A Novel Multilayer Composite Membrane for Wound Healing in Mice Skin Defect Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. Preparation of the ABL and the HPL
2.2.2. Preparation of the RFL
2.2.3. Preparation of the MC Membrane
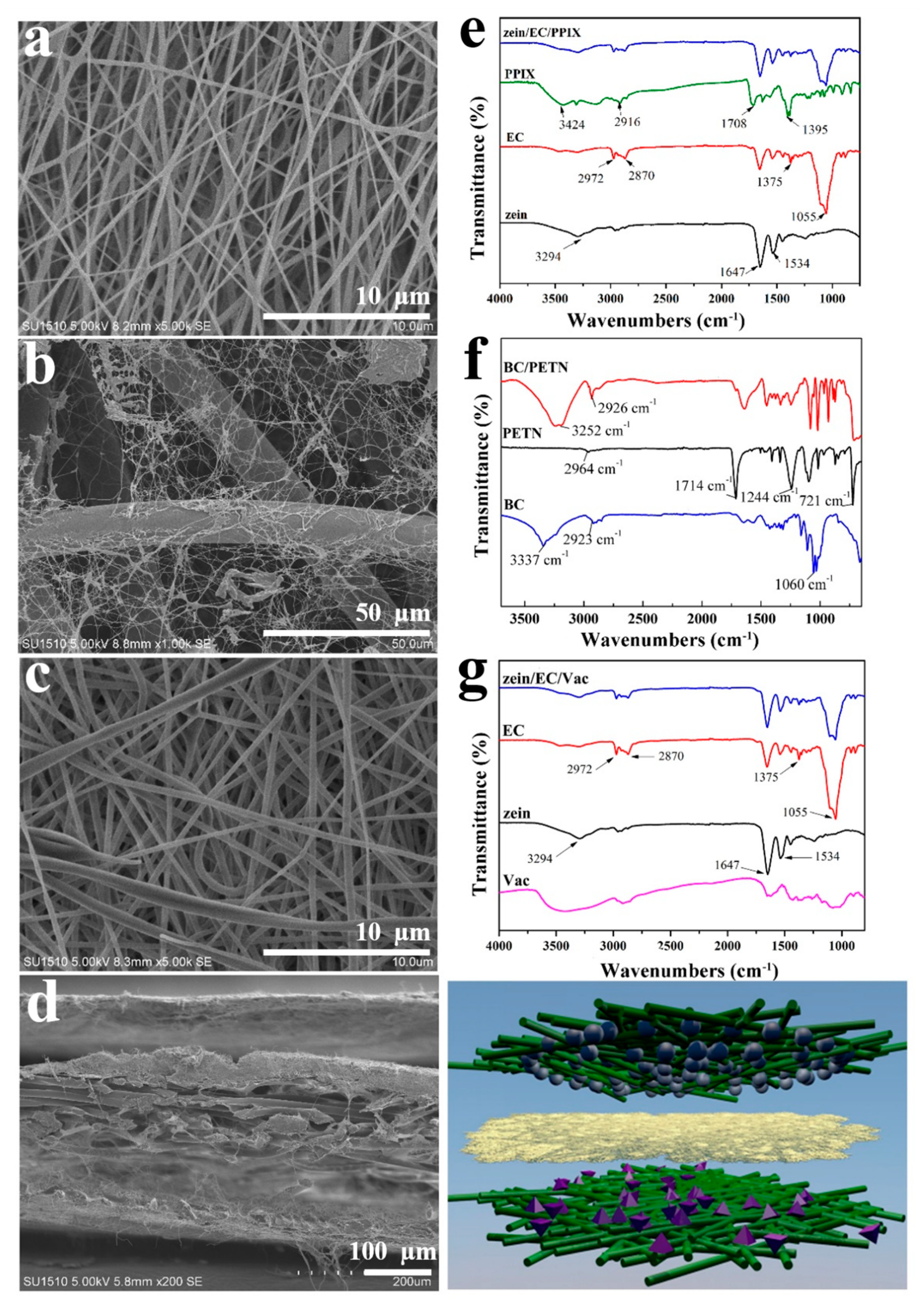
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Antibacterial Activity Assessment of the ABL
2.3.4. Mechanical Properties of the RFL
2.3.5. Hygroscopicity Test of the RFL
2.3.6. In Vitro Biocompatibility Assay of the HPL
2.4. In Vivo Animal Experiments
2.4.1. Establishment of a Skin Wound Model
2.4.2. Wound Observation
2.4.3. Histological Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Morphological and FTIR Analysis
3.2. Antibacterial Activity Assessment of the ABL
3.3. Mechanical Properties of the RFL
3.4. Hygroscopicity Test of the RFL
3.5. In Vitro Biocompatibility Evaluation of the HPL
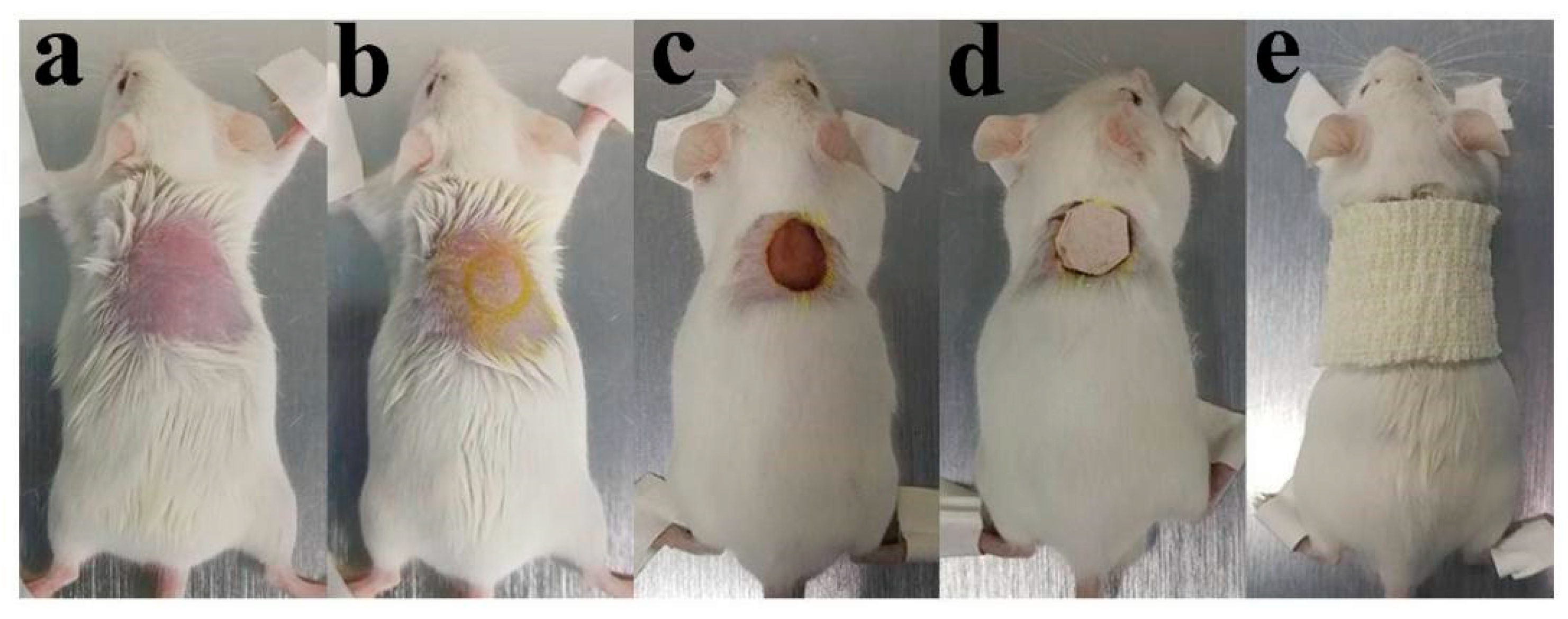
3.6. In Vivo Animal Experiment
3.6.1. Wound Observation
3.6.2. Histological Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2018, 99, 665–706. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings–a review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and characterization of novel eggshell membrane-chitosan blend films for potential wound-care dressing: From waste to medicinal products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef]
- Öztürk, E.; Ağalar, C.; Keçeci, K.; Denkbas, E.B. Preparation and characterization of ciprofloxacin-loaded alginate/chitosan sponge as a wound dressing material. J. Appl. Polym. Sci. 2006, 101, 1602–1609. [Google Scholar] [CrossRef]
- Chen, D.W.C.; Liao, J.Y.; Liu, S.J.; Chan, E.C. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: An in vitro and in vivo study. Int. J. Nanomed. 2012, 7, 763. [Google Scholar]
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; Ribeiro, S.J. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohyd. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2006, 4, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, E.; Karamali, F.; Loghmani, S.; Eslaminejad, M.B.; Nasr-Esfahani, M.H. Bio-engineered electrospun nanofibrous membranes using cartilage extracellular matrix particles. J. Mater. Chem. B. 2017, 5, 765–776. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng: C 2017, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Naragund, V.S.; Panda, P.K. Electrospinning of Polyacrylonitrile Nanofiber Membrane for Bacteria Removal. J. Mater. Sci. Appl. 2018, 4, 68–74. [Google Scholar]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical attributes of nanofibers: Preparation, drug loading, and tissue regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Gnanasekaran, G.; Sathishkumar, Y.; Lee, Y.S.; Kim, C.S. Electrospun antibacterial polyurethane–cellulose acetate-zein composite mats for wound dressing. Carbohyd. Polym. 2014, 102, 884–892. [Google Scholar] [CrossRef]
