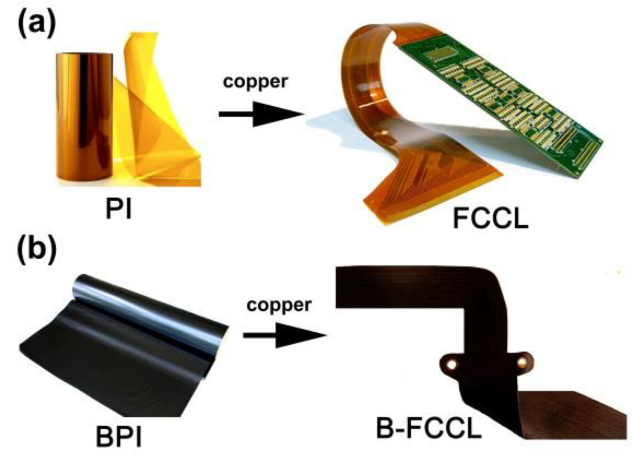
Preparation and Properties of Inherently Black Polyimide Films with Extremely Low Coefficients of Thermal Expansion and Potential Applications for Black Flexible Copper Clad Laminates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
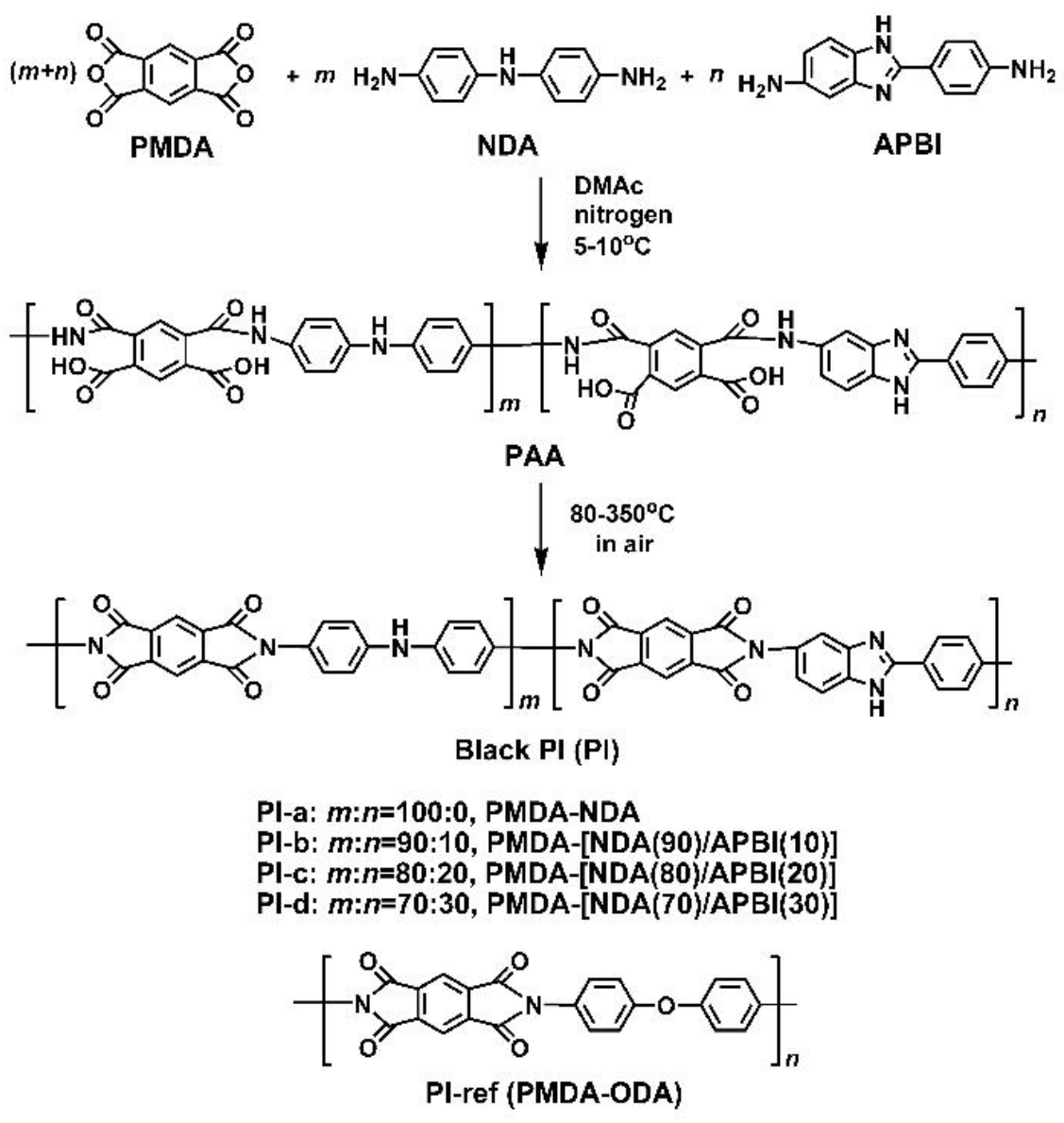
2.3. PI Synthesis and Film Preparation
3. Results and Discussion
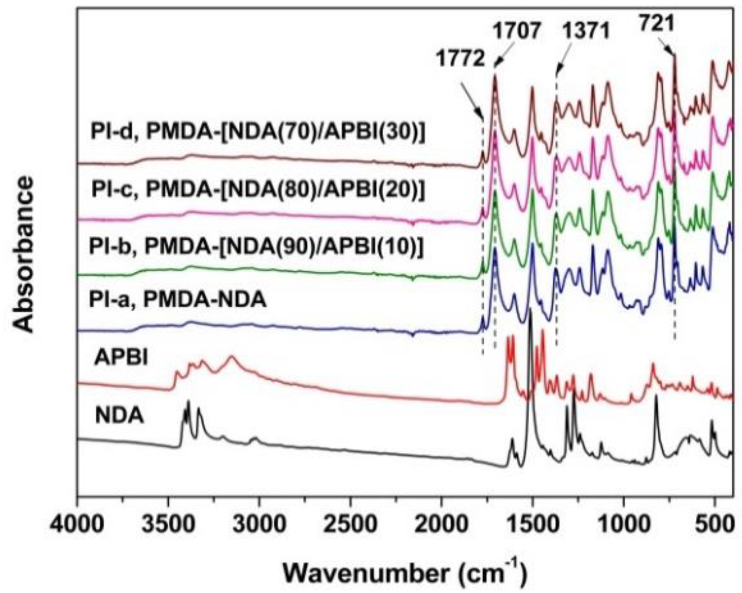
3.1. PI Synthesis and Film Preparation
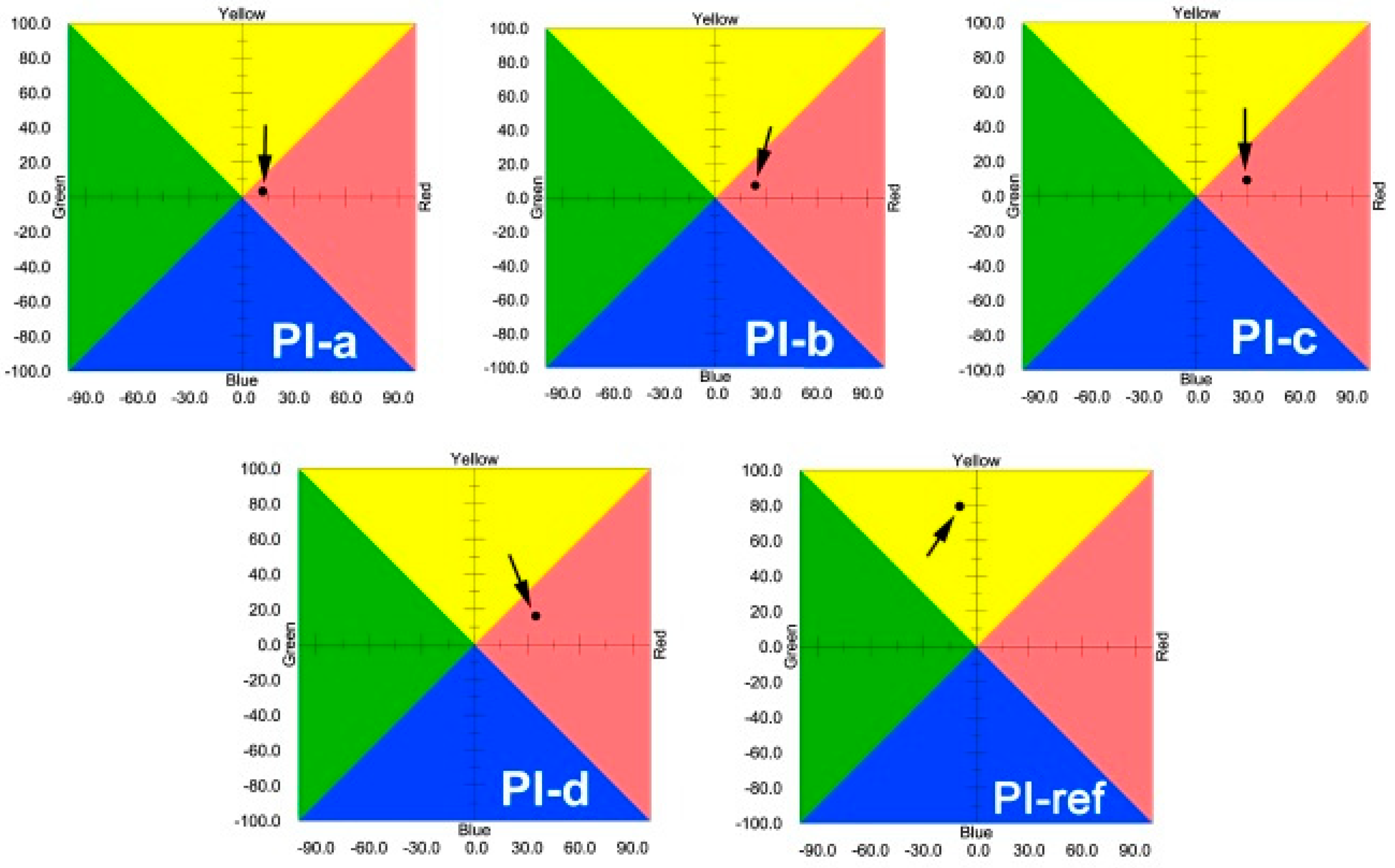
3.2. Optical Properties
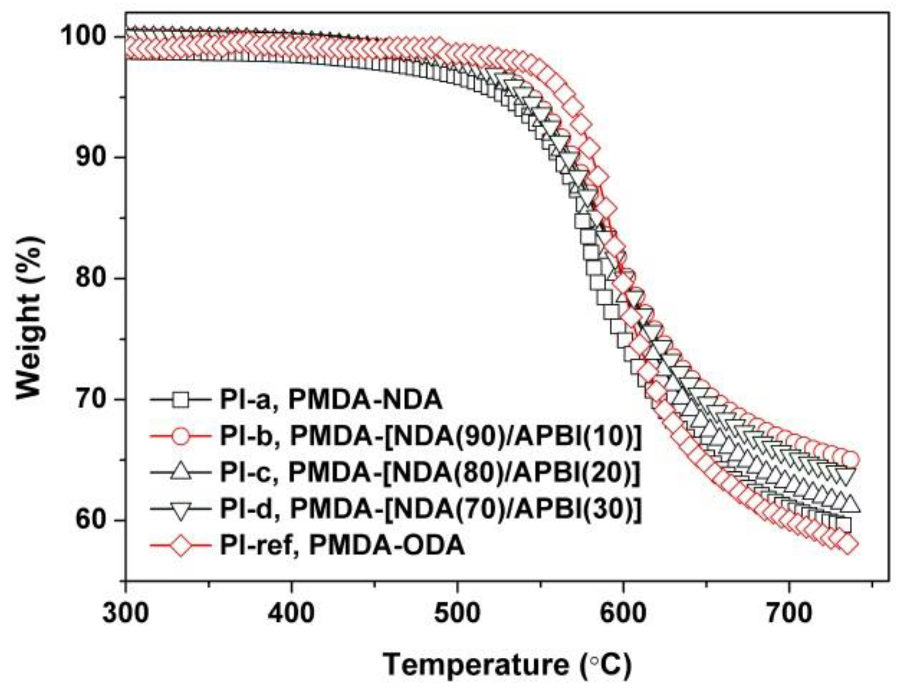
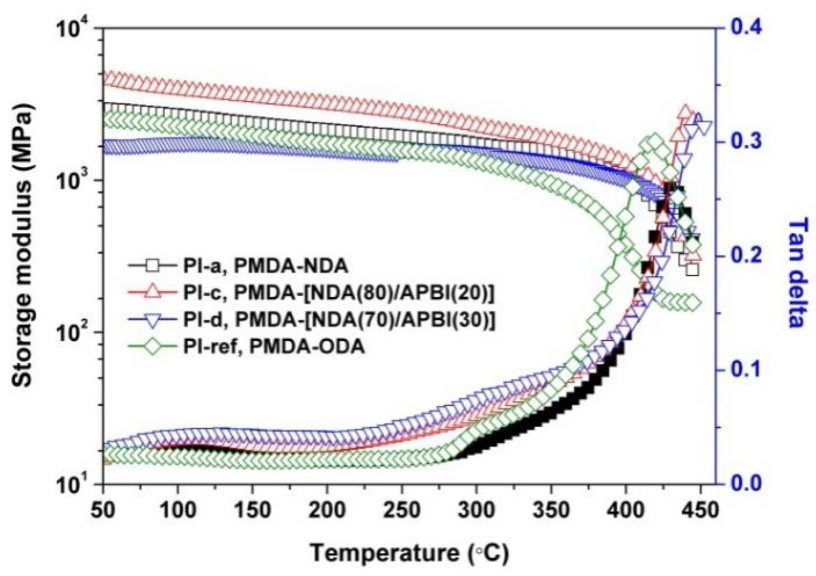
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films bearing heterocyclic structures and their applications. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A review of the latest development of polyimide based membranes for CO 2 separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Spechler, J.A.; Koh, T.W.; Herb, J.T.; Rand, B.P.; Arnold, C.B. A Transparent, Smooth, Thermally Robust, Conductive Polyimide for Flexible Electronics. Adv. Funct. Mater. 2015, 25, 7428–7434. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Liu, S.; Chi, Z.; Chen, X.; Xu, J. Flexible and highly fluorescent aromatic polyimide: Design, synthesis, properties, and mechanism. J. Mater. Chem. C 2016, 4, 10509–10517. [Google Scholar] [CrossRef]
- Cai, S.; Niu, H. Synthesis and characterization of triarylamine-based polyimides for electrochromic and optoelectronic conversion behaviors. J. Appl. Polym. Sci. 2019, 137, 48808. [Google Scholar] [CrossRef]
- Takizawa, K.; Fukuchi, S.; Takemasa, C.; Ishige, R.; Asai, S.; Ando, S. Enhancing photoconductivity of aromatic polyimide films by incorporating fluorinated dianhydrides and main chain triphenylamine structure. Polymer 2018, 157, 120–130. [Google Scholar] [CrossRef]
