In-Situ Synthesis of Hydrophobic Polyurethane Ternary Composite Induced by Hydroxyethyl Cellulose through a Green Method for Efficient Oil Removal
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
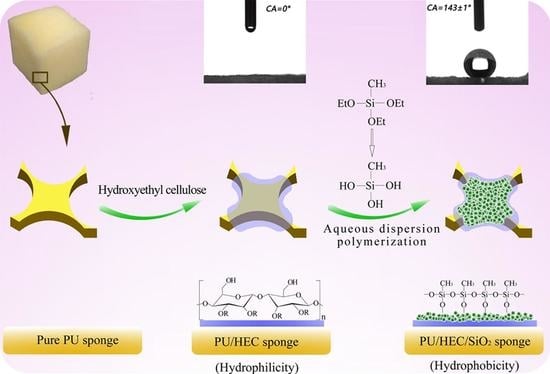
2.2. Preparation of Binary PU/HEC Composite
2.3. Preparation of Ternary PU/HEC/SiO2 Composite
2.4. Characterization
2.5. Oil-Water Separation Experiments
3. Results and Discussion
3.1. Morphological Characterization
3.2. FT-IR Spectra
3.3. Wettability
3.4. Oil-Water Separation Process
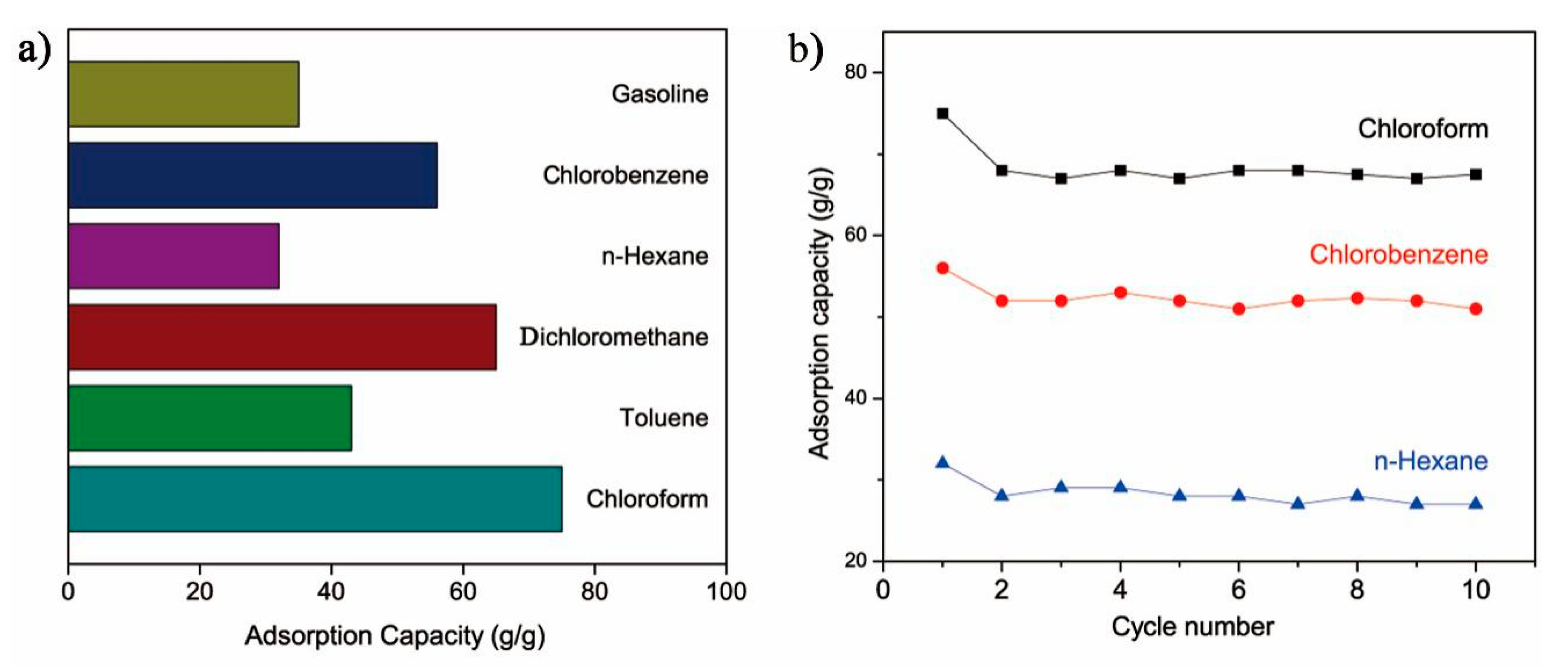
3.5. Recyclability of the Ternary PU/HEC/SiO2 Composite
3.6. Absorption Amount
3.7. Acid and Alkali Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nabi, G.D.; Wuerz, B.; Wick, L.Y.; Brussaard, C.P.D.; Huisman, J.; van der Meer, J.R.; Reddy, C.M.; Arey, J.S. First Day of an Oil Spill on the Open Sea: Early Mass Transfers of Hydrocarbons to Air and Water. Environ. Sci. Technol. 2014, 48, 9400–9411. [Google Scholar]
- Hong, P.K.A.; Xiao, T. Treatment of oil spill water by ozonation and sand filtration. Chemosphere 2013, 91, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Almojjly, A.; Johnson, D.; Oatley-Radcliffe, D.L.; Hilal, N. Removal of oil from oil-water emulsion by hybrid coagulation/sand filter as pre-treatment. J. Water Process Eng. 2018, 26, 17–27. [Google Scholar] [CrossRef]
- Ge, B.; Zhang, Z.; Zhu, X.; Men, X.; Zhou, X. A superhydrophobic/superoleophilic sponge for the selective absorption oil pollutants from water. Colloid Surf. A 2014, 457, 397–401. [Google Scholar] [CrossRef]
- Ma, L.; Luo, X.; Cai, N.; Xue, Y.; Zhu, S.; Fu, Z.; Yu, F. Facile fabrication of hierarchical porous resins via high internal phase emulsion and polymeric porogen. Appl. Surf. Sci. 2014, 305, 186–193. [Google Scholar] [CrossRef]
- Zhou, X.; Chuai, C. Synthesis and Characterization of a Novel High-Oil-Absorbing Resin. J. Appl. Polym. Sci. 2010, 115, 3321–3325. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Rong, J.; Xu, J.; Fang, J. The synthesis of hierarchical porous Al2O3/acrylic resin composites as durable, efficient and recyclable absorbents for oil/water separation. Chem. Eng. J. 2017, 309, 522–531. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Gore, P.M.; Naebe, M.; Wang, X.A.; Kandasubramanian, B. Progress in silk materials for integrated water treatments: Fabrication, modification and applications. Chem. Eng. J. 2019, 374, 437–470. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, T.X.; Li, S.; Huang, J.; Mao, J.; Yang, H.; Gao, S.; Chen, Z.; Lai, Y. A novel strategy for fabricating robust superhydrophobic fabrics by environmentally-friendly enzyme etching. Chem. Eng. J. 2019, 355, 290–298. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Jamil, S.M.; Mohtor, N.H. Superhydrophilic, low cost kaolin-based hollow fibre membranes for efficient oily-wastewater separation. Mater. Lett. 2017, 191, 119–122. [Google Scholar] [CrossRef]