- Vogt, P.M.; Andree, C.; Breuing, K.; Liu, P.Y.; Slama, J.; Helo, G.; Eriksson, E. Dry, moist, and wet skin wound repair. Ann. Plas. Surg. 1995, 34, 493–499. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A comprehensive review of advanced biopolymeric wound healing systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Malcolm Brown, R. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Chen, X.J.; Jiang, J.P.; Chen, W.H. Effect of PpIX-PDT irradiated by laser and LED. China I Laser Med. Surg. 2015, 4, 184–187. [Google Scholar]
- Dong, J.; Ghiladi, R.A.; Wang, Q.; Cai, Y.; Wei, Q. Protoporphyrin IX conjugated bacterial cellulose via diamide spacer arms with specific antibacterial photodynamic inactivation against Escherichia coli. Cellulose 2018, 25, 1673–1686. [Google Scholar] [CrossRef]
- Shankhwar, N.; Kumar, M.; Mandal, B.B.; Robi, P.S.; Srinivasan, A. Electrospun polyvinyl alcohol-polyvinyl pyrrolidone nanofibrous membranes for interactive wound dressing application. J. Biomater. Sci. Polym. Ed. 2016, 27, 247–262. [Google Scholar] [CrossRef]
- Dashdorj, U.; Reyes, M.K.; Unnithan, A.R.; Tiwari, A.P.; Tumurbaatar, B.; Park, C.H.; Kim, C.S. Fabrication and characterization of electrospun zein/Ag nanocomposite mats for wound dressing applications. Int. J. Biol. Macromol. 2015, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Labib, G. Overview on zein protein: A promising pharmaceutical excipient in drug delivery systems and tissue engineering. Expert Opin. Drug Deli. 2018, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Q.; Li, G.; Qiu, Y.; Wei, Q. Electrospun water-stable zein/ethyl cellulose composite nanofiber and its drug release properties. Mater. Sci. Eng. 2017, 74, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.M.; Kyazze, G.; Locke, I.C.; Tron, T.; Keshavarz, T. Poly (3-hydroxybutyrate)-ethyl cellulose based bio-composites with novel characteristics for infection free wound healing application. Int. J. Biol. Macromol. 2015, 81, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; lv, P.F.; Yao, Y.X.; Wei, Q.F. Preparation and properties of self-woven composites of bacterial cellulose /polyester. Text. Res. J. 2018, 2, 126–131. [Google Scholar]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of self-assembled bacterial cellulose–starch nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, L.X.; Liang, Q.Z.; Zhang, X.F.; Guan, H.L.; Xu, X.L.; Chen, X.S.; Jing, X.B. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control Release 2005, 105, 43–51. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Song, T.; Li, Y.; Pu, Y. Biodegradable nanofibrous membrane of zein/silk fibroin by electrospinning. Polym. Int. 2009, 58, 396–402. [Google Scholar] [CrossRef]
