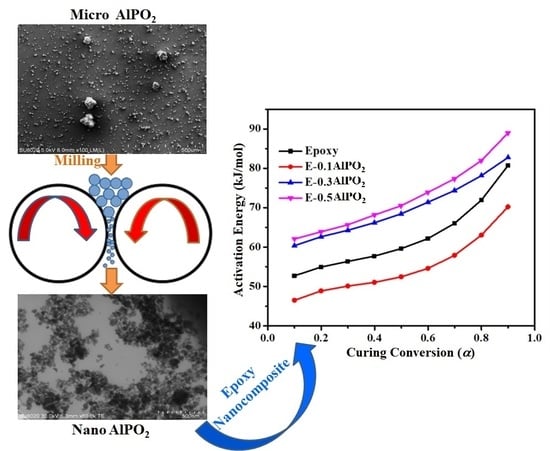
Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AHP Nanoparticles Preparation
2.3. Preparation of Epoxy/AHP Nanocomposites
2.4. Characterization Methods
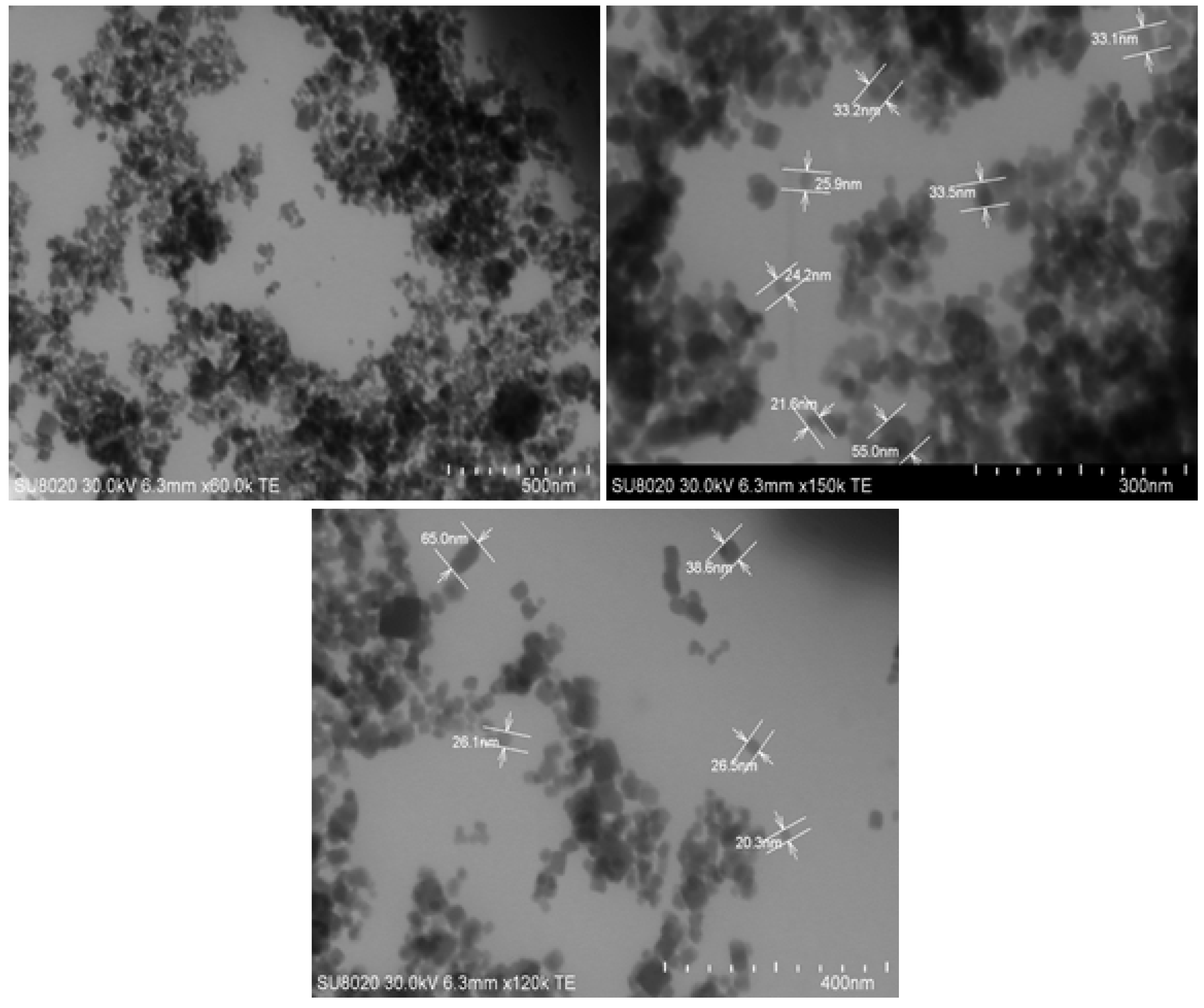
2.4.1. Electron Microscopy (SEM and TEM)
2.4.2. Nonisothermal Differential Scanning Calorimetry (DSC)
2.4.3. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Characterization of AHP Nanoparticles
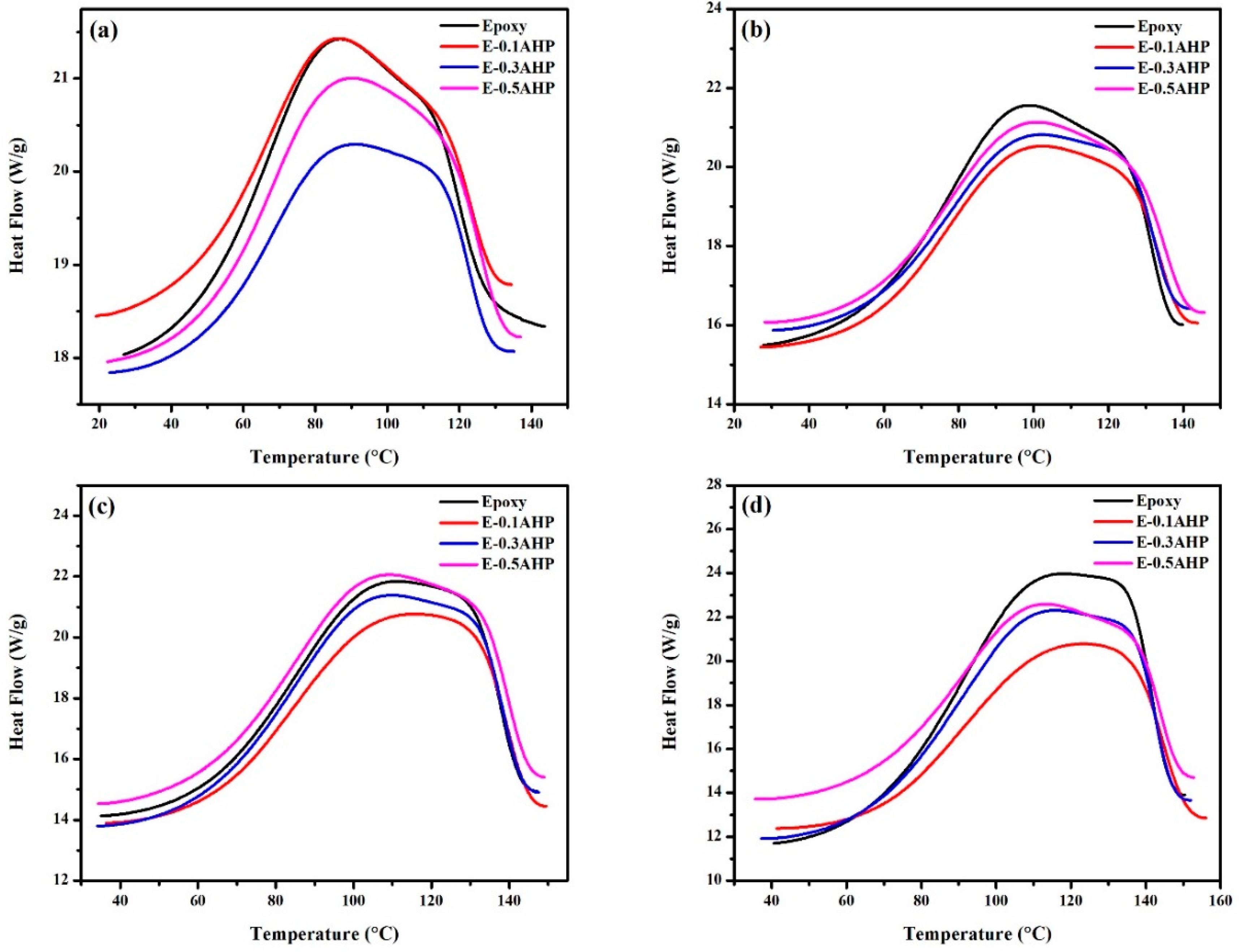
3.2. Qualitative Cure Analysis
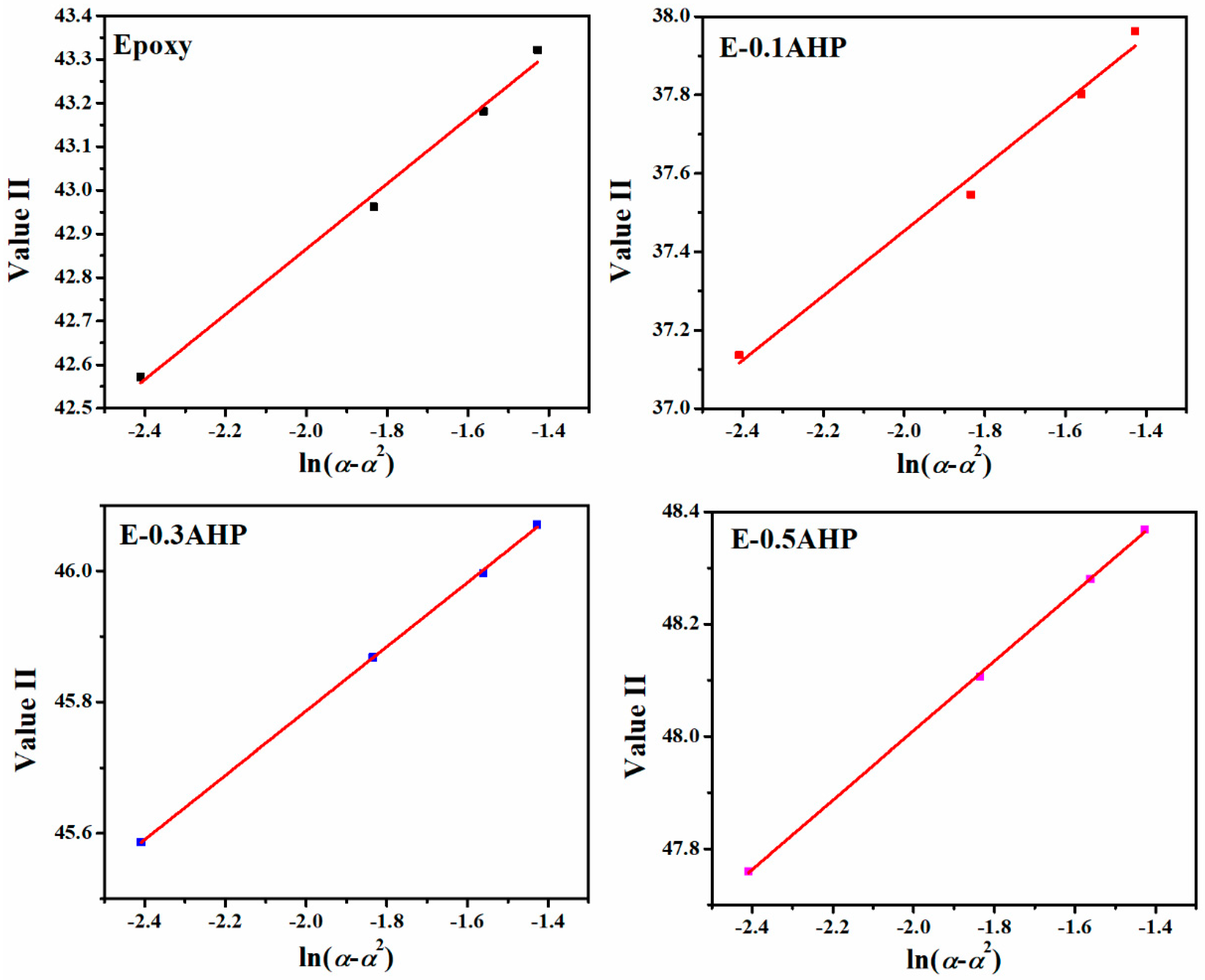
3.3. Quantitative Cure Analysis
3.4. Glass Transition Analysis
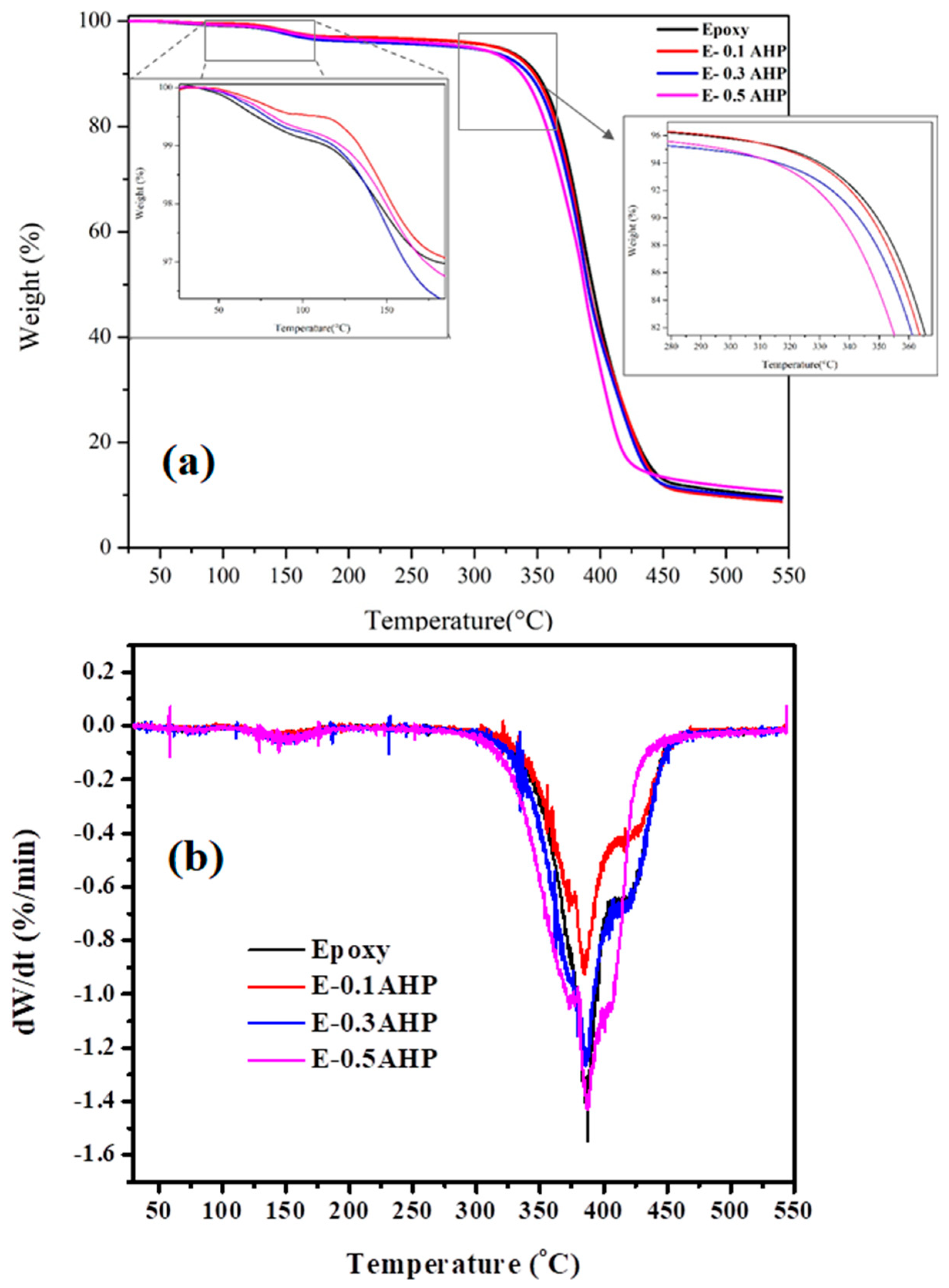
3.5. Thermal Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Isoconversional Kinetic Methods
Appendix A.1. Friedman Model
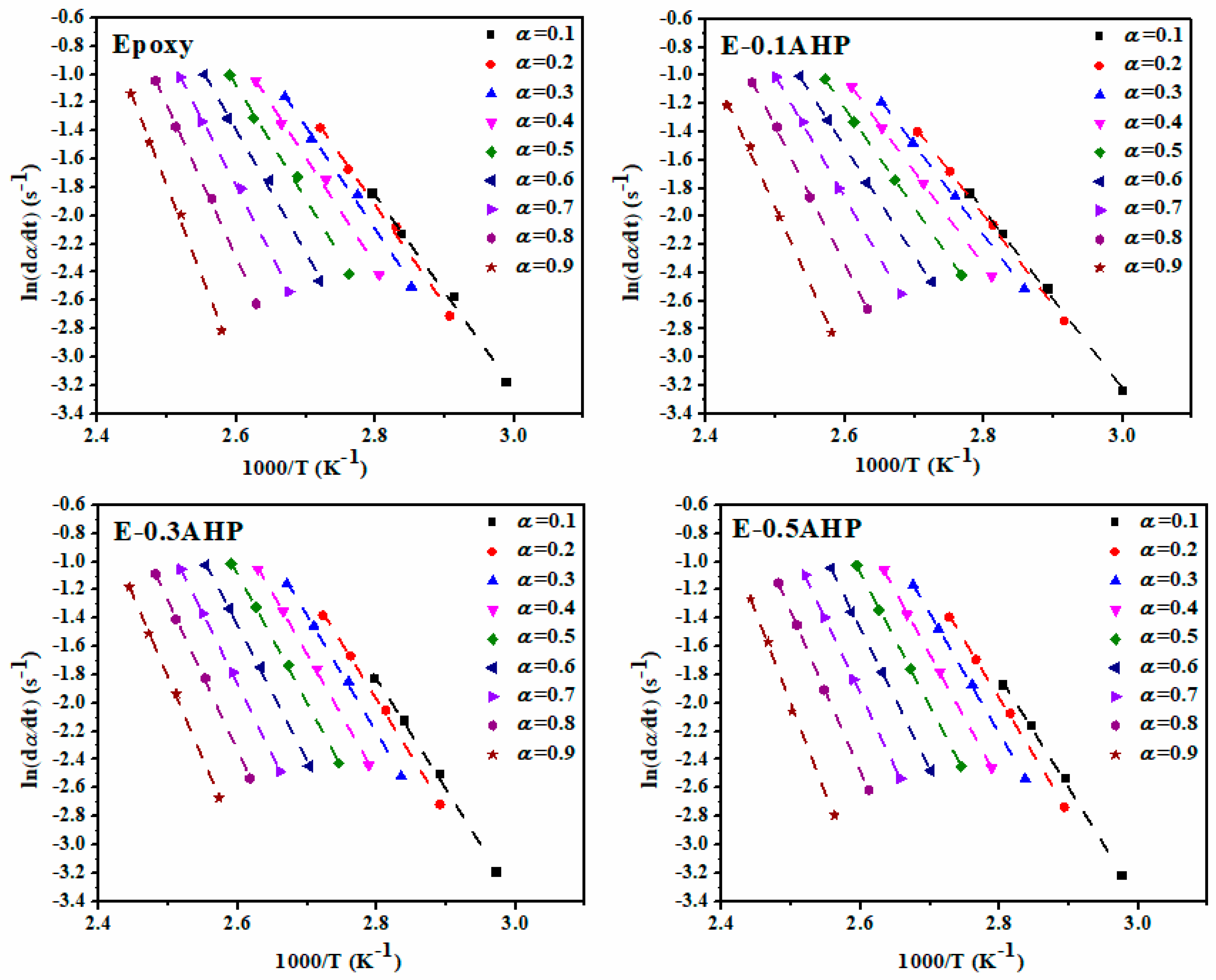
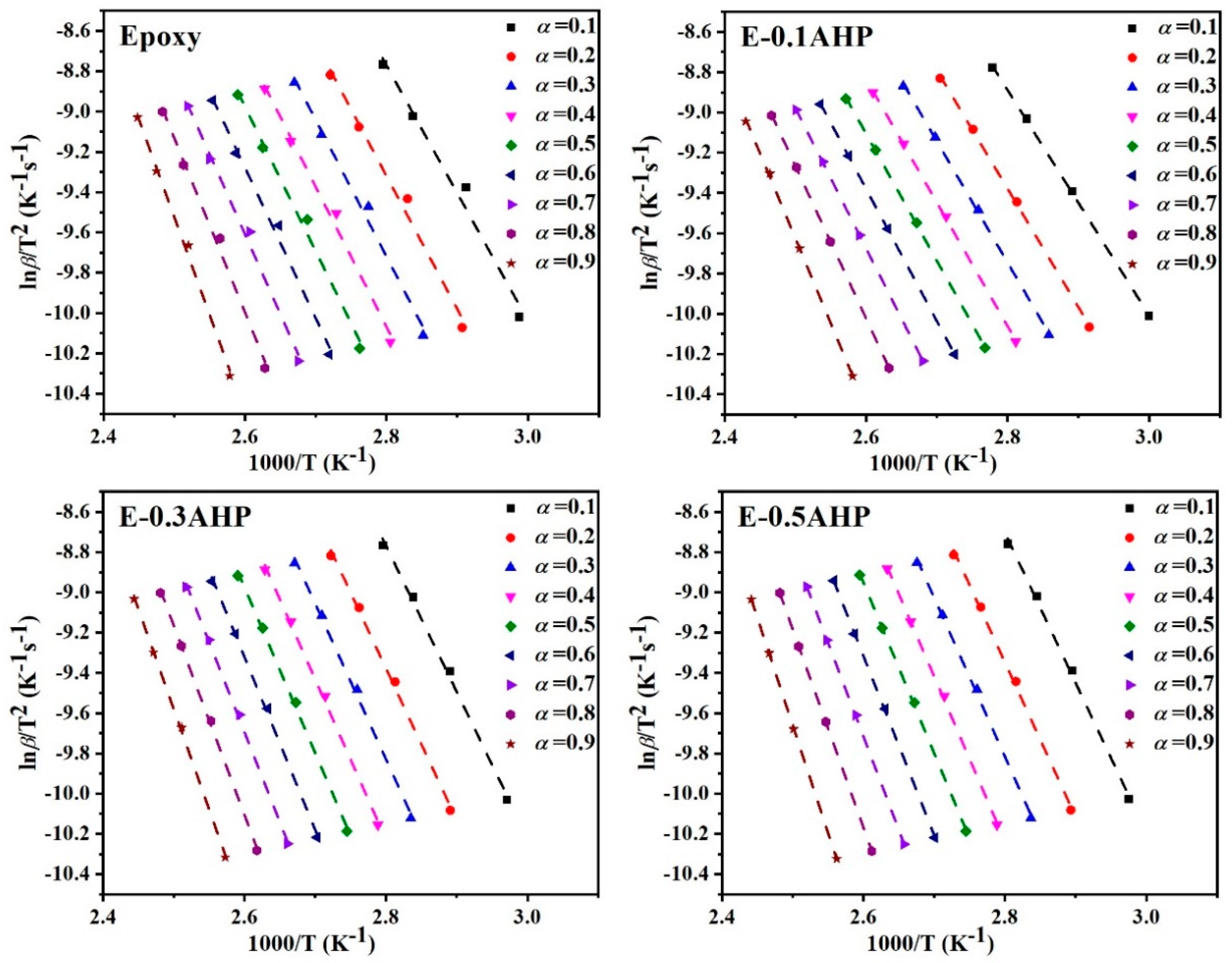
Appendix A.2. Kissinger Method
Appendix B. Curing Reaction Model Determination
Appendix B.1. Friedman Model

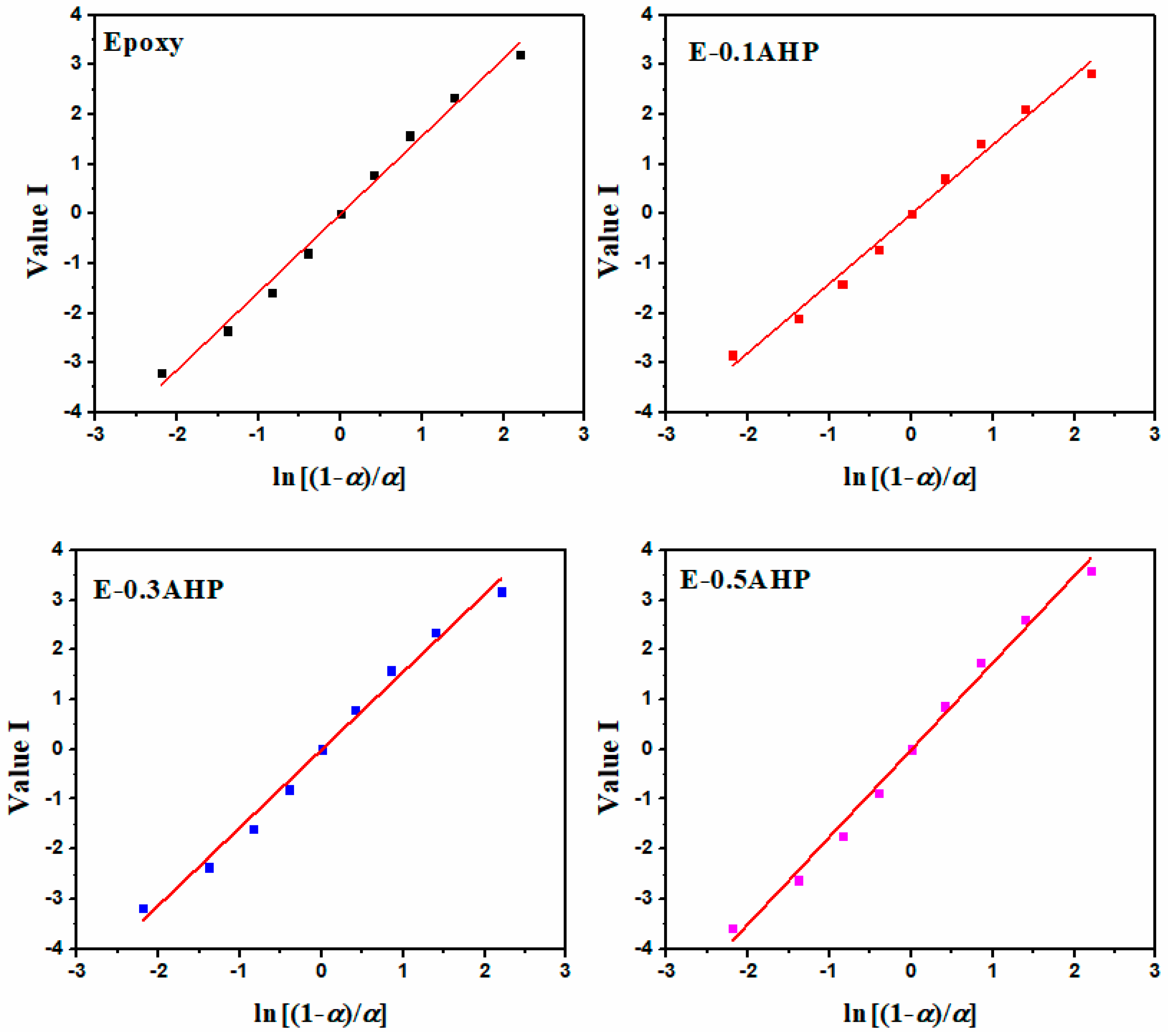
Appendix B.2. Malek Method

Appendix C. Determination of Degree of Reaction
References
- Ahmadi, Z. Nanostructured epoxy adhesives: A review. Prog. Org. Coat. 2019, 135, 449–453. [Google Scholar] [CrossRef]
- Ahmadi, Z. Epoxy in nanotechnology: A short review. Prog. Org. Coat. 2019, 132, 445–448. [Google Scholar] [CrossRef]
- Puglia, D.; Kenny, J.M. Chapter 7-Structure-property relationships of thermoset nanocomposites. In Thermosets, 2nd ed.; Guo, Q., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–276. [Google Scholar]
- Lange, J.; Luisier, A.; Hult, A. Influence of crosslink density, glass transition temperature and addition of pigment and wax on the scratch resistance of an epoxy coating. J. Coat. Technol. 1997, 69, 77–82. [Google Scholar] [CrossRef]
- Wu, G.M.; Liu, D.; Liu, G.F.; Chen, J.; Huo, S.P.; Kong, Z.W. Thermoset nanocomposites from waterborne bio-based epoxy resin and cellulose nanowhiskers. Carbohydr. Polym. 2015, 127, 229–235. [Google Scholar] [CrossRef]