- Nikkhah, A.A.; Zilouei, H.; Asadinezhad, A.; Keshavarz, A. Removal of oil from water using polyurethane foam modified with nanoclay. Chem. Eng. J. 2015, 262, 278–285. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Ultra-fast spill oil recovery using a mesoporous lignin based nanocomposite prepared from date palm pits (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 130, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Fu, Q.; Wang, X.; Zhu, J.; Yu, J.; Sun, G.; Ding, B. Superelastic and Superhydrophobic Nanofiber-Assembled Cellular Aerogels for Effective Separation of Oil/Water Emulsions. ACS Nano 2015, 9, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Palamà, I.E.; D’Amone, S.; Biasiucci, M.; Gigli, G.; Cortese, B. Bioinspired design of a photoresponsive superhydrophobic/oleophilic surface with underwater superoleophobic efficacy. J. Mater. Chem. A 2014, 2, 17666–17675. [Google Scholar]
- Chen, F.; Lu, Y.; Liu, X.; Song, J.; He, G.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P. Table Salt as a Template to Prepare Reusable Porous PVDF-MWCNT Foam for Separation of Immiscible Oils/Organic Solvents and Corrosive Aqueous Solutions. Adv. Funct. Mater. 2017, 27, 1702926–1702936. [Google Scholar] [CrossRef]
- Zhou, S.; Hao, G.; Zhou, X.; Jiang, W.; Wang, T.; Zhang, N.; Yu, L. One-pot synthesis of robust superhydrophobic, functionalized graphene/polyurethane sponge for effective continuous oil-water separation. Chem. Eng. J. 2016, 302, 155–162. [Google Scholar] [CrossRef]
- Wu, R.; Yu, B.; Liu, X.; Li, H.; Wang, W.; Chen, L.; Bai, Y.; Ming, Z.; Yang, S. One-pot hydrothermal preparation of graphene sponge for the removal of oils and organic solvents. Appl. Surf. Sci. 2016, 362, 56–62. [Google Scholar] [CrossRef]
- Vasquez, L.; Campagnolo, L.; Athanassiou, A.; Fragouli, D. Expanded Graphite-Polyurethane Foams for Water Oil-Filtration. ACS Appl. Mater. Interfaces 2019, 11, 30207–30217. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Y.; Miao, X.; Pei, Y.; Yan, Y.; Xiao, W.; Wu, L. Facile fabrication of superhydrophobic polyurethane sponge towards oil-water separation with exceptional flame-retardant performance. Sep. Purif. Technol. 2019, 229, 115801–115811. [Google Scholar] [CrossRef]
- Durgadevi, N.; Swarnalatha, V. Polythiophene functionalized hydrophobic cellulose kitchen wipe sponge and cellulose fabric for effective oil-water separation. RSC Adv. 2017, 7, 34866–34874. [Google Scholar] [CrossRef]
- Yue, X.; Li, J.; Zhang, T.; Qiu, F.; Yang, D.; Xue, M. in-situ one-step fabrication of durable superhydrophobic-superoleophilic cellulose/LDH membrane with hierarchical structure for efficiency oil/water separation. Chem. Eng. J. 2017, 328, 117–123. [Google Scholar] [CrossRef]
