The Role of Two-Step Blending in the Properties of Starch/Chitin/Polylactic Acid Biodegradable Composites for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
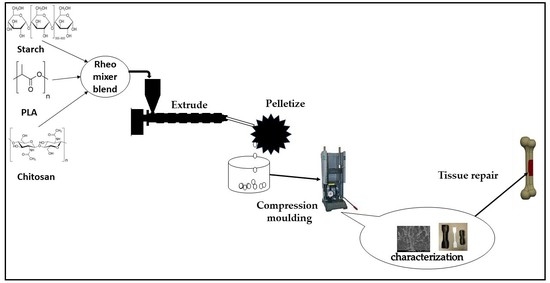
2.2. Composite Processing
2.3. Characterization
2.3.1. Tensile Test
2.3.2. Impact Test
2.3.3. Dynamic Mechanical Analysis
2.3.4. Microstructural Analysis
2.3.5. Bioabsorption and Biotoxicity Test
3. Results
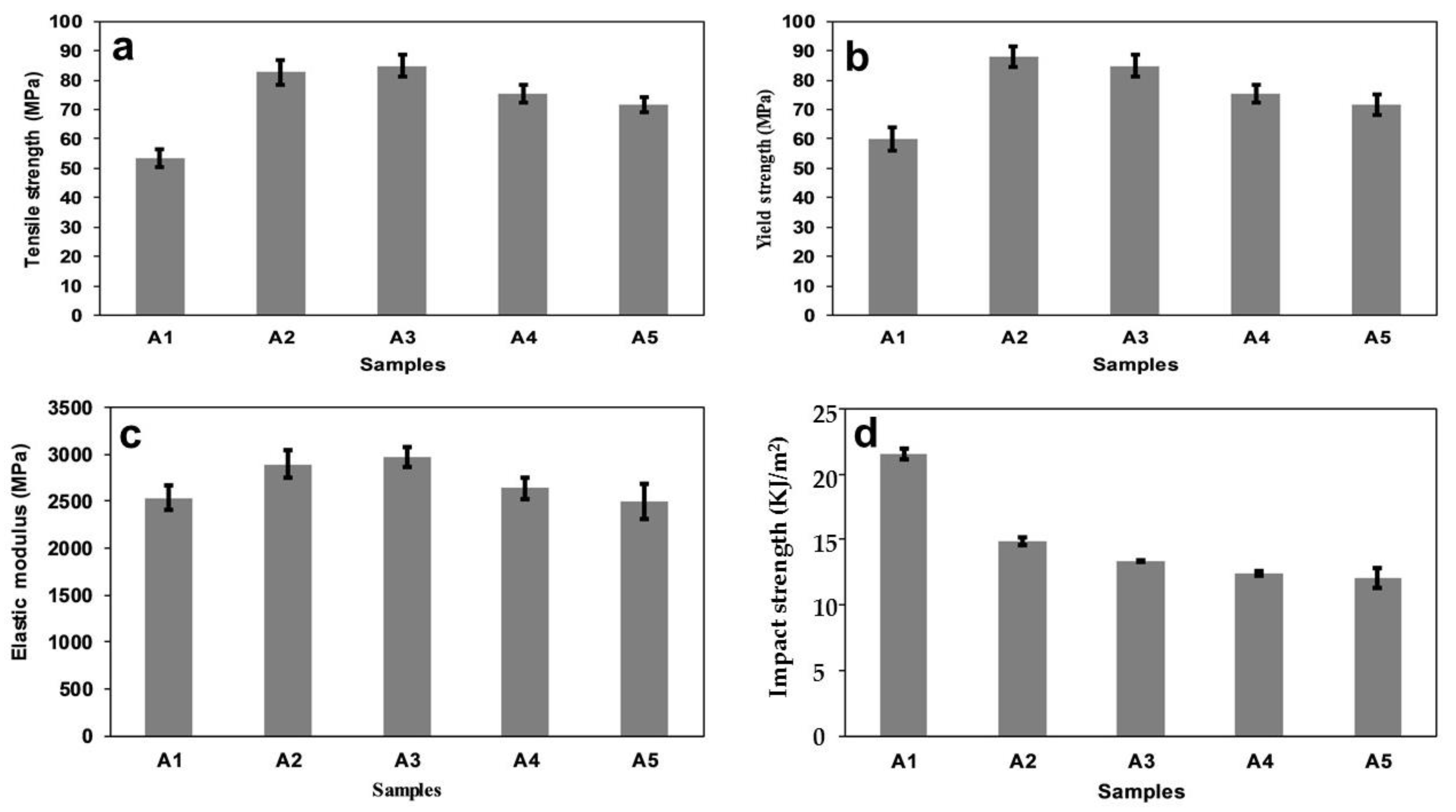
3.1. Tensile Properties
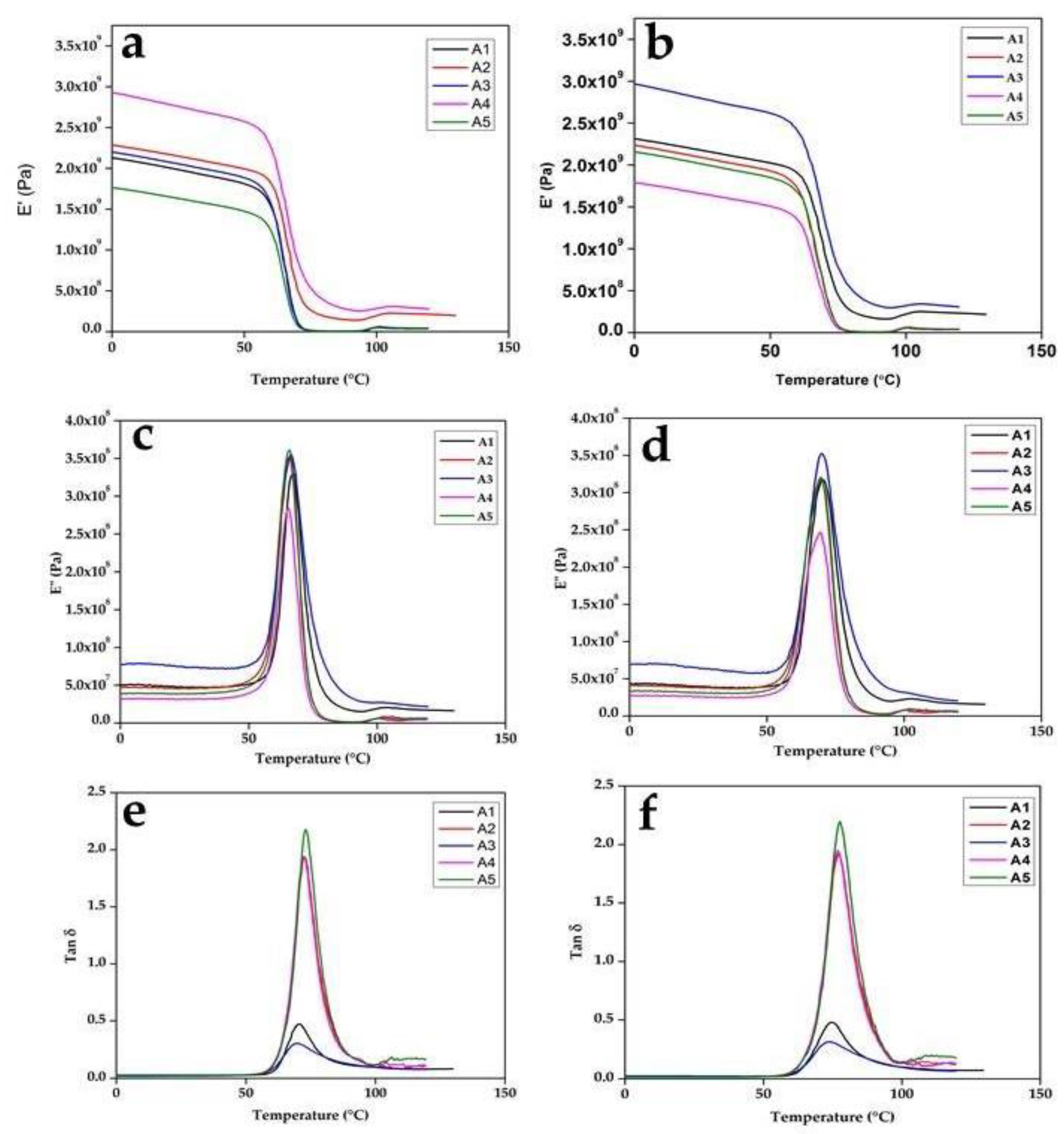
3.2. Dynamic Mechanical Analysis
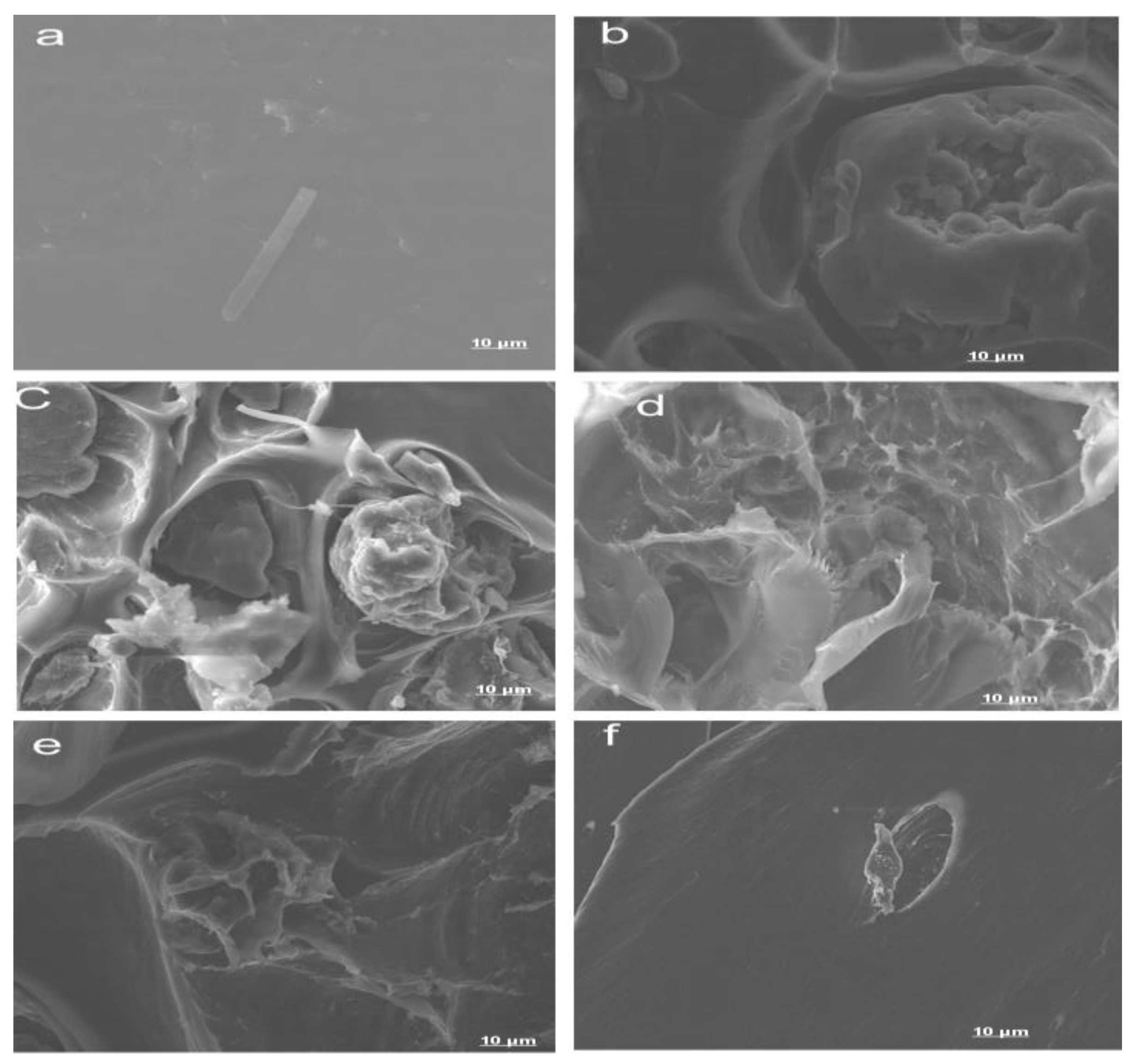
3.3. Morphological Properties
3.4. XRD Analysis
3.5. Biotoxicity Properties
4. Conclusions
- Starch/chitin/PLA composites were successfully developed using a two-step pre-mix;
- The mechanical properties of the polymer composites increased significantly more than did those of the neat PLA. The mechanical properties of the composites were improved using this method (with varying starch/chitin/PLA percentages). Sample A3 (PLA/6% C/2% S) had optimal strength and a suitable combination of the blend. This material can be used for trabecular tissue repair or any tissue repair (below the cut-off values of the tensile strength and modulus);
- The brittleness of neat PLA was reduced with the addition of the blend in all of the samples;
- The DMA of the composites showed a good storage modulus for stiffness, reduced loss factor, and good miscibility. It also showed a single peak, which showed the compatibility of the polymer blend. The glass temperature from the loss factor peak showed that the material was not suitable for high-temperature biomedical applications;
