Influence of Heating Rate on the Structure and Mechanical Properties of Aromatic BPDA–PDA Polyimide Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
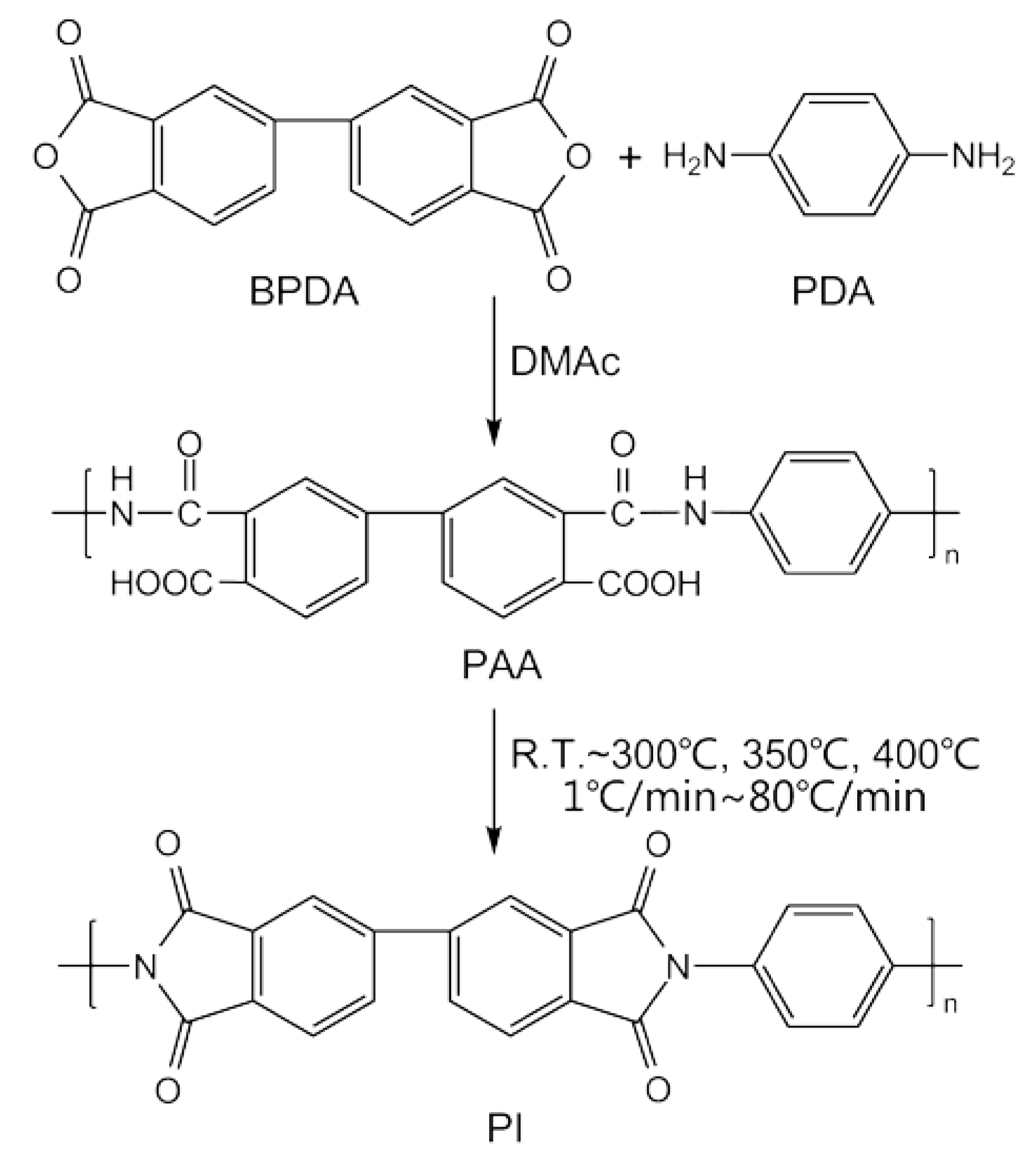
2.2. Preparation of PAA Fibers
2.3. Preparation of PI Fiber
2.4. Characterization
3. Results
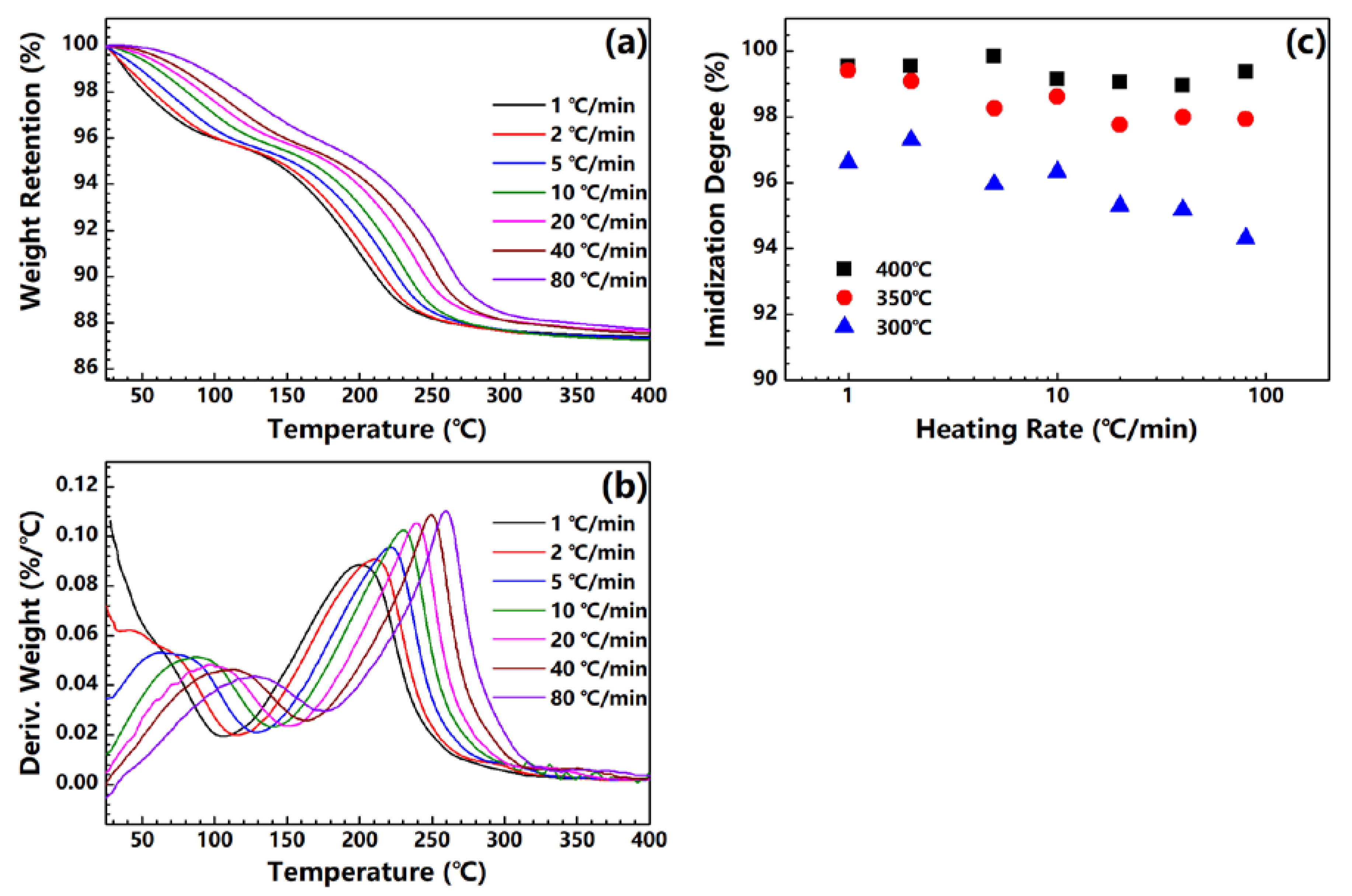
3.1. Imidization Process as Detected by TGA
3.2. DMA Measurement
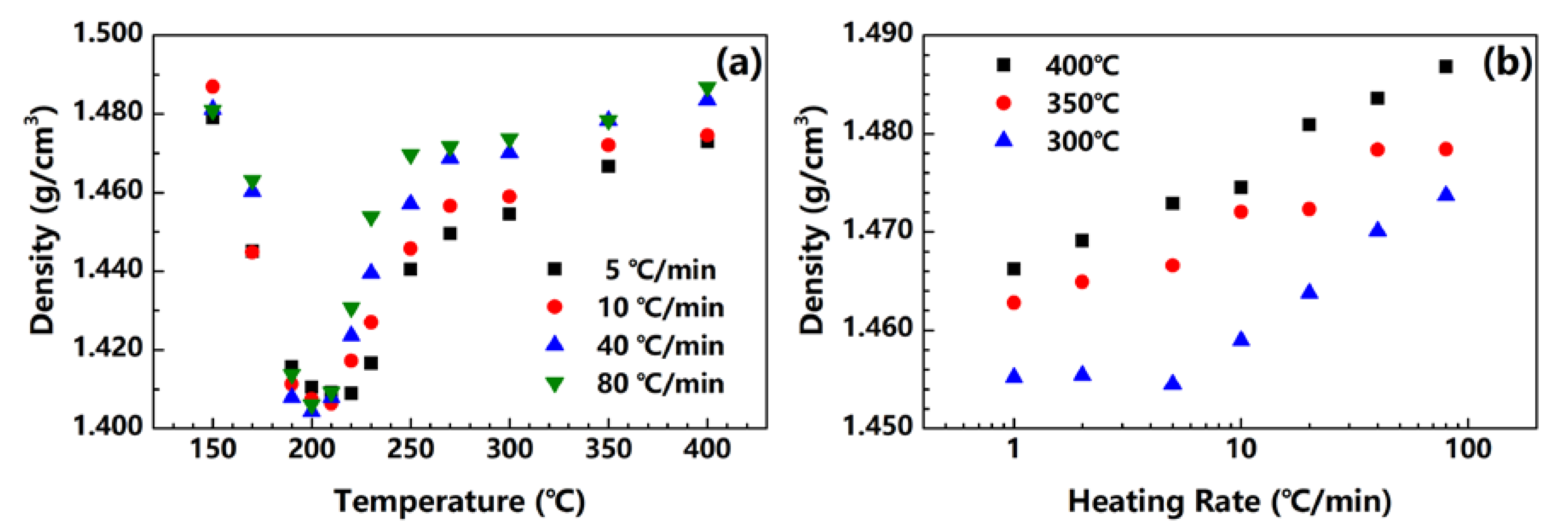
3.3. Density Measurement
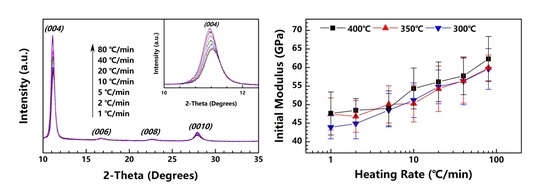
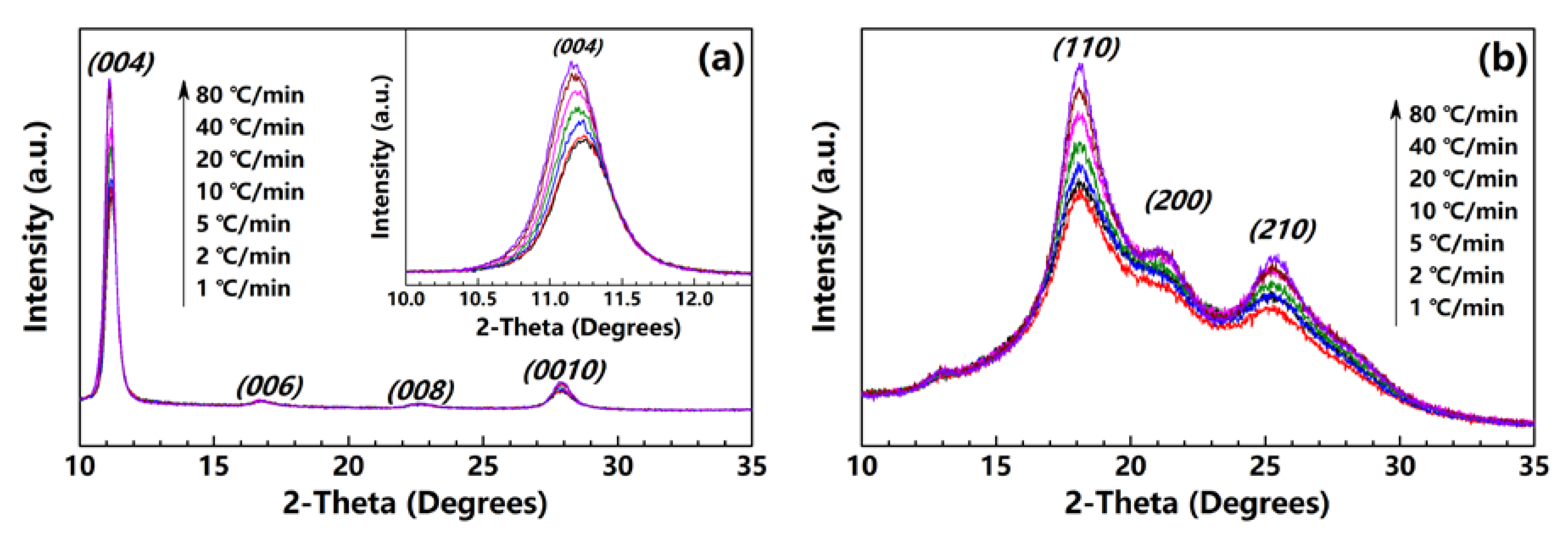
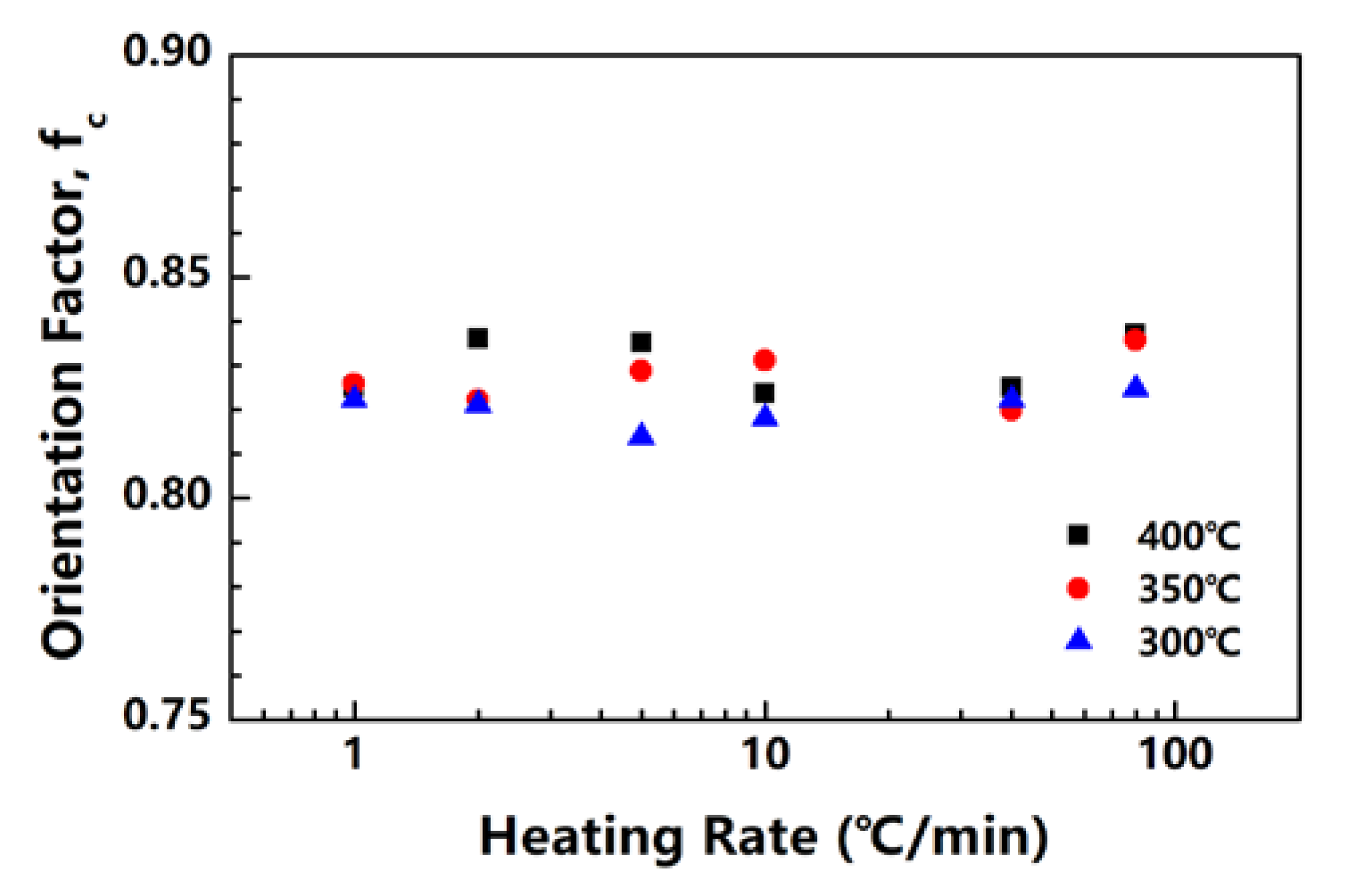
3.4. Crystallization Evolution and Orientation by XRD Patterns
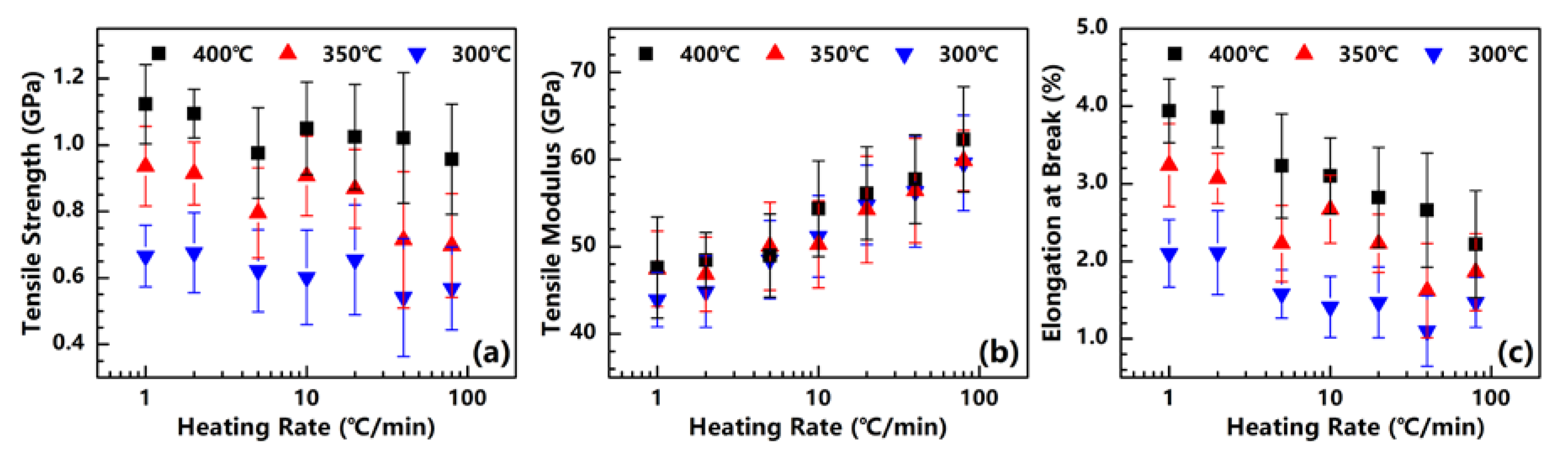
3.5. Mechanical Properties
3.6. Isothermal Annealing after Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dai, X.; Bao, F.; Jiao, L.; Yao, H.; Ji, X.; Qiu, X.; Men, Y. High-performance polyimide copolymer fibers derived from 5-anino-2-(2-hydroxy-4-aminobenzene)-benzoxazole: Preparation, structure and properties. Polymer 2018, 150, 254–266. [Google Scholar] [CrossRef]