- Song, S.; Ma, J.; Cao, K.; Chang, G.; Huang, Y.; Yang, J. Synthesis of a novel dicyclic silicon-/phosphorus hybrid and its performance on flame retardancy of epoxy resin. Polym. Degrad. Stab. 2014, 99, 43–52. [Google Scholar] [CrossRef]
- Bharadwaj, R.K.; Mehrabi, A.R.; Hamilton, C.; Trujillo, C.; Murga, M.; Fan, R.; Thompson, A.K. Structure–property relationships in cross-linked polyester–clay nanocomposites. Polymer 2002, 43, 3699–3705. [Google Scholar] [CrossRef]
- Wang, C.S.; Lin, C.H. Novel phosphorus-containing epoxy resins. Part II: Curing kinetics. Polymer 2000, 41, 8579–8586. [Google Scholar] [CrossRef]
- Narayan, R.; Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N. Cure, viscoelastic and mechanical properties of hydroxylated polyester melamine high solids coatings. J. Mater. Sci. 2002, 37, 4911–4918. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. ‘Cure Index’ for thermoset composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Vahedi, V.; Pasbakhsh, P. Instrumented impact properties and fracture behaviour of epoxy/modified halloysite nanocomposites. Polym. Test. 2014, 39, 101–114. [Google Scholar] [CrossRef]
- Tikhani, F.; Jouyandeh, M.; Jafari, S.H.; Chabokrow, S.; Ghahari, M.; Gharanjig, K.; Saeb, M.R. Cure Index demonstrates curing of epoxy composites containing silica nanoparticles of variable morphology and porosity. Prog. Org. Coat. 2019, 135, 176–184. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran SM, R.; Shabanian, M.; Ghiyasi, S.; Vahabi, H.; Badawi, M.; Saeb, M.R. Curing behavior of epoxy/Fe3O4nanocomposites: A comparison between the effects of bare Fe3O4, Fe3O4/SiO2/chitosan and Fe3O4/SiO2/chitosan/imide/phenylalanine-modified nanofillers. Prog. Org. Coat. 2018, 123, 10–19. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Wang, J.; Chow, W. A brief review on fire retardants for polymeric foams. J. Appl. Polym. Sci. 2005, 97, 366–376. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Liu, Y.L.; Tsiao, J. Phosphorus-Containing Epoxy Resins for Flame Retardancy V: Synergistic Effect of Phosphorus–Silicon on Flame Retardancy. J. Appl. Polym. Sci. 2000, 78, 1–7. [Google Scholar] [CrossRef]
