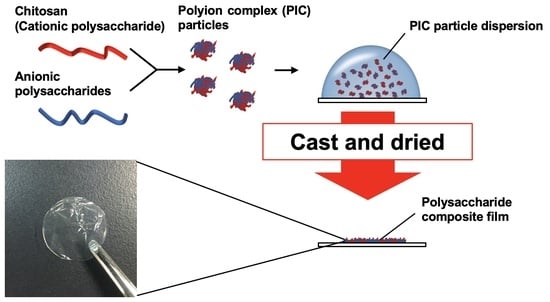
Fabrication and Characterization of Polysaccharide Composite Films from Polyion Complex Particles
Abstract
1. Introduction
2. Materials and Methods
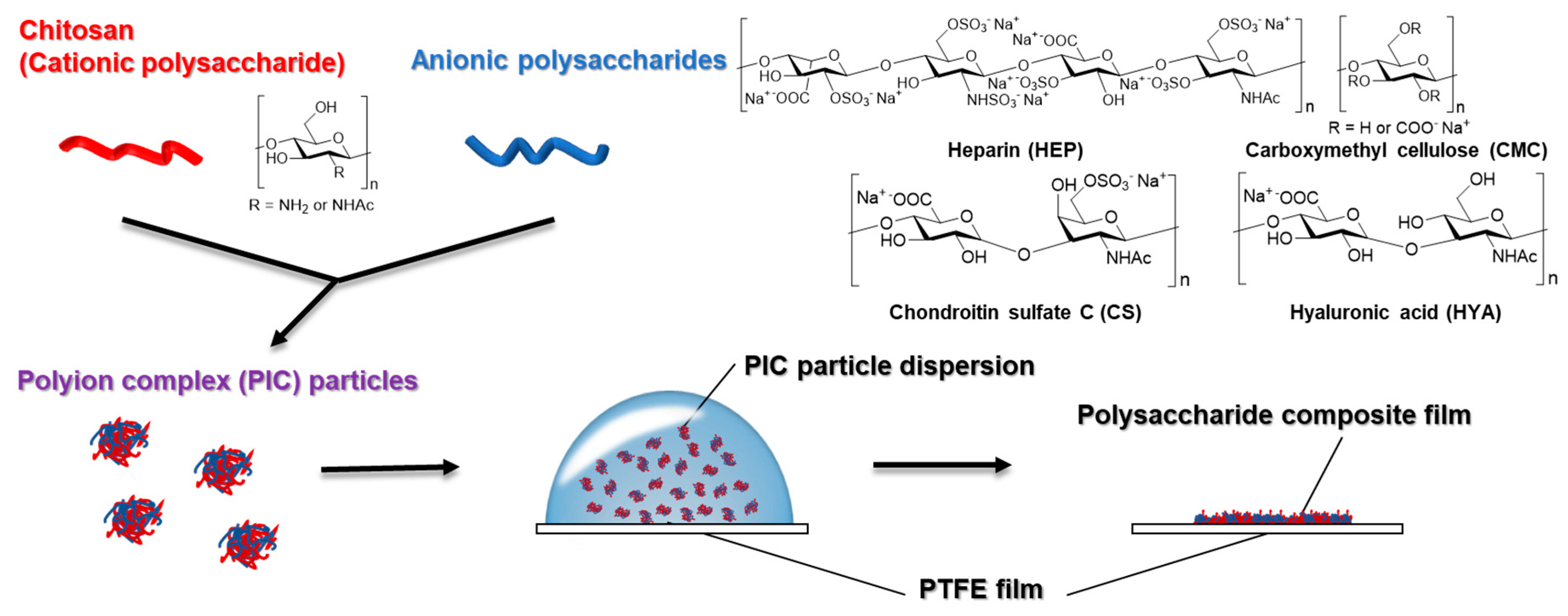
2.1. Materials
2.2. Cell Culture
2.3. Preparation of PIC Particles and Dispersion
2.4. Measurement of PIC Particle Diameters and ζ-potentials
2.5. Preparation of Films Composed of Two Kinds of Polysaccharides