- The composite, which was molded using a Carver press, showed a uniform distribution of the natural polymer blend in its physical appearance. The SEM images showed a network distribution of the chitin/starch blend in PLA. Though there were small voids, the images showed a homogenous blend of chitin/starch with PLA;
- The amorphous characteristics of neat PLA caused no significant change in the blend; and
- The material was absorbable and nontoxic according to the results of the acute toxicity test. Thus, full clinical analyses are recommended.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diwan, A.D.; Parvataneni, H.K.; Khan, S.N.; Sandhu, H.S.; Girardi, F.P.; Cammisa, F.P. Current concepts in intervertebral disk restoration. Orthop. Clin. 2000, 31, 453–464. [Google Scholar] [CrossRef]
- Lewis, G. Nucleus pulposus replacement and regeneration/repair technologies: Present status and future prospects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1702–1720. [Google Scholar] [CrossRef] [PubMed]
- Whatley, B.R.; Wen, X. Intervertebral disc (IVD): Structure, degeneration, repair and regeneration. Mater. Sci. Eng. C 2012, 32, 61–77. [Google Scholar] [CrossRef]
- Vasconcellos, L.A.; dos Santos, L.A. Calcium phosphate cement scaffolds with PLGA fibers. Mater. Sci. Eng. C 2013, 33, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Revati, R.; Majid, M.A.; Normahira, M. Biodegradable poly (lactic acid) scaffold for tissue engineering: A brief review. J. Polym. Sci. Technol. 2016, 1, 16–24. [Google Scholar]