- Ghiyasi, S.; Sari, M.G.; Shabanian, M.; Hajibeygi, M.; Zarrintaj, P.; Rallini, M.; Laoutid, F. Hyperbranched poly(ethyleneimine) physically attached to silica nanoparticles to facilitate curing of epoxy nanocomposite coatings. Prog. Org. Coat. 2018, 120, 100–109. [Google Scholar] [CrossRef]
- Nonahal, M.; Rastin, H.; Saeb, M.R.; Sari, M.G.; Moghadam, M.H.; Zarrintaj, P.; Ramezanzadeh, B. Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: Nonisothermal cure kinetics study. Prog. Org. Coat. 2018, 114, 233–243. [Google Scholar] [CrossRef]
- Yan, Y.W.; Huang, J.Q.; Guan, Y.H.; Shang, K.; Jian, R.K.; Wang, Y.Z. Flame retardance and thermal degradation mechanism of polystyrene modified with aluminum hypophosphite. Polym. Degrad. Stab. 2014, 99, 35–42. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Rao, W.H.; Kang, A.H.; Liao, W.; Wang, Y.Z. Highly effective flame retarded polystyrene by synergistic effects between expandable graphite and aluminum hypophosphite. Polym. Degrad. Stab. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, L.; Jiang, S.; Peng, X.; Kuang, T. Enhancement of flame retardancy of unsaturated polyester resin based on DOPO derivatives and aluminum hypophosphite. In Proceedings of the Annual Technical Conference-ANTEC, Conference Proceedings, Anaheim, CA, USA, 8–10 May 2017; pp. 591–594. [Google Scholar]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Puglia, D.; Saeb, M.R. Protocol for nonisothermal cure analysis of thermoset composites. Prog. Org. Coat. 2019, 131, 333–339. [Google Scholar] [CrossRef]