- Lei, S.; Shi, Z.; Ou, J.; Wang, F.; Xue, M.; Li, W.; Qiao, G.; Guan, X.; Zhang, J. Durable superhydrophobic cotton fabric for oil/water separation. Colloid Surf. A 2017, 533, 249–254. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Y.; Jiang, G.; Wang, R.; Wang, X.; Hu, R.; Xi, X. Transformation of hydrophilic cotton fabrics into superhydrophobic surfaces for oil/water separation. J. Text. Inst. 2013, 104, 305–311. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Pinto, J.; Fragouli, D.; Athanassiou, A. Facile Oil Removal from Water-in-Oil Stable Emulsions Using PU Foams. Materials 2018, 11, 2382. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Oil removal from water using biomaterials. Bioresour. Technol. 2010, 101, 6594–6600. [Google Scholar] [CrossRef]
- Santos, O.S.H.; da Silva, M.C.; Silva, V.R.; Mussel, W.N.; Yoshida, M.I. Polyurethane foam impregnated with lignin as a filler for the removal of crude oil from contaminated water. J. Hazard. Mater. 2017, 324, 406–413. [Google Scholar] [CrossRef]
- Xia, C.; Li, Y.; Fei, T.; Gong, W. Facile one-pot synthesis of superhydrophobic reduced graphene oxide-coated polyurethane sponge at the presence of ethanol for oil-water separation. Chem. Eng. J. 2018, 345, 648–658. [Google Scholar] [CrossRef]
- Jin, Y.; Jiang, P.; Ke, Q.; Cheng, F.; Zhu, Y.; Zhang, Y. Superhydrophobic and superoleophilic polydimethylsiloxane-coated cotton for oil-water separation process: An evidence of the relationship between its loading capacity and oil absorption ability. J. Hazard. Mater. 2015, 300, 175–181. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 0546–0550. [Google Scholar] [CrossRef]
- Extrand, C.W. Model for contact angles and hysteresis on rough and ultraphobic surfaces. Langmuir 2002, 18, 7991–7999. [Google Scholar] [CrossRef]
- Patankar, N.A. On the modeling of hydrophobic contact angles on rough surfaces. Langmuir 2003, 19, 1249–1253. [Google Scholar] [CrossRef]
- Larmour, I.A.; Bell, S.E.J.; Saunders, G.C. Remarkably simple fabrication of superhydrophobic surfaces using electroless galvanic deposition. Angew. Chem. Int. Ed. 2007, 46, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Zhu, Y.; Li, H.; He, K.; Zeng, G.; Chen, A.; Huang, Z.; Huang, T.; Yuan, L.; Chen, G. Synthesis and application of modified commercial sponges for oil-water separation. Chem. Eng. J. 2019, 373, 213–226. [Google Scholar] [CrossRef]
- Sun, S.; Tang, S.; Chang, X.; Wang, N.; Wang, D.; Liu, T.; Lei, Y.; Zhu, Y. A bifunctional melamine sponge decorated with silver-reduced graphene oxide nanocomposite for oil-water separation and antibacterial applications. Appl. Surf. Sci. 2019, 473, 1049–1061. [Google Scholar] [CrossRef]
- Lei, Z.; Deng, Y.; Wang, C. Multiphase surface growth of hydrophobic ZIF-8 on melamine sponge for excellent oil/water separation and effective catalysis in a Knoevenagel reaction. J. Mater. Chem. A 2018, 6, 3258–3263. [Google Scholar] [CrossRef]