- Pei, B.; Wang, W.; Fan, Y.; Wang, X.; Watari, F.; Li, X. Fiber-reinforced scaffolds in soft tissue engineering. Regen. Biomater. 2017, 4, 257–268. [Google Scholar] [CrossRef]
- Rosenzweig, D.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposus tissue regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Biodegradable elastomeric scaffolds for soft tissue engineering. J. Control. Release 2003, 87, 69–79. [Google Scholar] [CrossRef]
- P Pawar, R.; U Tekale, S.; U Shisodia, S.; T Totre, J.; J Domb, A. Biomedical applications of poly (lactic acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Eliades, T. Orthodontic materials research and applications: Part 2. Current status and projected future developments in materials and biocompatibility. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.-Q.; Li, H.; Zhu, G.; Li, D.-Y.; Fan, Y.-B.; Wu, S.-Q. The application of fiber-reinforced materials in disc repair. Biomed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Chong, E.; Owolabi, F.; Asniza, M.; Tye, Y.; Rizal, S.; Nurul Fazita, M.; Mohamad Haafiz, M.; Nurmiati, Z.; Paridah, M. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Silva, T.H.; Alves, A.; Ferreira, B.; Oliveira, J.M.; Reys, L.; Ferreira, R.; Sousa, R.; Silva, S.S.; Mano, J.; Reis, R. Materials of marine origin: A review on polymers and ceramics of biomedical interest. Int. Mater. Rev. 2012, 57, 276–306. [Google Scholar] [CrossRef]
