Optimal Design of Alkaline–Surfactant–Polymer Flooding under Low Salinity Environment
Abstract
:1. Introduction
2. Methodology
2.1. Properties of Formation Brine and Crude Oil
2.2. Experimental Procedure
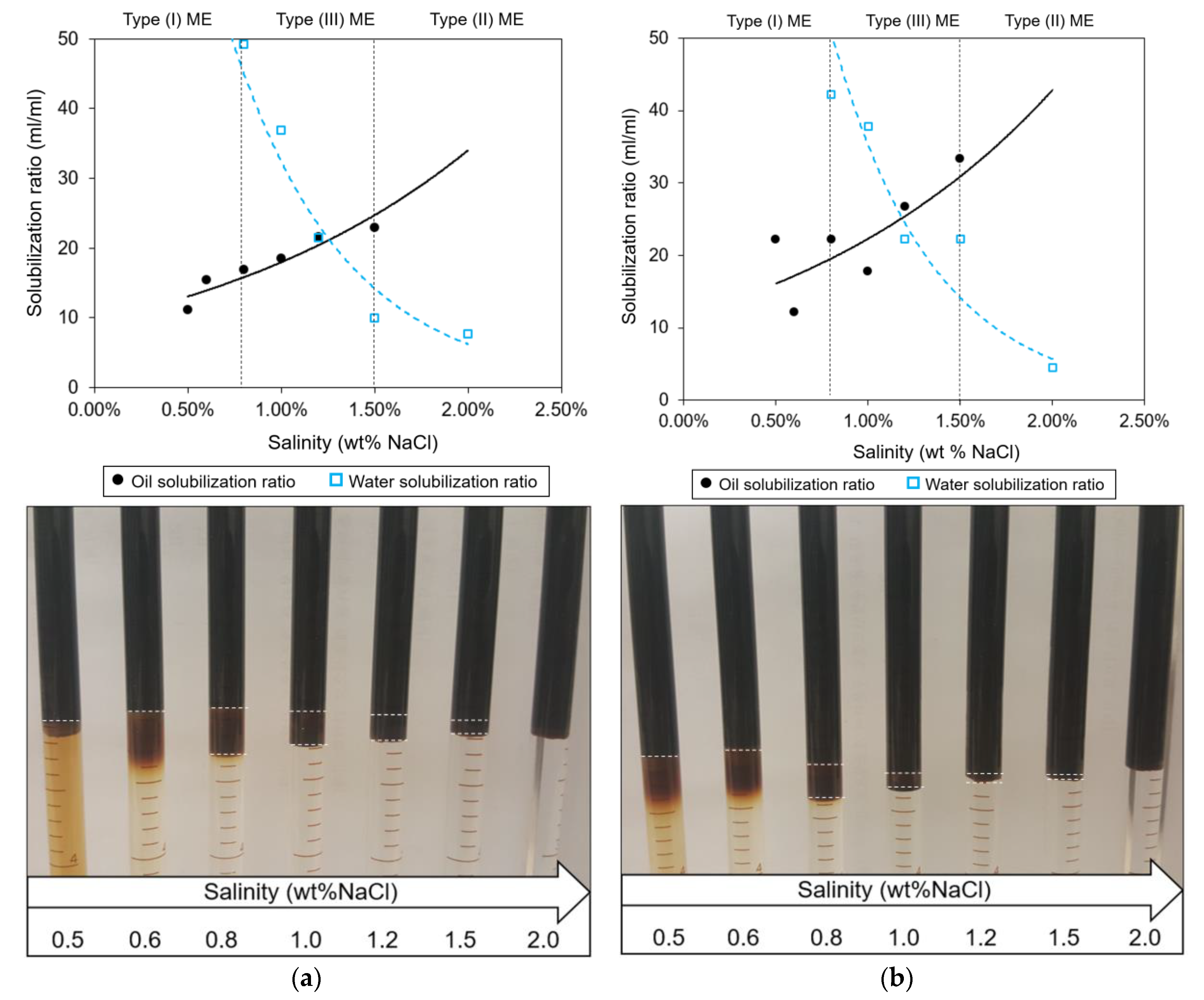
2.2.1. Phase Behavior Test
2.2.2. FR Test
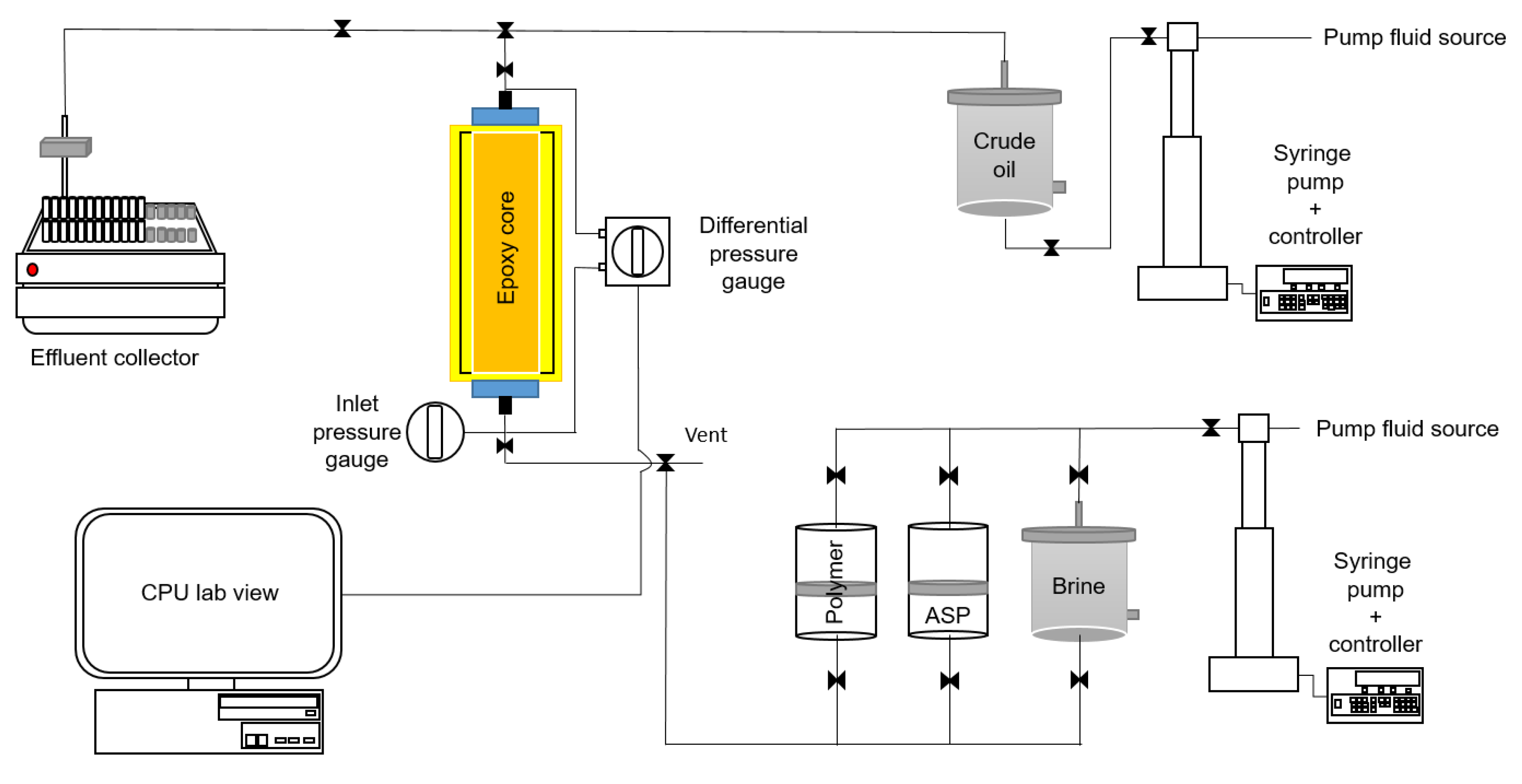
2.2.3. Coreflooding Test
3. Results and Discussion
3.1. Optimal ASP Formulation and Polymer Concentration
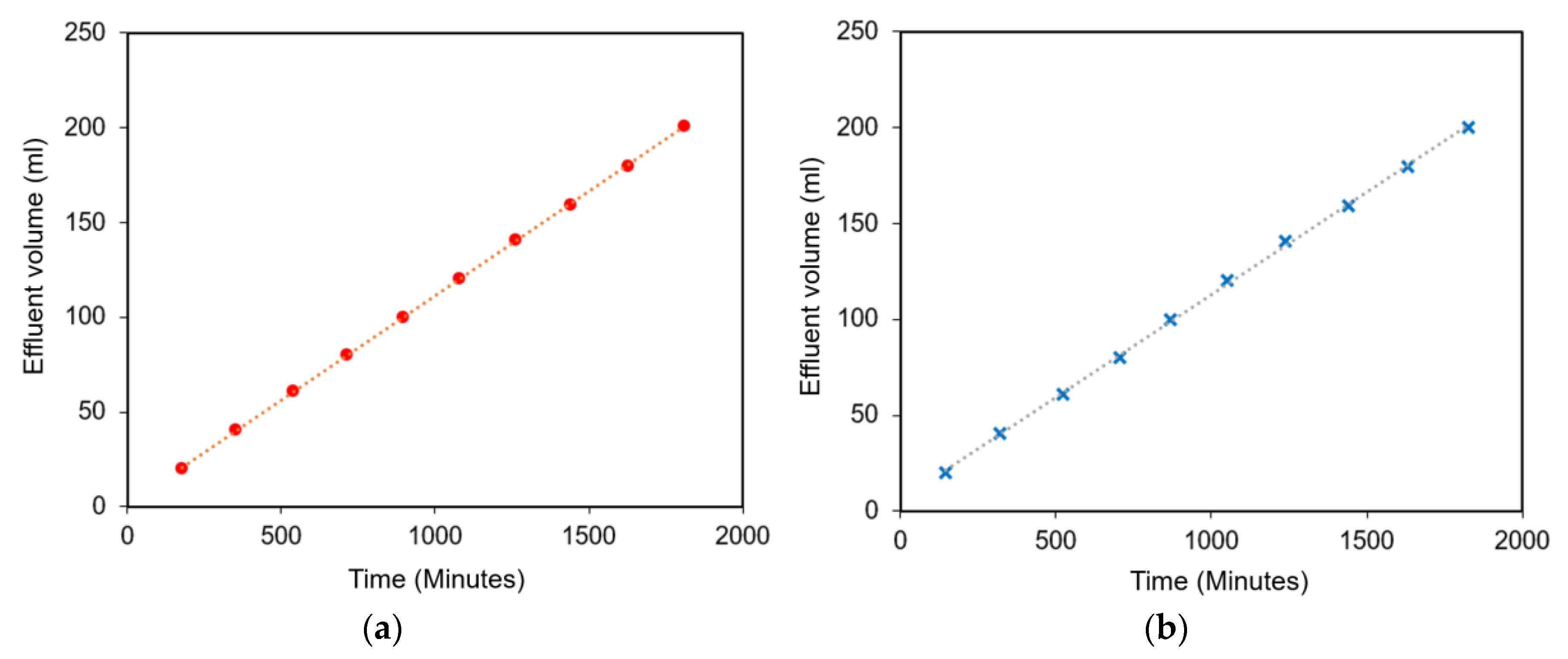
3.2. Coreflooding Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, B.; Li, X.; Xhang, W.; Wang, Y.; Fu, S. Study on demulsification–flocculation mechanism of oil–water emulsion in produced water from alkali/surfactant/polymer flooding. Polymers 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Burlington, NJ, USA, 2010; ISBN 9781856177450. [Google Scholar]
- Guo, H.; Li, Y.; Li, Y.; Kong, D.; Li, B.; Wang, F. Lessons learned from ASP flooding tests in China. In Proceedings of the SPE Reservoir Characterization and Simulation Conference and Exhibition, Abu Dhabi, UAE, 8–10 May 2017. SPE–186036. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Wang, F.; Gu, Y. Comparison of strong–alkali and weak–alkali ASP–flooding field tests in Daqing oil field. SPE Prod. Oper. 2018, 33, 353–362. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef] [Green Version]
- Sagi, A.R.; Puerto, M.C.; Bian, Y.; Miller, C.A.; Hirasaki, G.J.; Salehi, M.; Thomas, C.P.; Kwan, J.T. Laboratory studies for surfactant flood in low–temperature, low–salinity fractured carbonate reservoir. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. SPE–164062. [Google Scholar] [CrossRef] [Green Version]