- Mi, Z.; Liu, Z.; Wang, C.; Liu, Y.; Zhou, C.; Wang, D.; Zhao, X.; Zhou, H.; Zhang, Y.; Chen, C. Transparent and soluble polyimide films containing 4,4′-isopropylidenedicyclohexanol (Cis-HBPA) units: Preparation, characterization, thermal, mechanical, and dielectric properties. J. Polym. Sci. Part. A Polym. Chem. 2018, 56, 2115–2128. [Google Scholar] [CrossRef]
- Yang, Y.; Jung, Y.; Cho, M.D.; Lee, S.G.; Kwon, S. Transient color changes in oxidative-stable fluorinated polyimide film for flexible display substrates. RSC Adv. 2015, 5, 57339–57345. [Google Scholar] [CrossRef]
- Yu, H.C.; Jung, J.W.; Choi, J.Y.; Oh, S.Y.; Chung, C.M. Structure-property relationship study of partially aliphatic copolyimides for preparation of flexible and transparent polyimide films. J. Macromol. Sci. A 2017, 54, 97–104. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Choi, M.-C.; Nagappan, S.; Ha, C.S. Synthesis and characterization of highly transparent and hydrophobic fluorinated polyimides derived from perfluorodecylthio substituted diamine monomers. J. Polym. Sci. A Polym. Chem. 2014, 53, 479–488. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Mushtaq, N.; Fang, X. Synthesis of organosoluble and light-colored cardo polyimides via aromatic nucleophilic substitution polymerization. Polym. Adv. Technol. 2015, 26, 1519–1527. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Q.; Zhang, A.; Liu, F.; Zhang, N. Comparative studies on structure-property relationships of the polyimide films derived from the dianhydride containing alicyclic cis-ethylenic double bond. Macromol. Res. 2019, 27, 703–712. [Google Scholar] [CrossRef]
- Ni, H.; Liu, J.; Wang, Z.; Yang, S. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Jung, Y.; Jeong, B.; Yang, Y.; Kim, T.; Kwon, S. Correlation of stress and optical properties in highly transparent polyimides for future flexible display. Macromol. Res. 2017, 25, 971–975. [Google Scholar] [CrossRef]