- Isa, M.T.; Ameh, A.O.; Gabriel, J.O.; Adama, K.K. Extraction and Characterization of Chitin from Nigerian Sources’. Leonardo Electron. J. Pract. Technol. 2012, 21, 73–81. [Google Scholar]
- Adeosun, S.O.; Lawal, G.I.; Gbenebor, O.P. Characteristics of Biodegradable Implants. J. Miner. Mater. Charact. Eng. 2014, 2, 88–106. [Google Scholar] [CrossRef]
- Ali, A.; Yu, L.; Liu, H.; Khalid, S.; Meng, L.; Chen, L. Preparation and characterization of starch-based composite films reinforced by corn and wheat hulls. J. Appl. Polym. Sci. 2017, 134, 45159. [Google Scholar] [CrossRef]
- Olaiya, N.; Surya, I.; Oke, P.; Rizal, S.; Sadiku, E.; Ray, S.; Farayibi, P.; Hossain, M.S.; Abdul Khalil, H. Properties and Characterization of a PLA–Chitin–Starch Biodegradable Polymer Composite. Polymers 2019, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, R.; Biswas, S.; Rashid, T.U.; Afrin, S.; Jahan, R.A.; Haque, P.; Rahman, M.M. Preparation of Chitin-PLA laminated composite for implantable application. Bioact. Mater. 2017, 2, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Islam, M.; Hassan, A.; Mohamad Haafiz, M.; Arjmandi, R.; Inuwa, I.; Hasan, M. Mechanical properties and morphological characterization of PLA/chitosan/epoxidized natural rubber composites. Adv. Mater. Sci. Eng. 2013. [Google Scholar] [CrossRef]
- D3039/D3039M-00. Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials; ASTM International: Montgomery Country, PA, USA, 2000.