- Yin, C.Q.; Dong, J.; Tan, W.J.; Lin, J.Y.; Chen, D.J.; Zhang, Q.H. Strain-induced crystallization of polyimide fibers containing 2-(4-aminophenyl)-5-aminobenzimidazole moiety. Polymer 2015, 75, 178–186. [Google Scholar] [CrossRef]
- Yin, C.Q.; Dong, J.; Zhang, D.B.; Lin, J.Y.; Zhang, Q.H. Enhanced mechanical and hydrophobic properties of polyimide fibers containing benzimidazole and benzoxazole units. Eur. Polym. J. 2015, 67, 88–98. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, Z.; Dong, J.; Zhang, Q. Structure and properties of aromatic poly(benzimidazole-imide) copolymer fibers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Niu, H.; Huang, M.; Qi, S.; Han, E.; Tian, G.; Wang, X.; Wu, D. High-performance copolyimide fibers containing quinazolinone moiety: Preparation, structure and properties. Polymer 2013, 54, 1700–1708. [Google Scholar] [CrossRef]
- Niu, H.; Qi, S.; Han, E.; Tian, G.; Wang, X.; Wu, D. Fabrication of high-performance copolyimide fibers from 3,3’,4,4’-biphenyltetracarboxylic dianhydride, p-phenylenediamine and 2-(4-aminophenyl)-6-amino-4(3H)-quinazolinone. Mater. Lett. 2012, 89, 63–65. [Google Scholar] [CrossRef]
- Chen, W.J.; Chen, W.; Zhang, B.Q.; Yang, S.Y.; Liu, C.Y. Thermal imidization process of polyimide film: Interplay between solvent evaporation and imidization. Polymer 2017, 109, 205–215. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Real-Time Characterization of Physical Changes in Polyimide Film Formation: From Casting to Imidization. Macromolecules 2013, 46, 8616–8627. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Molecular mechanism of temporal physico/chemical changes that take place during imidization of polyamic acid: Coupled real-time rheo-optical and IR dichroism measurements. Polymer 2014, 55, 6569–6576. [Google Scholar] [CrossRef]