- Zin, N.; McIntosh, K.; Bakhshi, S.; Vázquez-Guardado, A.; Kho, T.; Fong, K.; Stocks, M.; Franklin, E.; Blakers, A. Polyimide for silicon solar cells with double-sided textured pyramids. Solar Energy Mater. Solar Cells 2018, 183, 200–204. [Google Scholar] [CrossRef]
- Gao, X.; Lin, L.; Liu, Y.; Huang, X. LTPS TFT process on polyimide substrate for flexible AMOLED. J. Disp. Technol. 2015, 11, 666–669. [Google Scholar] [CrossRef]
- San Sebastián, M.; Martínez-Martínez, V.; Maceiras, A.; Vilas, J.L.; López-Arbeloa, I.; León, L.M. Enhanced charge-transfer emission in polyimides by cyano-groups doping. J. Phys. Chem. B 2015, 119, 5685–5692. [Google Scholar] [CrossRef]
- Tseng, I.H.; Hsieh, T.T.; Lin, C.H.; Tsai, M.H.; Ma, D.L.; Ko, C.J. Phosphinated polyimide hybrid films with reduced melt-flow and enhanced adhesion for flexible copper clad laminates. Prog. Org. Coat. 2018, 124, 92–98. [Google Scholar] [CrossRef]
- Zhang, X.M.; Xiao, X.; Wu, X.; Liu, J.G. Preparation and properties of heat-sealable polyimide films with comparable coefficient of thermal expansion and good adhesion to copper matrix. Express Polym. Lett. 2017, 11, 983–990. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, T.; Han, Y.; Kim, H.; Han, H. Polyimide–epoxy composites with superior bendable properties for application in flexible electronics. J. Electron. Mater. 2017, 46, 4740–4749. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Y.; Yang, Y.; Li, L.; Song, C.; Su, X. Conductive mechanism of carbon black/polyimide composite films. J. Polym. Eng. 2018, 38, 147–156. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Lee, J.; Han, P.; Park, D.; Han, H. Fabrication of polyimide composite films based on carbon black for high-temperature resistance. Polym. Compos. 2014, 35, 2214–2220. [Google Scholar] [CrossRef]