- Hongyan, C.; Jie, C.; Jian, F.; Hexin, L.; Qing, W.; Wenli, L. ASP flooding: A solution for chemical enhanced oil recovery in high temperature, low salinity reservoir. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018. SPE–192376. [Google Scholar] [CrossRef]
- Sheng, J.J. Optimum phase type and optimum salinity profile in surfactant flooding. J. Petrol. Sci. Eng. 2010, 75, 143–153. [Google Scholar] [CrossRef]
- Levitt, D.; Chamerois, M.; Bourrel, M.; Gauer, P.R.; Morel, D.C. The effect of a non–negative salinity gradient on ASP flood performance. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–21 July 2011. SPE–144938. [Google Scholar] [CrossRef]
- Gregersen, C.S.; Kazempour, M.; Alvarado, V. ASP design for the Minnelusa formation under low–salinity conditions: impacts of anhydrite on ASP performance. Fuel 2013, 105, 368–382. [Google Scholar] [CrossRef]
- Battistutta, E.; van Kuijk, S.R.; Groen, K.V.; Zitha, P.L.J. Alkaline–surfactant–polymer (ASP) flooding of crude oil at under–optimum salinity conditions. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. SPE–174666. [Google Scholar] [CrossRef]
- Chen, Z.; Han, X.; Kurnia, I.; Yu, J.; Zhang, G.; Li, L. Adoption of phase behavior tests and negative salinity gradient concept to optimize Daqing oilfield alkaline–surfactant–polymer flooding. Fuel 2018, 232, 71–80. [Google Scholar] [CrossRef]
- Riswati, S.S.; Bae, W.; Park, C.; Permadi, A.K.; Efriza, I.; Min, B. Experimental analysis to design optimum phase type and salinity gradient of Alkaline Surfactant Polymer flooding at low saline reservoir. J. Petrol. Sci. Eng. 2019, 173, 1005–1019. [Google Scholar] [CrossRef]