- Kailani, M.H.; Sung, C.S.P. Chemical imidization study by spectroscopic techniques. 1. Model amic acids. Macromolecules 1998, 31, 5771–5778. [Google Scholar] [CrossRef]
- Pryde, C.A. Ftir Studies of Polyimides.2. Factors Affecting Quantitative Measurement. J. Polym. Sci. Polym. Chem. 1993, 31, 1045–1052. [Google Scholar] [CrossRef]
- Pryde, C.A. IR Studies of Polyimides.1. Effects of Chemical and Physical Changes during Cure. J. Polym. Sci. Polym. Chem. 1989, 27, 711–724. [Google Scholar] [CrossRef]
- Terui, Y.; Matsuda, S.I.; Ando, S. Molecular structure and thickness dependence of chain orientation in aromatic polyimide films. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2109–2120. [Google Scholar] [CrossRef]
- Nomura, H.; Asano, M. Molecular-Orientation in Polyimide Films Having Rodlike Molecular Skeleton Formed on Silicon Substrate. Jpn. J. Appl. Phys. 1993, 32, 3933–3937. [Google Scholar] [CrossRef]
- Kotera, M.; Samyul, B.; Araie, K.; Sugioka, Y.; Nishino, T.; Maji, S.; Noda, M.; Senoo, K.; Koganezawa, T.; Hirosawa, I. Microstructures of BPDA-PPD polyimide thin films with different thicknesses. Polymer 2013, 54, 2435–2439. [Google Scholar] [CrossRef]
- Ishii, J.; Takata, A.; Oami, Y.; Yokota, R.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (6). Mechanism of negative in-plane CTE generation in non-stretched polyimide films. Eur. Polym. J. 2010, 46, 681–693. [Google Scholar] [CrossRef]
- Ishii, J.; Shimizu, N.; Ishihara, N.; Ikeda, Y.; Sensui, N.; Matano, T.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (4): Casting- and melt-induced in-plane orientation. Eur. Polym. J. 2010, 46, 69–80. [Google Scholar] [CrossRef]
- Ebisawa, S.; Ishii, J.; Sato, M.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (5). Effect of ordered structure formation in polyimide precursors on CTE. Eur. Polym. J. 2010, 46, 283–297. [Google Scholar] [CrossRef]