- Atar, N.; Grossman, E.; Gouzman, I.; Bolker, A.; Murray, V.J.; Marshall, B.C.; Qian, M.; Minton, T.K.; Hanein, Y. Atomic-oxygen-durable and electrically-conductive CNT-POSS-Polyimide flexible films for space applications. ACS Appl. Mater. Interf. 2015, 7, 12047–12056. [Google Scholar] [CrossRef] [PubMed]
- Okafor, P.A.; Huxel, B.; Iroh, J.O. Electrochemical behavior of multifunctional graphene-polyimide nanocomposite film in two different electrolyte solutions. J. Appl. Polym. Sci. 2015, 132, 42673. [Google Scholar] [CrossRef]
- Yoonessi, M.; Gaier, J.R.; Sahimi, M.; Daulton, T.L.; Kaner, R.B.; Meador, M.A. Fabrication of graphene–polyimide nanocomposites with superior electrical conductivity. ACS Appl. Mater. Interf. 2017, 9, 43230–43238. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Liu, J.; Zhi, X.; Huangfu, M.; Jiang, G.; Wu, X.; Zhang, X. Molecular design, synthesis and characterization of intrinsically black polyimide films with high thermal stability and good electrical properties. J. Polym. Res. 2019, 26, 171. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Seo, K.; Nam, K.H.; Lee, S.; Han, H. Polyimide/organosilicate nanocomposites: Residual stress behavior on Si wafer for multichip packaging. Mater. Lett. 2019, 247, 171–173. [Google Scholar] [CrossRef]
- Huangfu, M.; Zhang, Y.; Zhang, X.; Liu, J.; Liu, Y.; Guo, Y.; Huang, Q.Y.; Zhang, X. Preparation and thermal evaluation of novel polyimide protective coatings for quartz capillary chromatographic columns operated over 320 °C for high-temperature gas chromatography analysis. Polymers 2019, 11, 946. [Google Scholar] [CrossRef]
- Chern, Y.T. High subglass transitions appearing in the rigid polyimides derived from the novel 1,6-bis(4-aminophenyl)diamantane. Macromolecules 1998, 31, 1898–1905. [Google Scholar] [CrossRef]