- Akbari, V.; Naja, F.; Vahabi, H.; Jouyandeh, M.; Badawi, M. Progress in Organic Coatings Surface chemistry of halloysite nanotubes controls the curability of low fi lled epoxy nanocomposites. Prog. Org. Coat. 2019, 135, 555–564. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Hamad, S.M.; Karimzadeh, I.; Aghazadeh, M.; Karami, Z.; Akbari, V.; Ganjali, M.R. Unconditionally blue: Curing epoxy with polyethylene glycol (PEG) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105285. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karabela, M.M.; Varkopoulou, E.A.; Sideridou, I.D. Cure Kinetics Study of Two Epoxy Systems with Fourier Tranform Infrared Spectroscopy (FTIR) and Differential Scanning Calorimetry (DSC). J. Macromol. Sci. Part A 2012, 49, 630–638. [Google Scholar] [CrossRef]
- Ehlers, J.E.; Rondan, N.G.; Huynh, L.K.; Pham, H.; Marks, M.; Truong, T.N. Theoretical Study on Mechanisms of the Epoxy Macromolecules. Macromolecules 2007, 40, 4370–4377. [Google Scholar] [CrossRef]
- Zhao, S.F.; Zhang, G.P.; Sun, R.; Wong, C.P. Curing kinetics, mechanism and chemorheological behavior of methanol etherified amino/novolac epoxy systems. Express Polym. Lett. 2014, 8, 95–106. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Gabrionf, X.; Laheurteg, P.; Vahabiab, H.; Saebe, M.R. Synthesis, characterization, and high potential of 3D metal–organic framework (MOF) nanoparticles for curing with epoxy. J. Alloys Compd. 2020, 829, 154547. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Bushy-surface hybrid nanoparticles for developing epoxy superadhesives. Appl. Surf. Sci. 2019, 479, 1148–1160. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Sun, S.; Chen, J. A New Type of Flame Retarded Epoxy Resin Based on Metal Phosphinates. Polym. Polym. Compos. 2012, 20, 151–154. [Google Scholar] [CrossRef]
- Hu, Q.; Peng, P.; Peng, S.; Liu, J.; Liu, X.; Zou, L.; Chen, J. Flame-retardant epoxy resin based on aluminum monomethylphosphinate. J. Therm. Anal. Calorim. 2017, 128, 201–210. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Akbari, V. Cure Index for labeling curing potential of epoxy/LDH nanocomposites: A case study on nitrate anion intercalated Ni-Al-LDH. Prog. Org. Coat. 2019, 136, 105228. