synth. Met. 2015, 209, 206–211. [Google Scholar] [CrossRef]
- Bakker, E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. [Google Scholar] [CrossRef]





| Type of Sensor | MWCNT | MIP (mg) | NIP (mg) | PVC (mg) | TPB (mg) | DOP (mg) | THF (mL) | Slope (mV/decade) | Detection Limit (mol/L) | r2 |
|---|---|---|---|---|---|---|---|---|---|---|
| I | - | 12.0 | - | 68.0 | 2.0 | 118.0 | 2.0 | 55.2 ± 0.4 | 4.7 × 10−6 | 0.999 |
| II | - | - | - | 68.0 | 2.0 | 118.0 | 2.0 | 55.4 ± 0.5 | 1.2 × 10−5 | 0.999 |
| III | - | 12.0 | - | 68.0 | - | 118.0 | 2.0 | 32.8 ± 1.4 | 1.0 × 10−5 | 0.995 |
| IV | presence | 12.0 | - | 68.0 | 2.0 | 118.0 | 2.0 | 56.2 ± 0.6 | 4.3 × 10−6 | 0.999 |
| V | - | - | 12.0 | 68.0 | - | 118.0 | 2.0 | 29.7 ± 1.4 | 1.1 × 10−5 | 0.999 |
| Interfering Ion | ||
|---|---|---|
| With MWCNT | Without MWCNT | |
| K+ | −3.41 | −3.95 |
| Na+ | −3.74 | −3.97 |
| Ba2+ | −3.62 | −3.84 |
| Ca2+ | −3.44 | −3.83 |
| Arginine | −3.85 | −4.22 |
| Caffeine | −3.84 | −3.48 |
| Glucose | −3.72 | −3.87 |
| Lactose | −3.71 | −3.84 |
| Paracetamol | −3.60 | −3.65 |
| Fluxatine, | −3.12 | −3.11 |
| Venlafaxine, | −2.94 | −3.01 |
| Ephedrine | −3.66 | −3.67 |
| Pharmaceutical Product and Source | Nominal Content is Taken, mg tablet−1 | Found, mg tablet−1 | t-Student Test b | F-Test b | |||
|---|---|---|---|---|---|---|---|
| Proposed Method | Mean a (%) ± SD | Reference Method [12] | Mean a (%) ± SD | ||||
| Fluxamine tablets (Pharaonia pharmaceuticals “Pharo Pharma”, Egypt) | 50 | 50.9 | 101.9 ± 0.8 | 50.1 | 100.1 ± 1.3 | 1.7 | 5.1 |
| 100 | 99.1 | 99.1 ± 0.9 | 99.9 | 99.9 ± 1.3 | 1.1 | 3.6 | |
| Faverin® tablets (Abbott Healthcare SAS) | 50 | 49.4 | 98.8 ± 0.9 | 50.1 | 100.1 ± 1.5 | 1.4 | 5.5 |
| 100 | 100.3 | 100.3 ± 0.9 | 99.9 | 99.9 ± 1.4 | 0.2 | 5.2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Hashem, H.; S. M. Hassan, S.; H. Kamel, A.; Amr, A.E.-G.E.; AbdelBary, E.M. Cost-Effective Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) and Based on Imprinted Receptors for Fluvoxamine Assessment. Polymers 2020, 12, 673. https://doi.org/10.3390/polym12030673
M. Hashem H, S. M. Hassan S, H. Kamel A, Amr AE-GE, AbdelBary EM. Cost-Effective Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) and Based on Imprinted Receptors for Fluvoxamine Assessment. Polymers. 2020; 12(3):673. https://doi.org/10.3390/polym12030673
Chicago/Turabian StyleM. Hashem, Heba, Saad S. M. Hassan, Ayman H. Kamel, Abd El-Galil E. Amr, and E. M. AbdelBary. 2020. "Cost-Effective Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) and Based on Imprinted Receptors for Fluvoxamine Assessment" Polymers 12, no. 3: 673. https://doi.org/10.3390/polym12030673
APA StyleM. Hashem, H., S. M. Hassan, S., H. Kamel, A., Amr, A. E.-G. E., & AbdelBary, E. M. (2020). Cost-Effective Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) and Based on Imprinted Receptors for Fluvoxamine Assessment. Polymers, 12(3), 673. https://doi.org/10.3390/polym12030673