- ASTM American Society for Testing and Materials. Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics; ASTM International: Montgomery Country, PA, USA, 2010. [Google Scholar]
- Standard Practice for Plastics: Dynamic Mechanical Properties: Determination and Report of Procedures; ASTM International: Montgomery Country, PA, USA, 2012.
- Palipoch, S.; Punsawad, C. Biochemical and histological study of rat liver and kidney injury induced by cisplatin. J. Toxicol. Pathol. 2013, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hemvichian, K.; Suwanmala, P.; Kangsumrith, W.; Sudcha, P.; Inchoto, K.; Pongprayoon, T.; Güven, O. Enhancing compatibility between poly (lactic acid) and thermoplastic starch using admicellar polymerization. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Shirai, M.A.; Olivato, J.B.; Demiate, I.M.; Müller, C.M.O.; Grossmann, M.V.E.; Yamashita, F. Poly (lactic acid)/thermoplastic starch sheets: Effect of adipate esters on the morphological, mechanical and barrier properties. Polímeros 2016, 26, 66–73. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Shanks, R.; Kong, I. Thermoplastic Elastomers; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and mechanobiology of trabecular bone: A review. J. Biomech. Eng. 2015, 137, 010802. [Google Scholar] [CrossRef]
- Kiangkitiwan, N.; Srikulkit, K. Poly (lactic acid) filled with cassava starch-g-soybean oil maleate. Sci. World J. 2013. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. Effect of kenaf fibre modification on morphology and mechanical properties of thermoplastic polyurethane materials. Ind. Crop. Prod. 2015, 74, 566–576. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, T.-L.; Chen, Y.-L.; Wu, J.-H. Assessing the effect of wood acetylation on mechanical properties and extended creep behavior of wood/recycled-polypropylene composites. Constr. Build. Mater. 2016, 108, 139–145. [Google Scholar] [CrossRef]
- Maubane, L.; Ray, S.S.; Jalama, K. The effect of starch amylose content on the morphology and properties of melt-processed butyl-etherified starch/poly [(butylene succinate)-co-adipate] blends. Carbohydr. Polym. 2017, 155, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kaid, F.; Alabsi, A.; Alafifi, N.; Ali-Saeed, R.; Ameen Al-koshab, M.; Ramanathan, A.; Ali, A. Histological, Biochemical, and Hematological Effects of Goniothalamin on Selective Internal Organs of Male Sprague-Dawley Rats. J. Toxicol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, D.; Lai, T.K.; Jafarzadeh, S.; Gopakumar, D.A.; Hasan, M.; Owolabi, F.T.; Aprilia, N.S.; Rizal, S.; Khalil, H.A. Development of Seaweed-based Bamboo Microcrystalline Cellulose Films Intended for Sustainable Food Packaging Applications. BioResources 2019, 14, 3389–3410. [Google Scholar]
- Lv, S.; Liu, X.; Gu, J.; Jiang, Y.; Tan, H.; Zhang, Y. Microstructure analysis of polylactic acid-based composites during degradation in soil. Int. Biodeterior. Biodegrad. 2017, 122, 53–60. [Google Scholar] [CrossRef]
- Ayana, B.; Suin, S.; Khatua, B. Highly exfoliated eco-friendly thermoplastic starch (TPS)/poly (lactic acid)(PLA)/clay nanocomposites using unmodified nanoclay. Carbohydr. Polym. 2014, 110, 430–439. [Google Scholar]