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Thomas, S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105227. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized CoxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105252. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Akbari, V.; Karami, Z.; Aghazadeh, M.; Zarrintaje, P.; Saebd, M.R. Curing epoxy with polyethylene glycol (PEG) surface-functionalized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105283. [Google Scholar] [CrossRef]
- Karami, Z.; Aghazadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Vahabi, H.; Ganjali, M.R.; Saeb, M.R. Epoxy/Zn-Al-CO3 LDH nanocomposites: Curability assessment. Prog. Org. Coat. 2020, 138, 105355. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Hamad, S.M.; Ganjali, M.R.; Aghazadeh, M.; Torre, L.; Saeb, M.R. Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog. Org. Coat. 2019, 136, 105278. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Saeb, M.R. Development of Mg-Zn-Al-CO3 ternary LDH and its curability in epoxy/amine system. Prog. Org. Coat. 2019, 136, 105264. [Google Scholar] [CrossRef]
- Karami, Z. Well-cured silicone/halloysite nanotubes nanocomposite coatings. Prog. Org. Coat. 2019, 129, 357–365. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vyazovkin, S. Learning about epoxy cure mechanisms from isoconversional analysis of DSC data. Thermochim. Acta 2002, 388, 289–298. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Surface engineering of nanoparticles with macromolecules for epoxy curing: Development of super-reactive nitrogen-rich nanosilica through surface chemistry manipulation. Appl. Surf. Sci. 2018, 447, 152–164. [Google Scholar] [CrossRef]
- Málek, J. The kinetic analyss of non-isothermal data. Thermochim. Acta 1992, 200, 257–269. [Google Scholar] [CrossRef]