- Desai, J.; Alexander, K.; Riga, A. Characterization of polymeric dispersions of dimenhydrinate in ethyl cellulose for controlled release. Int. J. Pharm. 2006, 308, 115–123. [Google Scholar]
- Qiu, B.; Xu, C.; Sun, D.; Yi, H.; Guo, J.; Zhang, X.; Luo, Z. Polyaniline coated ethyl cellulose with improved hexavalent chromium removal. ACS Sustain. Chem. Eng. 2014, 2, 2070–2080. [Google Scholar] [CrossRef]
- Dong, J.; Ghiladi, R.A.; Wang, Q.; Cai, Y.; Wei, Q. Protoporphyrin-IX conjugated cellulose nanofibers that exhibit high antibacterial photodynamic inactivation efficacy. Nanotechnology 2018, 29, 265601. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Yao, Y.; Li, D.; Zhou, H.; Naeem, M.A.; Feng, Q.; Wei, Q. Self-assembly of nitrogen-doped carbon dots anchored on bacterial cellulose and their application in iron ion detection. Carbohyd. Polym. 2017, 172, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yoo, J.Y.; Kwak, H.S.; Nam, B.U.; Lee, J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Phys. 2005, 5, 472–474. [Google Scholar] [CrossRef]
- Liang, J.; Xia, Q.; Wang, S.; Li, J.; Huang, Q.; Ludescher, R.D. Influence of glycerol on the molecular mobility, oxygen permeability and microstructure of amorphous zein films. Food Hydrocoll. 2015, 44, 94–100. [Google Scholar] [CrossRef]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef]
- Bereket, W.; Hemalatha, K.; Getenet, B.; Wondwossen, T.; Solomon, A.; Zeynudin, A.; Kannan, S. Update on bacterial nosocomial infections. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1039–1044. [Google Scholar]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose—the natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Żywicka, A.; Peitler, D.; Rakoczy, R.; Junka, A.F.; Fijałkowski, K. Wet and Dry Forms of Bacterial Cellulose Synthetized by Different Strains of Gluconacetobacter xylinus as Carriers for Yeast Immobilization. Appl. Biochem. Biotech. 2016, 180, 805–816. [Google Scholar] [CrossRef]
- Karabay, O.; Koçoglu, E.; Tahtaci, M. The role of mobile phones in the spread of bacteria associated with nosocomial infections. J. Infect Dev. Ctries 2007, 1, 72–73. [Google Scholar]
- Karayannidis, G.P.; Papachristos, N.; Bikiaris, D.N.; Papageorgiou, G.Z. Synthesis, crystallization and tensile properties of poly(ethylene terephthalate-co-2,6-naphthalate)s with low naphthalate units content. Polymer 2003, 44, 7801–7808. [Google Scholar] [CrossRef]