- Kim, D.W.; Eum, K.; Kim, H.; Kim, D.; de Mello, M.D.; Park, K.; Tsapatsis, M. Continuous ZIF-8/reduced graphene oxide nanocoating for ultrafast oil/water separation. Chem. Eng. J. 2019, 372, 509–515. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Bayer, I.S.; Anyfantis, G.C.; Martiradonna, L.; Cozzoli, P.D.; Cingolani, R.; Athanassiou, A. Magnetically Driven Floating Foams for the Removal of Oil Contaminants from Water. ACS Nano 2012, 6, 5413–5419. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.S.; Xiong, W.; Wang, M.; Fan, L.; Rabiee-Golgir, H.; Jiang, L.; Hou, W.; Huang, X.; Jiang, L.; et al. Highly Efficient and Recyclable Carbon Soot Sponge for Oil Cleanup. ACS Appl. Mater. Interfaces 2014, 6, 5924–5929. [Google Scholar] [CrossRef]
- Vintu, M.; Unnikrishnan, G. Indolocarbazole based polymer coated super adsorbent polyurethane sponges for oil/organic solvent removal. J. Environ. Manag. 2019, 248, 109344. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. A novel hierarchical hollow SiO2@MnO2 cubes reinforced elastic polyurethane foam for the highly efficient removal of oil from water. Chem. Eng. J. 2017, 327, 539–547. [Google Scholar] [CrossRef]
- Wang, B.; Lei, B.; Tang, Y.; Xiang, D.; Li, H.; Ma, Q.; Zhao, C.; Li, Y. Facile fabrication of robust superhydrophobic cotton fabrics modified by polysiloxane nanowires for oil/water separation. J. Coat. Technol. Res. 2017, 15, 611–621. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xiang, J.; Guan, Y.; Zhang, F. Fabrication of elastic silica-bacterial cellulose composite aerogels with nanoscale interpenetrating network by ultrafast evaporative drying. Compos. Sci. Technol. 2018, 155, 72–80. [Google Scholar] [CrossRef]
- Lu, X.; Cui, Z.; Wei, W.; Xie, J.; Jiang, L.; Huang, J.; Liu, J. Constructing polyurethane sponge modified with silica/graphene oxide nanohybrids as a ternary sorbent. Chem. Eng. J. 2016, 284, 478–486. [Google Scholar] [CrossRef]
- Barbara Cortese, B.; Caschera, D.; Federici, F.; Gabriel, M.; Ingoc, G.; Gigli, G. Superhydrophobic fabrics for oil–water separation through a diamond like carbon (DLC) coating. J. Mater. Chem. A 2014, 2, 6781–6789. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yue, X.; Xiao, Z.; Li, H.; Yu, X.; Xiang, J. In-Situ Synthesis of Hydrophobic Polyurethane Ternary Composite Induced by Hydroxyethyl Cellulose through a Green Method for Efficient Oil Removal. Polymers 2020, 12, 509. https://doi.org/10.3390/polym12030509
Chen J, Yue X, Xiao Z, Li H, Yu X, Xiang J. In-Situ Synthesis of Hydrophobic Polyurethane Ternary Composite Induced by Hydroxyethyl Cellulose through a Green Method for Efficient Oil Removal. Polymers. 2020; 12(3):509. https://doi.org/10.3390/polym12030509
Chicago/Turabian StyleChen, Junyong, Xian Yue, Zhou Xiao, Huaxin Li, Xianbo Yu, and Junhui Xiang. 2020. "In-Situ Synthesis of Hydrophobic Polyurethane Ternary Composite Induced by Hydroxyethyl Cellulose through a Green Method for Efficient Oil Removal" Polymers 12, no. 3: 509. https://doi.org/10.3390/polym12030509
APA StyleChen, J., Yue, X., Xiao, Z., Li, H., Yu, X., & Xiang, J. (2020). In-Situ Synthesis of Hydrophobic Polyurethane Ternary Composite Induced by Hydroxyethyl Cellulose through a Green Method for Efficient Oil Removal. Polymers, 12(3), 509. https://doi.org/10.3390/polym12030509