- Saeb, M.R.; Ghaffari, M.; Rastin, H.; Khonakdar, H.A.; Simon, F.; Najafi, F.; Formela, K. Biowaste chicken eggshell powder as a potential cure modifier for epoxy/anhydride systems: Competitiveness with terpolymer-modified calcium carbonate at low loading levels. RSC Adv. 2017, 7, 2218–2230. [Google Scholar] [CrossRef]
- Toldy, A.; Szolnoki, B.; Csontos, I.; Marosi, G. Green synthesis and characterization of phosphorus flame retardant crosslinking agents for epoxy resins. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Qiu, C.; Yao, Y.; Sakai, E.; Yang, L. Mechanical properties and degrading behaviors of aluminum hypophosphite-poly (Lactic Acid)(PLA) nanocomposites. Polym. Plast. Technol. Mater. 2019, 58, 126–138. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Jiao, C. In situ synthesis of flame retardant organic–inorganic hybrids by a molten blending method based on thermoplastic polyurethane elastomer and polybutyl titanate. RSC Adv. 2016, 6, 92276–92284. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Song, L. Thermal degradation and flame retardance of biobased polylactide composites based on aluminum hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]
- Yi, J.; Yin, H.; Cai, X. Effects of common synergistic agents on intumescent flame retardant polypropylene with a novel charring agent. J. Therm. Anal. Calorim. 2013, 111, 725–734. [Google Scholar] [CrossRef]

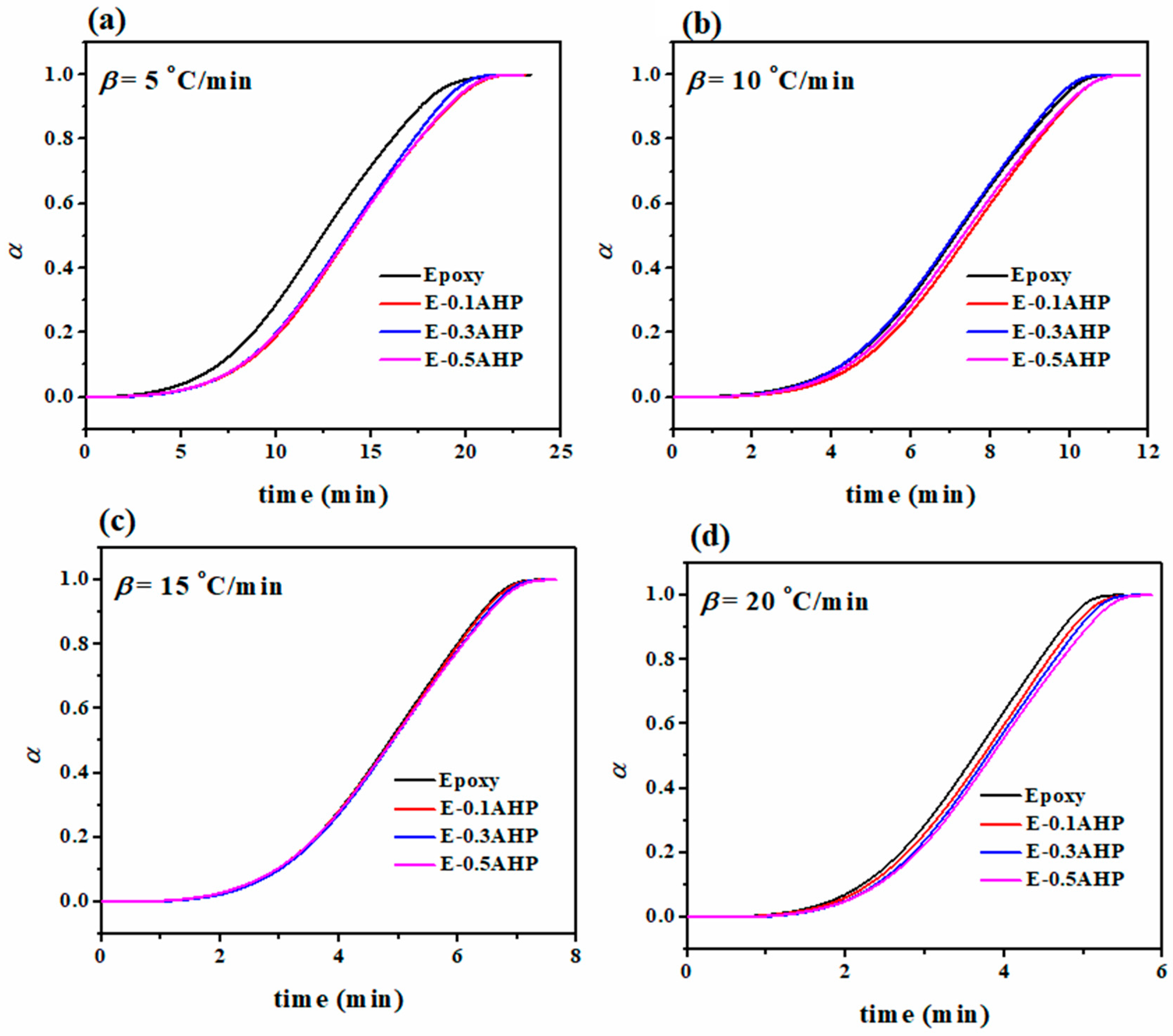



| Designation | β (°C/min) | Tonset | Tp (°C) | Tendset | ΔT (°C) | ΔH∞ (J/g) | ΔT* | ΔH* | CI | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 26.8 | 86.9 | 143.7 | 116.8 | 179.9 | n.a. | n.a. | n.a. | n.a. | |
| Epoxy | 10 | 27.7 | 98.8 | 139.9 | 112.1 | 192.5 | n.a. | n.a. | n.a. | n.a. |
| 15 | 35.1 | 111.7 | 147.3 | 112.2 | 132.5 | n.a. | n.a. | n.a. | n.a. | |
| 20 | 40.6 | 118.42 | 150.5 | 110.0 | 145.9 | n.a. | n.a. | n.a. | n.a. | |
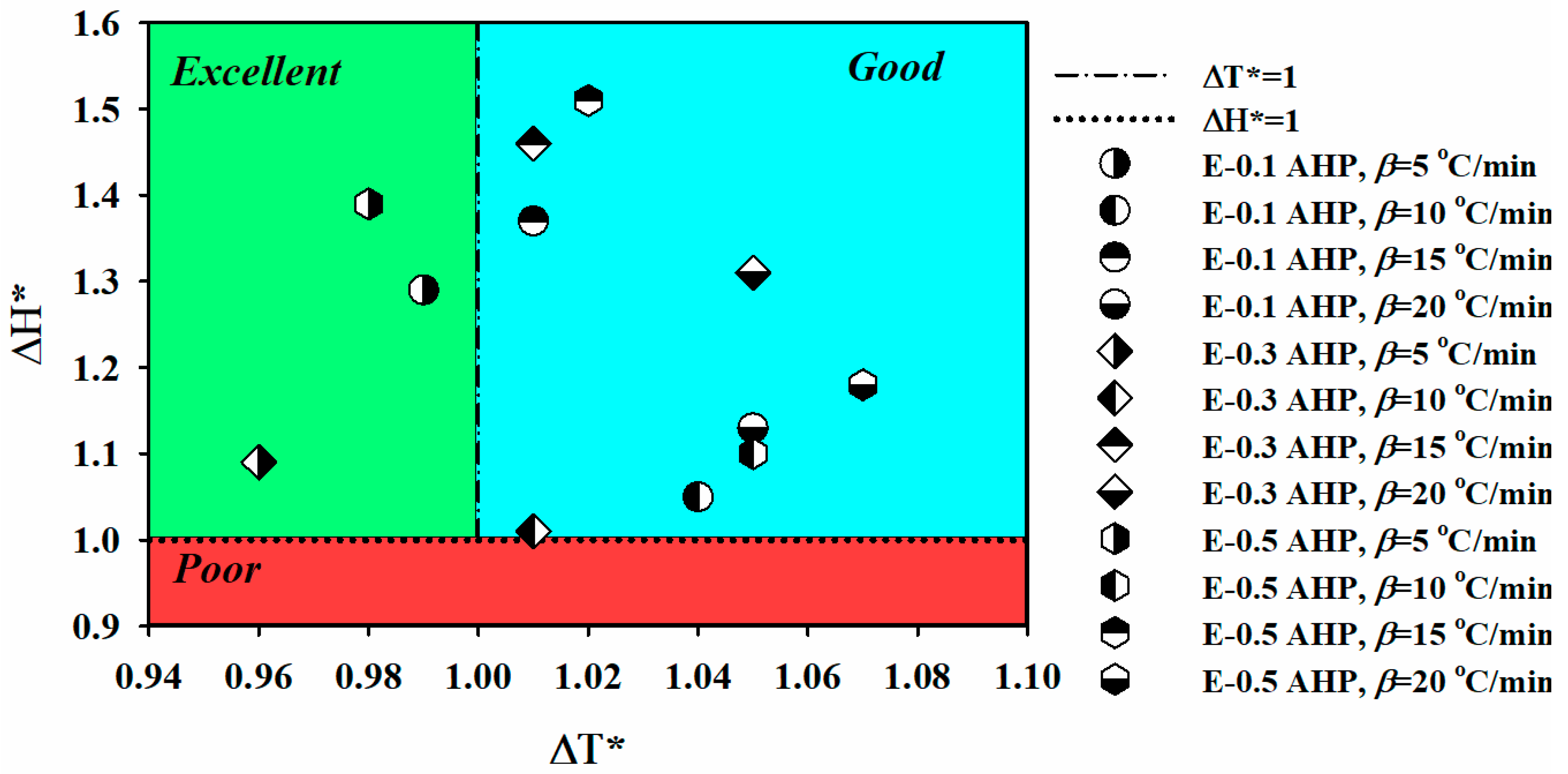
| E-0.1 AHP | 5 | 19.0 | 86.5 | 134.5 | 115.4 | 232.8 | 0.99 | 1.29 | 1.28 | Excellent |
| 10 | 26.9 | 102.3 | 143.9 | 117.0 | 201.2 | 1.04 | 1.05 | 1.02 | Good | |