- Song, G.; Zhang, X.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C.; Dang, G. Negative in-plane CTE of benzimidazole-based polyimide film and its thermal expansion behavior. Polymer 2014, 55, 3242–3246. [Google Scholar] [CrossRef]
- Choi, H.; Chung, I.S.; Hong, K.; Park, C.E.; Kim, S.Y. Soluble polyimides from unsymmetrical diamine containing benzimidazole ring and trifluoromethyl pendent group. Polymer 2008, 49, 2644–2649. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; Dang, G.; Chen, C. Synthesis and characterization of thermally stable, high-modulus polyimides containing benzimidazole moieties. J. Polym. Sci. A Polym. Chem. 2009, 47, 2024–2031. [Google Scholar] [CrossRef]
- Lian, M.; Lu, X.; Lu, Q. Synthesis of superheat-resistant polyimides with high Tg and low coefficient of thermal expansion by introduction of strong intermolecular interaction. Macromolecules 2018, 51, 10127–10135. [Google Scholar] [CrossRef]
- Chung, I.S.; Park, C.E.; Ree, M.; Kim, S.Y. Soluble polyimides containing benzimidazole rings for interlevel dielectrics. Chem. Mater. 2001, 13, 2801–2806. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Y.; Wang, D.; Chen, C.; Zhou, H.; Zhao, X.; Dang, G. Intermolecular interactions of polyimides containing benzimidazole and benzoxazole moieties. Polymer 2013, 54, 2335–2340. [Google Scholar] [CrossRef]




| PI | [η]inh a (dL/g) | Mnb (g/mol) | Mwb (g/mol) | PDI b |
|---|---|---|---|---|
| PI-a | 0.98 | 58,281 | 129,744 | 2.23 |
| PI-b | 0.86 | 38,852 | 72,264 | 1.86 |
| PI-c | 0.73 | 34,599 | 59,856 | 1.73 |
| PI-d | 0.71 | 32,971 | 54,072 | 1.64 |
| PI | Optical Properties a | Thermal Properties b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Λ (nm) | T600 (%) | T760 (%) | L* | a* | b* | Tg (°C) | T5% (°C) | Rw730 (%) | CTE (×10−6/K) | |
| PI-a | 592 | 0 | 36.8 | 2.20 | 11.99 | 3.33 | 431.6 | 515.7 | 59.7 | 18.8 |