- Turoti, M.; Olayemi, J.Y.; Adeniyi, J.B.; Peters, O. The photooxidative degradation of poly(vinylchloride)-2. The stabilising action of dibutyltin maleate and trisnitro (1,3-dihydroxyl-2-hydroxymethyl-2-nitropropane) on PVC from ultraviolet light radiation. Polym. Degrad. Stabil. 1998, 61, 297–302. [Google Scholar] [CrossRef]
- Field, C.K.; Kerstein, M.D. Overview of wound healing in a moist environment. Am. J. Surg. 1994, 167, 2–6. [Google Scholar] [CrossRef]
- Abdelrahman, T.; Newton, H. Wound dressings: Principles and practice. Surgery 2011, 29, 491–495. [Google Scholar] [CrossRef]
- Min, S.; Gao, X.; Han, C.; Chen, Y.; Yang, M.; Zhu, L.; Yao, J. Preparation of a silk fibroin spongy wound dressing and its therapeutic efficiency in skin defects. J. Biomat. Sci-Polym E. 2012, 23, 97–110. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Zhang, A.; Huang, Y.; Tavakoli, J.; Tang, Y. Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture. Polymers 2018, 10, 581. [Google Scholar] [CrossRef]
- Xie, F.; Cai, W.; Liu, Y.; Li, Y.; Du, B.; Feng, L.; Qiu, L. Vaccarin attenuates the human EA. hy926 endothelial cell oxidative stress injury through inhibition of Notch signaling. Int. J. Mol. Med. 2015, 35, 135–142. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Zhu, X.; Li, S.; Shi, X.; Li, Z.; Sun, H. Protective Effects and Mechanisms of Vaccarin on Vascular Endothelial Dysfunction in Diabetic Angiopathy. Int. J. Mol. Sci. 2019, 20, 4587. [Google Scholar] [CrossRef]
- Young, A.; Mcnaught, C.E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Menon, G.K. New insights into skin structure: Scratching the surface. Adv. Drug Deliver Rev. 2002, 54, 3–17. [Google Scholar] [CrossRef]






| Sample | Breaking Strength (MPa) | Elongation at Break (%) | Young Modulus (MPa) |
|---|---|---|---|
| Dried BC | 7.85 ± 2.07 | 6.33 ± 1.64 | 93.6 ± 16.4 |
| Wet BC | 3.39 ± 0.65* | 34.39 ± 5.32* | 19.4 ± 3.27* |
| Dried BC/PETN | 3.40 ± 0.34* | 28.88 ± 2.03* | 11.77 ± 1.90* |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Wang, Q.; Chen, Y.; Xia, S.; Huang, W.; Wei, Q. A Novel Multilayer Composite Membrane for Wound Healing in Mice Skin Defect Model. Polymers 2020, 12, 573. https://doi.org/10.3390/polym12030573
Qiu Y, Wang Q, Chen Y, Xia S, Huang W, Wei Q. A Novel Multilayer Composite Membrane for Wound Healing in Mice Skin Defect Model. Polymers. 2020; 12(3):573. https://doi.org/10.3390/polym12030573
Chicago/Turabian StyleQiu, Yuyu, Qingqing Wang, Yajun Chen, Shufang Xia, Wei Huang, and Qufu Wei. 2020. "A Novel Multilayer Composite Membrane for Wound Healing in Mice Skin Defect Model" Polymers 12, no. 3: 573. https://doi.org/10.3390/polym12030573
APA StyleQiu, Y., Wang, Q., Chen, Y., Xia, S., Huang, W., & Wei, Q. (2020). A Novel Multilayer Composite Membrane for Wound Healing in Mice Skin Defect Model. Polymers, 12(3), 573. https://doi.org/10.3390/polym12030573