| 15 | 36.4 | 115.6 | 149.6 | 113.2 | 181.7 | 1.01 | 1.37 | 1.38 | Good | |
| 20 | 41.2 | 123.4 | 156.2 | 115.0 | 165.2 | 1.05 | 1.13 | 1.19 | Good | |
| E-0.3 AHP | 5 | 22.8 | 91.1 | 135.2 | 112.4 | 196.5 | 0.96 | 1.09 | 1.05 | Excellent |
| 10 | 30.3 | 102.3 | 142.5 | 112.2 | 195.0 | 1.01 | 1.01 | 0.95 | Good | |
| 15 | 34.1 | 110.0 | 147.6 | 113.5 | 192.8 | 1.01 | 1.46 | 1.47 | Good | |
| 20 | 37.2 | 115.7 | 152.1 | 115.0 | 190.7 | 1.05 | 1.31 | 1.38 | Good | |
| E-0.5 AHP | 5 | 22.3 | 90.6 | 137.0 | 114.8 | 250.5 | 0.98 | 1.39 | 1.36 | Excellent |
| 10 | 28.1 | 101.0 | 145.8 | 117.7 | 213.5 | 1.05 | 1.10 | 1.09 | Good | |
| 15 | 34.3 | 109.3 | 149.0 | 114.7 | 199.7 | 1.02 | 1.51 | 1.54 | Good | |
| 20 | 35.5 | 113.3 | 152.8 | 117.3 | 172.2 | 1.07 | 1.18 | 1.26 | Good |
| Designation | Heating Rate (°C/min) | αp∞ | αm | αp |
|---|---|---|---|---|
| Epoxy | 5 | 0.47 | 0.14 | 0.51 |
| 10 | 0.70 | 0.15 | 0.55 | |
| 15 | 0.79 | 0.15 | 0.70 | |
| 20 | 0.83 | 0.17 | 0.79 | |
| E-0.1AHP | 5 | 0.47 | 0.19 | 0.51 |
| 10 | 0.59 | 0.18 | 0.59 | |
| 15 | 0.70 | 0.17 | 0.72 | |
| 20 | 0.72 | 0.18 | 0.72 | |
| E-0.3AHP | 5 | 0.50 | 0.06 | 0.59 |
| 10 | 0.57 | 0.07 | 0.60 | |
| 15 | 0.62 | 0.08 | 0.62 | |
| 20 | 0.65 | 0.08 | 0.64 | |
| E-0.5AHP | 5 | 0.50 | 0.04 | 0.55 |
| 10 | 0.54 | 0.05 | 0.58 | |
| 15 | 0.60 | 0.06 | 0.59 | |
| 20 | 0.62 | 0.06 | 0.57 |
| Designation | β (°C/min) | Ēa (kJ/mol) | ln A (s−1) | Mean (s−1) | m | Mean | n | Mean |
|---|---|---|---|---|---|---|---|---|
| Friedman | ||||||||
| Epoxy | 5 | 72.5 | 22.2 | 22.0 | 0.40 | 0.39 | 1.17 | 0.94 |
| 10 | 22.1 | 0.39 | 1.00 | |||||
| 15 | 21.9 | 0.41 | 0.83 | |||||
| 20 | 21.9 | 0.37 | 0.78 | |||||
| E-0.1AHP | 5 | 64.4 | 19.6 | 19.3 | 0.27 | 0.29 | 1.12 | 0.89 |
| 10 | 19.3 | 0.31 | 0.91 | |||||
| 15 | 19.2 | 0.31 | 0.77 | |||||
| 20 | 19.2 | 0.26 | 0.74 | |||||
| E-0.3AHP | 5 | 77.2 | 23.4 | 23.3 | 0.53 | 0.49 | 1.03 | 0.93 |
| 10 | 23.4 | 0.51 | 0.94 | |||||
| 15 | 23.3 | 0.47 | 0.90 | |||||