- Luo, L.B.; Yao, J.; Wang, X.; Li, K.; Huang, J.Y.; Li, B.Y.; Wang, H.N.; Feng, Y.; Liu, X.Y. The evolution of macromolecular packing and sudden crystallization in rigid-rod polyimide via effect of multiple H-bonding on charge transfer (CT) interactions. Polymer 2014, 55, 4258–4269. [Google Scholar] [CrossRef]
- Yang, W.; Liu, F.; Zhang, J.; Zhang, E.; Qiu, X.; Ji, X. Influence of thermal treatment on the structure and mechanical properties of one aromatic BPDA-PDA polyimide fiber. Eur. Polym. J. 2017, 96, 429–442. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Parrish, W.; Depero, I.E.; Ree, M. Chain Conformations of Aromatic Polyimides and Their Ordering in Thin-Films. MRS Online Proc. Lib. Arch. 1991, 227, 387–393. [Google Scholar] [CrossRef]
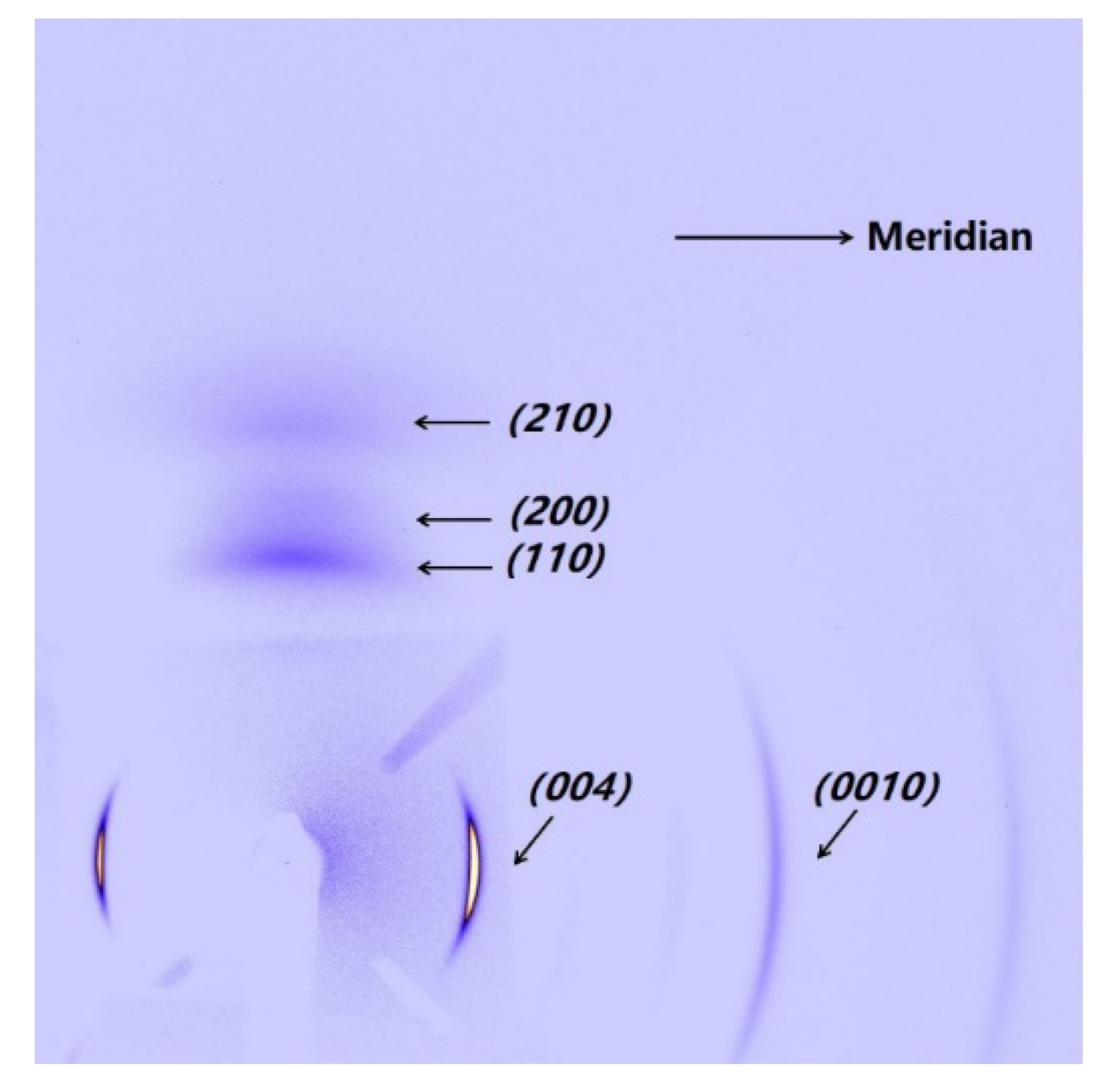



| End Temperature (°C) | Heating Rate (°C/min) | 2-Theta (Degrees) | FWHM (Degrees) | d-spacing (Angstrom) | Crystal Size (Angstrom) |
|---|---|---|---|---|---|
| 400 | 1 | 11.22 | 0.46 | 31.56 | 173.36 |
| 2 | 11.21 | 0.45 | 31.59 | 175.08 | |