- Cheaburu Yilmaz, C.; Pamfil, D.; Vasile, C.; Bibire, N.; Lupuşoru, R.-V.; Zamfir, C.-L.; Lupușoru, C. Toxicity, biocompatibility, pH-responsiveness and methotrexate release from PVA/hyaluronic acid cryogels for psoriasis therapy. Polymers 2017, 9, 123. [Google Scholar] [CrossRef]
- Thoolen, B.; Maronpot, R.R.; Harada, T.; Nyska, A.; Rousseaux, C.; Nolte, T.; Malarkey, D.E.; Kaufmann, W.; Küttler, K.; Deschl, U. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010, 38, 5S–81S. [Google Scholar] [CrossRef]

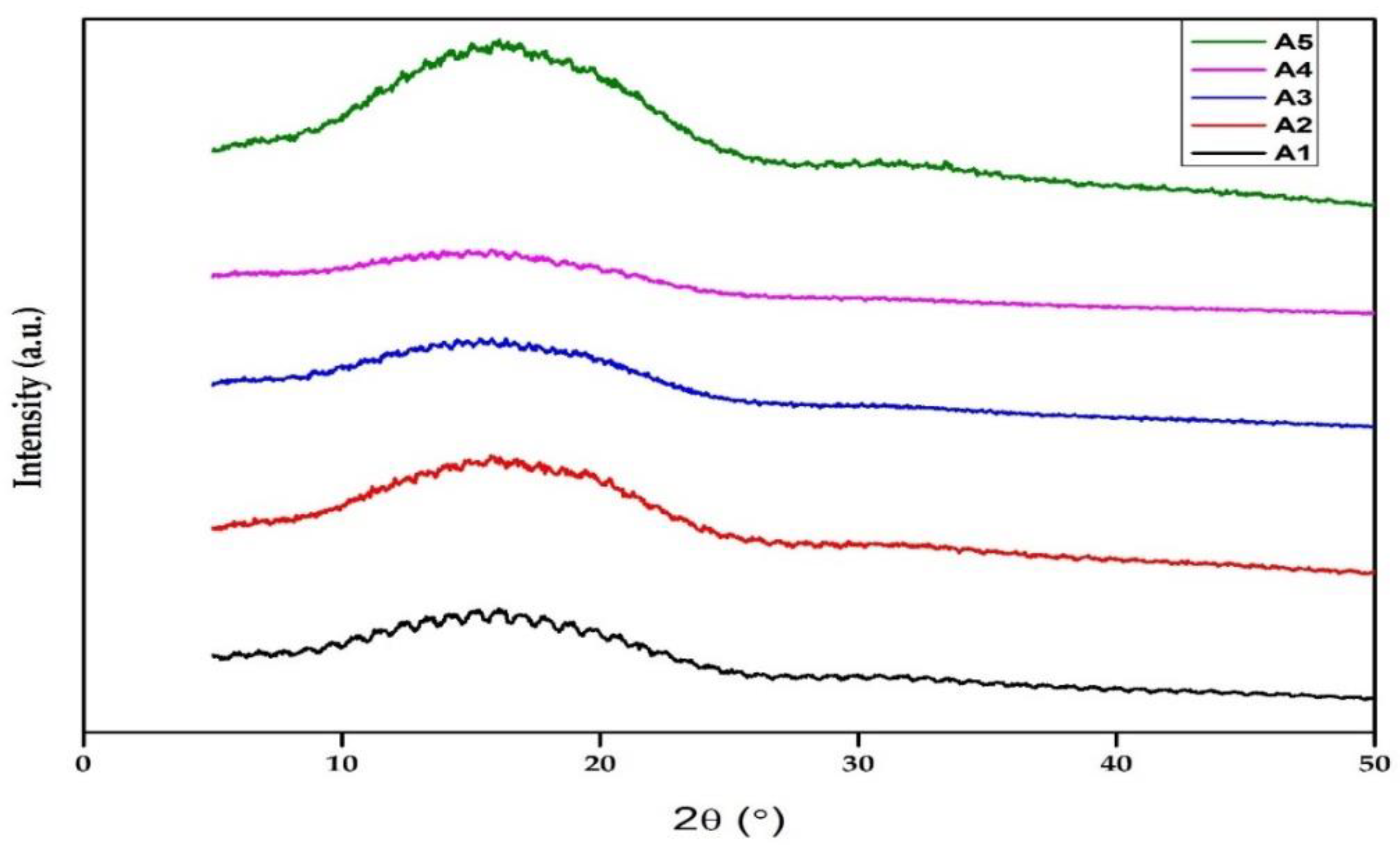
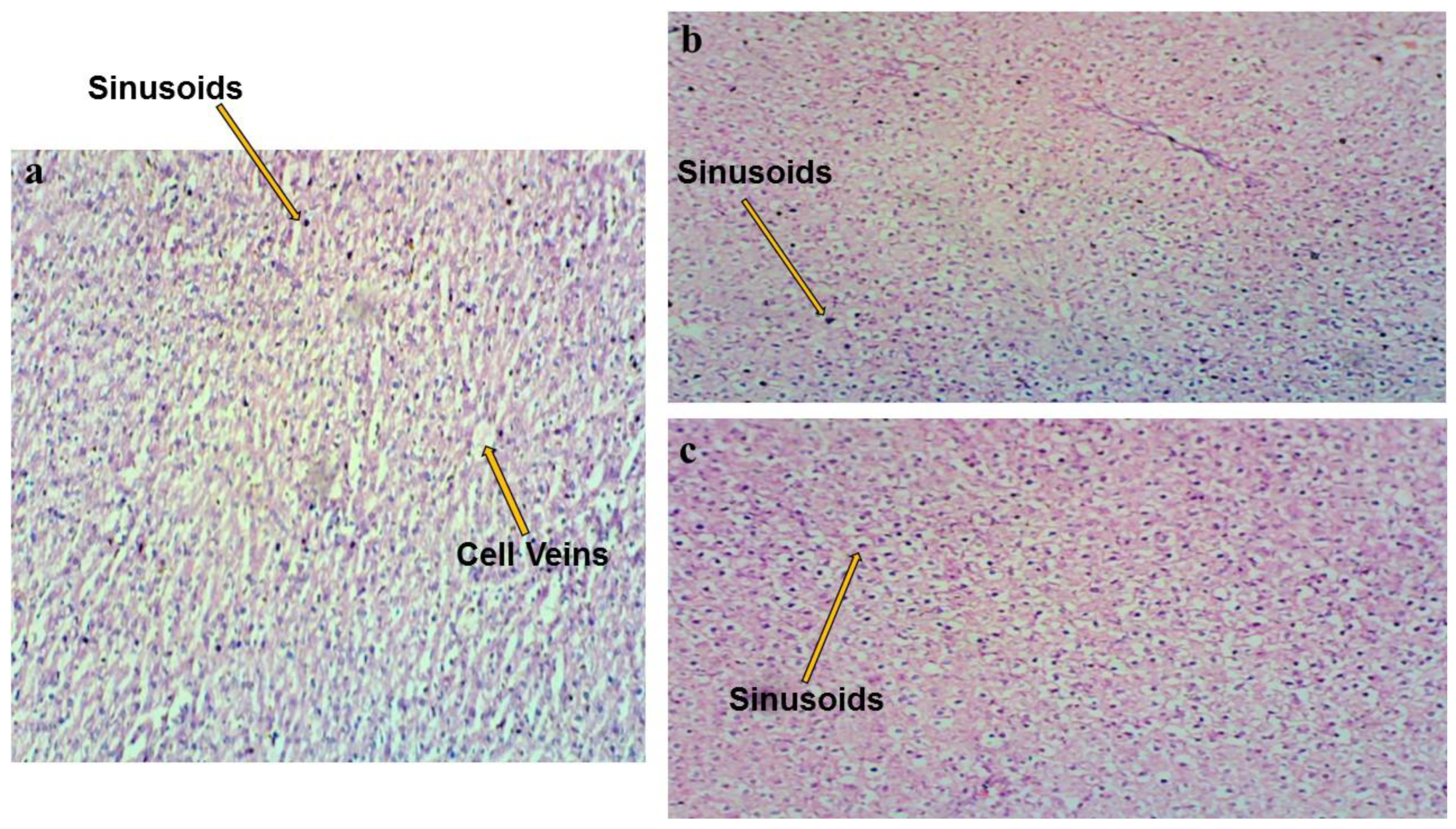
| Samples | DOC (%) |
|---|---|
| A1 | 7.18 |
| A2 | 7.10 |
| A3 | 7.55 |
| A4 | 8.56 |
| A5 | 8.42 |
| Rat Color Identification | Sample | Weight Increase (%) | Weight Increase (%) | Weight Increase (%) | Weight Increase (%) |
|---|---|---|---|---|---|
| 3 Days | 6 Days | 9 Days | 14 Days | ||
| Blue rat | A2 | 26 | 38 | 90 | 94 |
| Green rat | A3 | 7 | 19 | 20 | 111 |
| Black rat | A4 | 23 | 52 | 81 | 99 |
| Red rat | A5 | 12 | 24 | 64 | 67 |
| Control | Feed | 35 | 74 | 95 | 134 |
| Rat | PCV | Hb | WBC | RBC | MCV | MCH | MCHC | Differential Count | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (g/L) | (109/L) | (1012 g/L) | (fL) | (pg) | (g/dL) | N | L | E | M | B | |
| Control | 48 | 16.1 | 6.8 | 4.57 | 105.03 | 35.54 | 33.54 | 50 | 48 | 1 | 1 | 0 |
| Green | 34 | 11.4 | 5.9 | 3.37 | 100.89 | 33.82 | 38 | 35 | 62 | 2 | 1 | 0 |