| 20 | 23.3 | 0.44 | 0.85 | |||||
| E-0.5AHP | 5 | 80.7 | 24.6 | 24.5 | 0.56 | 0.52 | 1.19 | 1.06 |
| 10 | 24.6 | 0.54 | 1.09 | |||||
| 15 | 24.5 | 0.53 | 0.98 | |||||
| 20 | 24.5 | 0.46 | 0.97 | |||||
| Kissinger | ||||||||
| Epoxy | 5 | 62.4 | 18.9 | 18.8 | 0.26 | 0.25 | 1.08 | 0.87 |
| 10 | 18.9 | 0.25 | 0.92 | |||||
| 15 | 18.7 | 0.27 | 0.77 | |||||
| 20 | 18.8 | 0.24 | 0.71 | |||||
| E-0.1AHP | 5 | 54.9 | 16.5 | 16.4 | 0.14 | 0.16 | 1.04 | 0.82 |
| 10 | 16.4 | 0.18 | 0.83 | |||||
| 15 | 16.3 | 0.18 | 0.71 | |||||
| 20 | 16.4 | 0.13 | 0.69 | |||||
| E-0.3AHP | 5 | 69.3 | 21.0 | 21.0 | 0.43 | 0.39 | 0.97 | 0.88 |
| 10 | 21.0 | 0.41 | 0.89 | |||||
| 15 | 21.1 | 0.371 | 0.85 | |||||
| 20 | 21.0 | 0.34 | 0.80 | |||||
| E-0.5AHP | 5 | 72.5 | 21.9 | 21.9 | 0.44 | 0.41 | 1.11 | 0.99 |
| 10 | 21.9 | 0.42 | 1.03 | |||||
| 15 | 21.9 | 0.41 | 0.92 | |||||
| 20 | 22.0 | 0.34 | 0.91 | |||||
| Sample | Tg (°C) |
|---|---|
| Epoxy | 73.2 |
| E-0.1AHP | 79.5 |
| E-0.3AHP | 73.1 |
| E-0.5AHP | 69.5 |
| Samples | T5%(°C) | T10%(°C) | Residue Weight Ratio at 550 °C |
|---|---|---|---|
| Epoxy | 319 | 351 | 9.8% |
| E-0.1AHP | 320 | 348 | 9.0% |
| E-0.3AHP | 295 | 343 | 9.4% |
| E-0.5AHP | 299 | 337 | 10.7% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. https://doi.org/10.3390/polym12030644
Tikhani F, Moghari S, Jouyandeh M, Laoutid F, Vahabi H, Saeb MR, Dubois P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers. 2020; 12(3):644. https://doi.org/10.3390/polym12030644
Chicago/Turabian StyleTikhani, Farimah, Shahab Moghari, Maryam Jouyandeh, Fouad Laoutid, Henri Vahabi, Mohammad Reza Saeb, and Philippe Dubois. 2020. "Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2)" Polymers 12, no. 3: 644. https://doi.org/10.3390/polym12030644
APA StyleTikhani, F., Moghari, S., Jouyandeh, M., Laoutid, F., Vahabi, H., Saeb, M. R., & Dubois, P. (2020). Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers, 12(3), 644. https://doi.org/10.3390/polym12030644