| 5 | 11.18 | 0.46 | 31.67 | 173.14 | |
| 10 | 11.16 | 0.44 | 31.73 | 181.12 | |
| 20 | 11.15 | 0.44 | 31.76 | 179.92 | |
| 40 | 11.14 | 0.43 | 31.79 | 184.91 | |
| 80 | 11.11 | 0.44 | 31.87 | 181.08 | |
| 350 | 1 | 11.24 | 0.49 | 31.50 | 165.05 |
| 2 | 11.22 | 0.48 | 31.56 | 163.55 | |
| 5 | 11.19 | 0.47 | 31.64 | 167.11 | |
| 10 | 11.19 | 0.47 | 31.64 | 167.40 | |
| 20 | 11.17 | 0.45 | 31.70 | 175.97 | |
| 40 | 11.16 | 0.45 | 31.73 | 174.83 | |
| 80 | 11.15 | 0.46 | 31.76 | 172.58 | |
| 300 | 1 | 11.24 | 0.46 | 31.50 | 173.08 |
| 2 | 11.23 | 0.45 | 31.53 | 176.27 | |
| 5 | 11.22 | 0.44 | 31.56 | 181.86 | |
| 10 | 11.20 | 0.43 | 31.62 | 182.40 | |
| 20 | 11.19 | 0.43 | 31.64 | 184.04 | |
| 40 | 11.18 | 0.49 | 31.67 | 184.08 | |
| 80 | 11.17 | 0.43 | 31.70 | 184.78 |
| Annealing Time (s) | 2-Theta (Degrees) | FWHM (Degrees) | d-spacing (Angstrom) | Crystal Size (Angstrom) |
|---|---|---|---|---|
| 0 | 11.11 | 0.44 | 31.87 | 181.08 |
| 5 | 11.12 | 0.44 | 31.84 | 179.42 |