- Kusumah, G.S.; Vazquez, O. Evolution of pH and retention of different alkali species for ASP flooding field applications. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 5 April 2017. SPE– 185886. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, T.; Yin, H.; Fu, C.; Lu, J. The role of chemicals loss in sandstone formation in ASP flooding enhanced oil recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018. SPE–191545. [Google Scholar] [CrossRef]
- Broze, G. Handbook of Detergents, Part A: Properties; CRC Press: Abingdon, UK, 1999; ISBN 9780824714178. [Google Scholar]
- Riehm, D.A.; McCormick, A.V. The role of dispersants’ dynamic interfacial tension in effective crude oil spill dispersion. Mar. Pollut. Bull. 2014, 84, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Huh, C. Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine. J. Colloid Interf. Sci. 1979, 71, 408–426. [Google Scholar] [CrossRef]
- Chang, L.Y. Prediction of Microemulsion Phase Behavior from Surfactant and Co–solvent Structures. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2018. [Google Scholar]
- Koh, H.; Lee, V.B.; Pope, G.A. Experimental investigation of the effect of polymers on residual oil saturation. SPE J. 2018, 23, 1–17. [Google Scholar] [CrossRef]
- Sahni, V.; Dean, R.M.; Britton, C.; Kim, D.H.; Weerasooriya, U.; Pope, G.A. The role of co–solvents and co–surfactants in making chemical floods robust. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. SPE–130007. [Google Scholar] [CrossRef]


| Components | Concentration (mol %) | Weight Percentage (wt %) |
|---|---|---|
| Hydrogen sulfide (H2S) | 0 | 0 |