| PI-b | 578 | 0.4 | 43.1 | 4.23 | 23.67 | 7.26 | 435.7 | 545.1 | 65.3 | 17.2 |
| PI-c | 578 | 0.5 | 57.1 | 5.51 | 29.31 | 9.22 | 440.6 | 539.4 | 61.5 | 11.0 |
| PI-d | 561 | 1.6 | 58.2 | 9.58 | 34.92 | 16.20 | 445.5 | 541.7 | 64.0 | 8.9 |
| PI-ref | 407 | 74.6 | 80.9 | 88.65 | −9.39 | 79.41 | 418.8 | 581.0 | 61.6 | 29.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.-y.; Zhang, Y.; Jiang, G.-l.; Zhi, X.-x.; Xiao, X.; Wu, L.; Jia, Y.-J.; Liu, J.-g.; Zhang, X.-m. Preparation and Properties of Inherently Black Polyimide Films with Extremely Low Coefficients of Thermal Expansion and Potential Applications for Black Flexible Copper Clad Laminates. Polymers 2020, 12, 576. https://doi.org/10.3390/polym12030576
Tan Y-y, Zhang Y, Jiang G-l, Zhi X-x, Xiao X, Wu L, Jia Y-J, Liu J-g, Zhang X-m. Preparation and Properties of Inherently Black Polyimide Films with Extremely Low Coefficients of Thermal Expansion and Potential Applications for Black Flexible Copper Clad Laminates. Polymers. 2020; 12(3):576. https://doi.org/10.3390/polym12030576
Chicago/Turabian StyleTan, Yao-yao, Yan Zhang, Gang-lan Jiang, Xin-xin Zhi, Xiao Xiao, Lin Wu, Yan-Jiang Jia, Jin-gang Liu, and Xiu-min Zhang. 2020. "Preparation and Properties of Inherently Black Polyimide Films with Extremely Low Coefficients of Thermal Expansion and Potential Applications for Black Flexible Copper Clad Laminates" Polymers 12, no. 3: 576. https://doi.org/10.3390/polym12030576
APA StyleTan, Y.-y., Zhang, Y., Jiang, G.-l., Zhi, X.-x., Xiao, X., Wu, L., Jia, Y.-J., Liu, J.-g., & Zhang, X.-m. (2020). Preparation and Properties of Inherently Black Polyimide Films with Extremely Low Coefficients of Thermal Expansion and Potential Applications for Black Flexible Copper Clad Laminates. Polymers, 12(3), 576. https://doi.org/10.3390/polym12030576