| 10 | 11.14 | 0.47 | 31.79 | 167.97 |
| 25 | 11.16 | 0.51 | 31.73 | 154.80 |
| 60 | 11.16 | 0.52 | 31.73 | 151.82 |
| 120 | 11.16 | 0.51 | 31.73 | 154.80 |
| 300 | 11.16 | 0.52 | 31.73 | 151.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Liu, F.; Chen, H.; Dai, X.; Liu, W.; Qiu, X.; Ji, X. Influence of Heating Rate on the Structure and Mechanical Properties of Aromatic BPDA–PDA Polyimide Fiber. Polymers 2020, 12, 510. https://doi.org/10.3390/polym12030510
Yang W, Liu F, Chen H, Dai X, Liu W, Qiu X, Ji X. Influence of Heating Rate on the Structure and Mechanical Properties of Aromatic BPDA–PDA Polyimide Fiber. Polymers. 2020; 12(3):510. https://doi.org/10.3390/polym12030510
Chicago/Turabian StyleYang, Wenke, Fangfang Liu, Hongxiang Chen, Xuemin Dai, Wei Liu, Xuepeng Qiu, and Xiangling Ji. 2020. "Influence of Heating Rate on the Structure and Mechanical Properties of Aromatic BPDA–PDA Polyimide Fiber" Polymers 12, no. 3: 510. https://doi.org/10.3390/polym12030510