2.6. Film Characterization
2.7. Film Swelling Behavior
2.8. Tensile Strength Measurement
2.9. Adhesion and Proliferation of Cells on Polysaccharide Composite Film
2.10. Preparation of Films Composed of Three Kinds of Polysaccharides
3. Results
3.1. Preparation and Characterization of PIC Particles
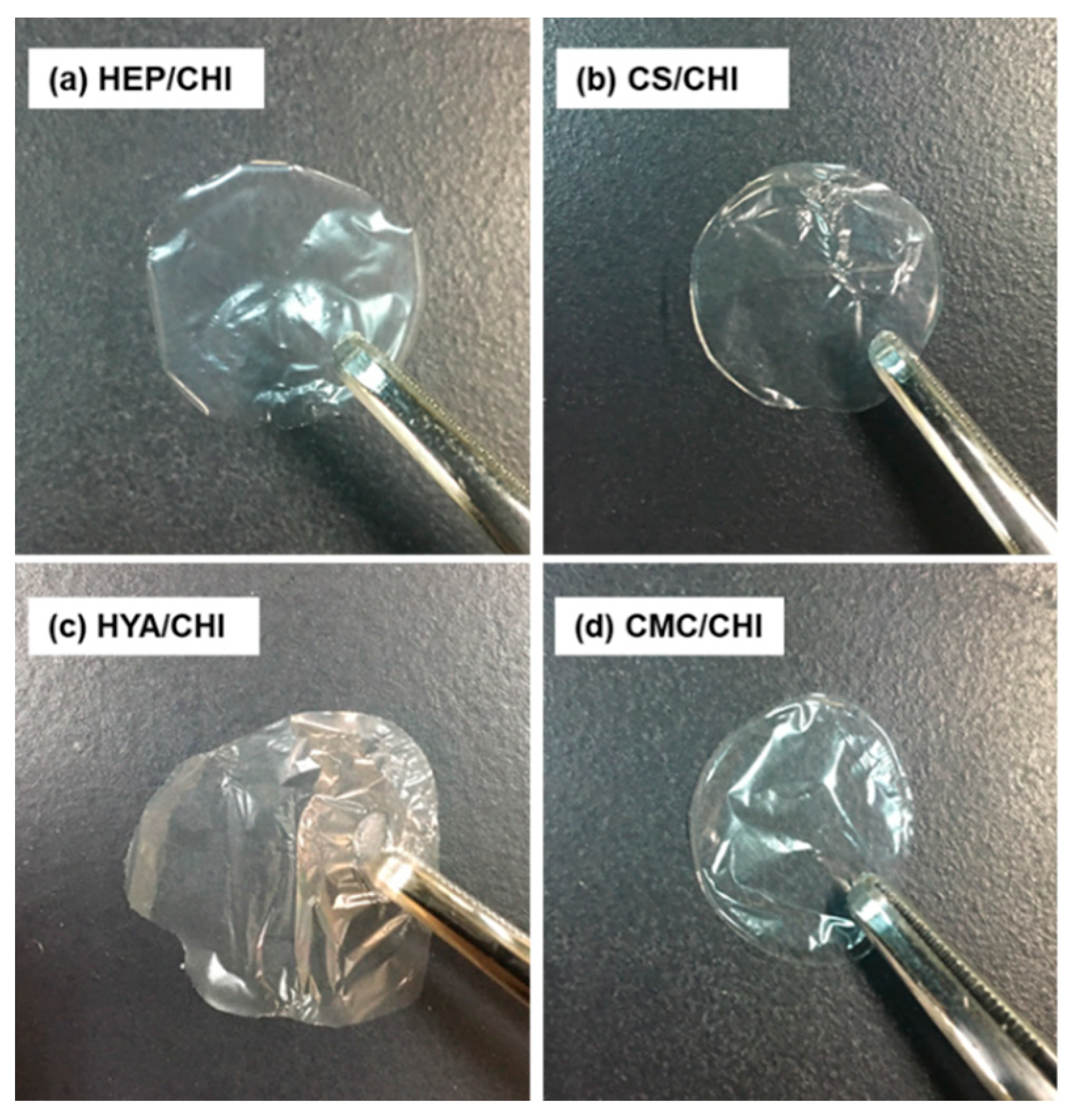
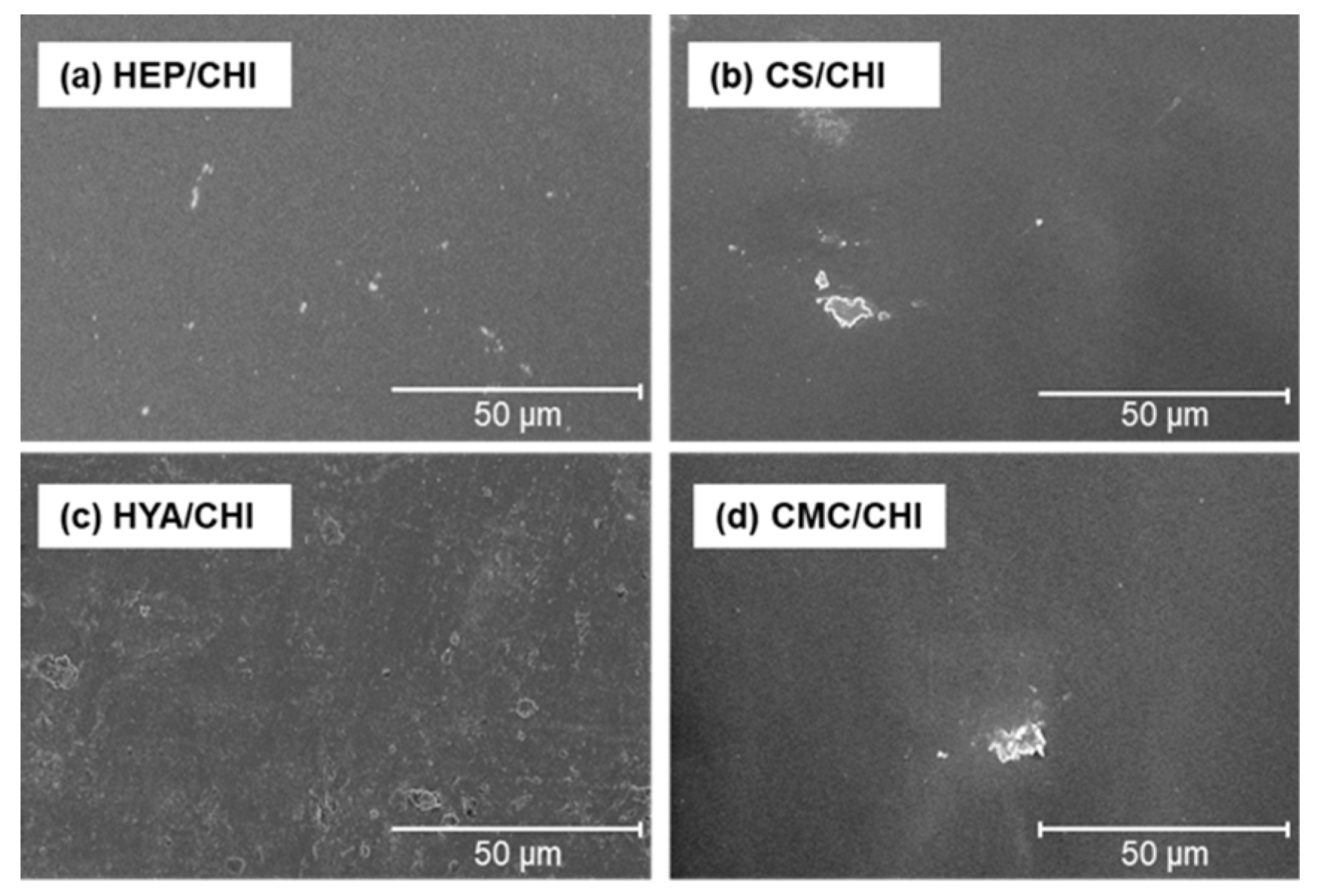