| Red | 30 | 10.2 | 5.4 | 3.32 | 90.36 | 3.4 | 34 | 50 | 48 | 1 | 1 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaiya, N.G.; Nuryawan, A.; Oke, P.K.; Khalil, H.P.S.A.; Rizal, S.; Mogaji, P.B.; Sadiku, E.R.; Suprakas, S.R.; Farayibi, P.K.; Ojijo, V.; et al. The Role of Two-Step Blending in the Properties of Starch/Chitin/Polylactic Acid Biodegradable Composites for Biomedical Applications. Polymers 2020, 12, 592. https://doi.org/10.3390/polym12030592
Olaiya NG, Nuryawan A, Oke PK, Khalil HPSA, Rizal S, Mogaji PB, Sadiku ER, Suprakas SR, Farayibi PK, Ojijo V, et al. The Role of Two-Step Blending in the Properties of Starch/Chitin/Polylactic Acid Biodegradable Composites for Biomedical Applications. Polymers. 2020; 12(3):592. https://doi.org/10.3390/polym12030592
Chicago/Turabian StyleOlaiya, Niyi Gideon, Arif Nuryawan, Peter Kayode Oke, H. P. S. Abdul Khalil, Samsul Rizal, P. B. Mogaji, E. R. Sadiku, S. R. Suprakas, Peter Kayode Farayibi, Vincent Ojijo, and et al. 2020. "The Role of Two-Step Blending in the Properties of Starch/Chitin/Polylactic Acid Biodegradable Composites for Biomedical Applications" Polymers 12, no. 3: 592. https://doi.org/10.3390/polym12030592
APA StyleOlaiya, N. G., Nuryawan, A., Oke, P. K., Khalil, H. P. S. A., Rizal, S., Mogaji, P. B., Sadiku, E. R., Suprakas, S. R., Farayibi, P. K., Ojijo, V., & Paridah, M. T. (2020). The Role of Two-Step Blending in the Properties of Starch/Chitin/Polylactic Acid Biodegradable Composites for Biomedical Applications. Polymers, 12(3), 592. https://doi.org/10.3390/polym12030592