| Carbon dioxide (CO2) | 0.3783 | 0.1044 |
| Nitrogen (N2) | 0.4645 | 0.0816 |
| Methane (CH4) | 11.2789 | 1.1348 |
| Ethane (C2H6) | 0.1622 | 0.0306 |
| Propane (C3H8) | 0.2503 | 0.0692 |
| Isobutane (i-C4H10) | 0.1832 | 0.0668 |
| n-butane (n-C4H10) | 0.4448 | 0.1621 |
| Isopentane (i-C5H12) | 2.3216 | 1.0505 |
| n-pentane (n-C5H12) | 2.6256 | 1.1881 |
| Hexane (C6H14) | 13.2666 | 7.1701 |
| Heptane plus (+) (C7H16+) | 68.6240 | 88.9418 |
| Total | 100 | 100 |
| Surfactant | Trade Name (Abbreviation) | Active Matter (%) | Appearance |
|---|---|---|---|
| Dioctyl sulfosuccinate (C8–C8) | ASCODOSS (DOSS) | 63.0–67.0 | Clear liquid |
| Linear alkylbenzene sulfonate (C11–C13) | ASCO96 (LAS) | minimum: 96.0 | Viscous amber liquid |
| Properties | Diethylene Glycol Monobutyl Ether (DGBE) | Isobutyl Alcohol (IBA) |
|---|---|---|
| Chemical formula | C8H18O3 | C4H10O |
| Molar mass (g/mol) | 162.229 | 74.122 |
| Vapor pressure | - | 9 mmHg (20 °C) |
| Density (g/cm3) | 0.955 | 0.803 |
| Melting point (°C) | −68 | −108 |
| Boiling point (°C) | 231 | 108 |
| Flash point (°C) | 100 | 27 |
| Appearance | Colorless clear liquid | Colorless liquid |
| Solubility in water | Soluble in water, ethanol, ethyl ether, and acetone | 8.7 mL/100 mL |
| Sample | Diameter (cm) | Length (cm) | Porosity (%) | Permeability (md) | Pore Volume (PV; cm3) |
|---|---|---|---|---|---|
| Sample 1 | 3.80 | 14.8 | 19 | 200 | 31.86 |
| Sample 2 | 3.79 | 14.2 | 17 | 180 | 27.83 |
| Case | ASP Formulation | Results | ||||
|---|---|---|---|---|---|---|
| Alkali (wt % Na2CO3) | Surfactant (wt %, LAS:DOSS Ratio) | Co-solvent (wt %, Co-solvent) | Optimum salinity (wt % NaCl) | Optimum Solubilization Ratio (mL/mL) | Interfacial Tension (mN/m) | |
| PB1 | 0 | 2 (1:2) | 4, IBA | 3.0 | 3.0 | 0.0333 |
| PB2 | 0 | 2 (1:4) | 4, IBA | 2.0 | 2.5 | 0.0480 |
| PB3 | 1 | 2 (1:4) | 4, IBA | 1.0 | 4.0 | 0.0188 |
| PB4 | 1 | 1 (1:4) | 2, IBA | 1.0 | 5.0 | 0.0120 |
| PB5 | 1 | 1 (1:4) | 5, DGBE | 1.25 | 21.5 | 6.49 × 10−4 |
| PB6 | 1 | 0.9 (1:4) | 5, DGBE | 1.20 | 25.0 | 4.80 × 10−4 |
| Sample | Salinity (wt % NaCl) | PV Injection (ASP Mixture) | Residual Oil Recovery (%) | ||
|---|---|---|---|---|---|
| Preflush | ASP Mixture | Polymer Drive | |||
| Sample 1 | 0.6 | 1.5 | 0.6 | 0.37 | 86.20 |
| Sample 2 | 0.6 | 1.5 | 0.6 | 0.10 | 48.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novriansyah, A.; Bae, W.; Park, C.; Permadi, A.K.; Sri Riswati, S. Optimal Design of Alkaline–Surfactant–Polymer Flooding under Low Salinity Environment. Polymers 2020, 12, 626. https://doi.org/10.3390/polym12030626
Novriansyah A, Bae W, Park C, Permadi AK, Sri Riswati S. Optimal Design of Alkaline–Surfactant–Polymer Flooding under Low Salinity Environment. Polymers. 2020; 12(3):626. https://doi.org/10.3390/polym12030626
Chicago/Turabian StyleNovriansyah, Adi, Wisup Bae, Changhyup Park, Asep K. Permadi, and Shabrina Sri Riswati. 2020. "Optimal Design of Alkaline–Surfactant–Polymer Flooding under Low Salinity Environment" Polymers 12, no. 3: 626. https://doi.org/10.3390/polym12030626
APA StyleNovriansyah, A., Bae, W., Park, C., Permadi, A. K., & Sri Riswati, S. (2020). Optimal Design of Alkaline–Surfactant–Polymer Flooding under Low Salinity Environment. Polymers, 12(3), 626. https://doi.org/10.3390/polym12030626