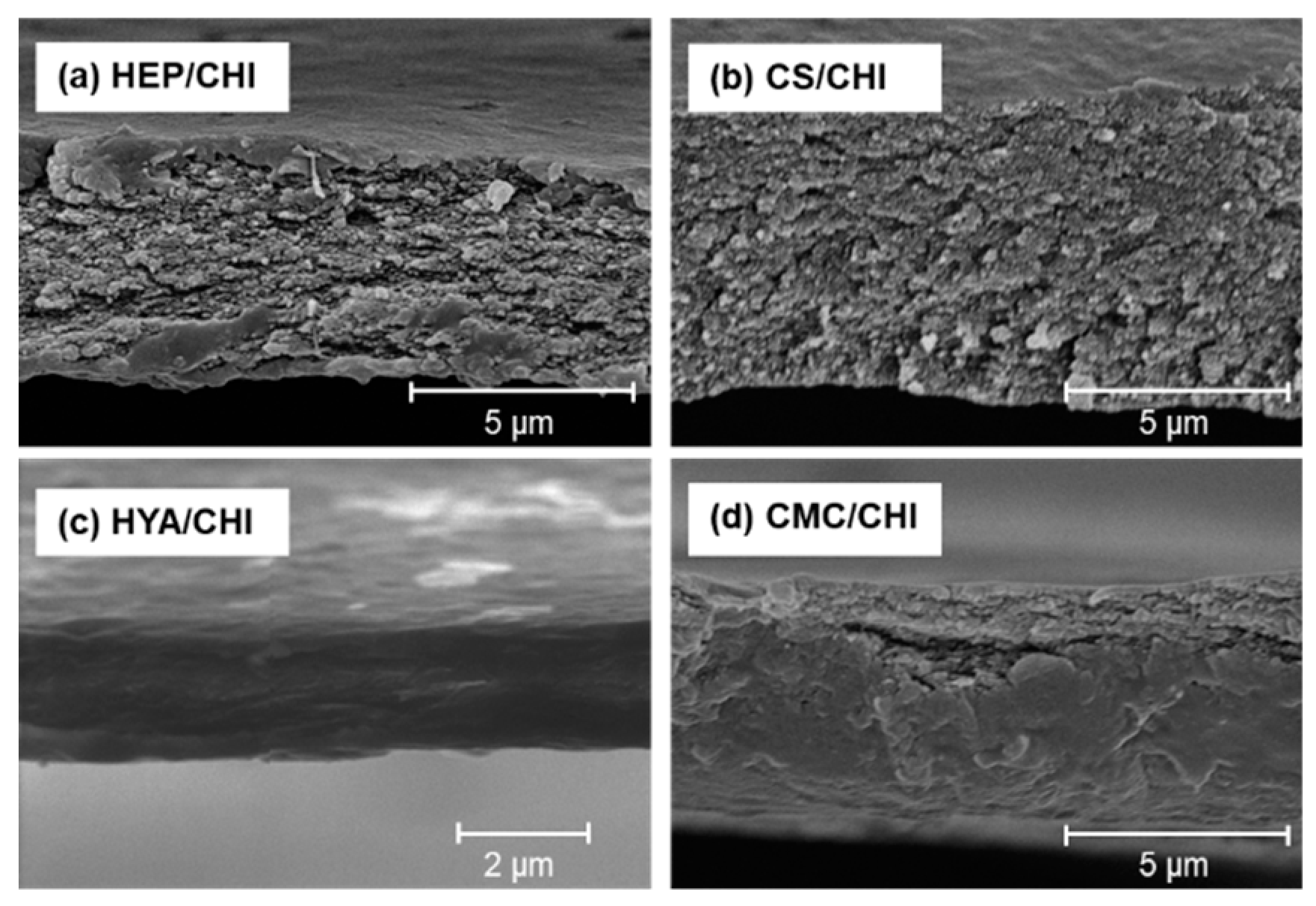
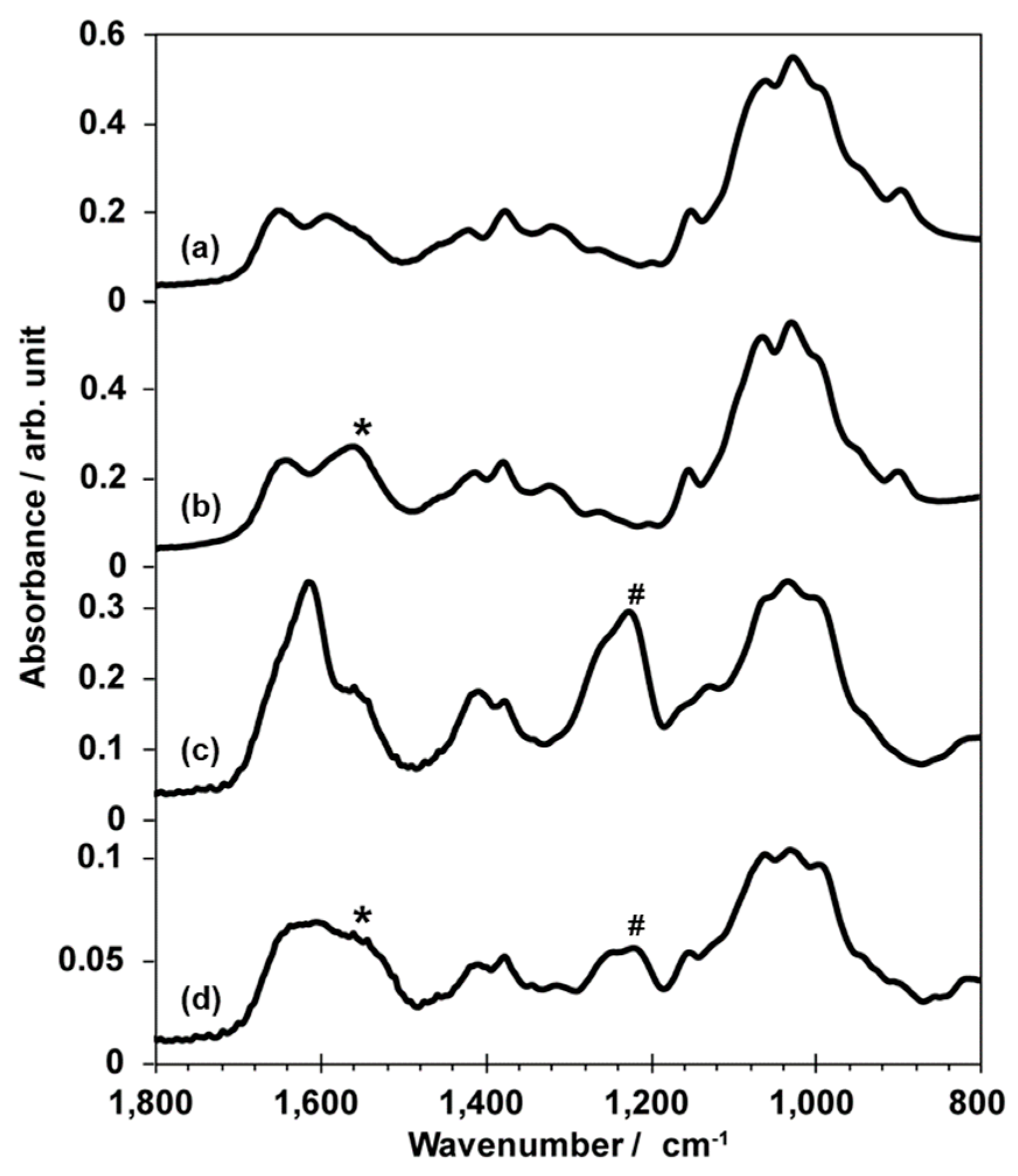
3.2. Characterization of Films Composed of Two Kinds of Polysaccharides
3.3. Adhesion of Cells to Polysaccharide Composite Films
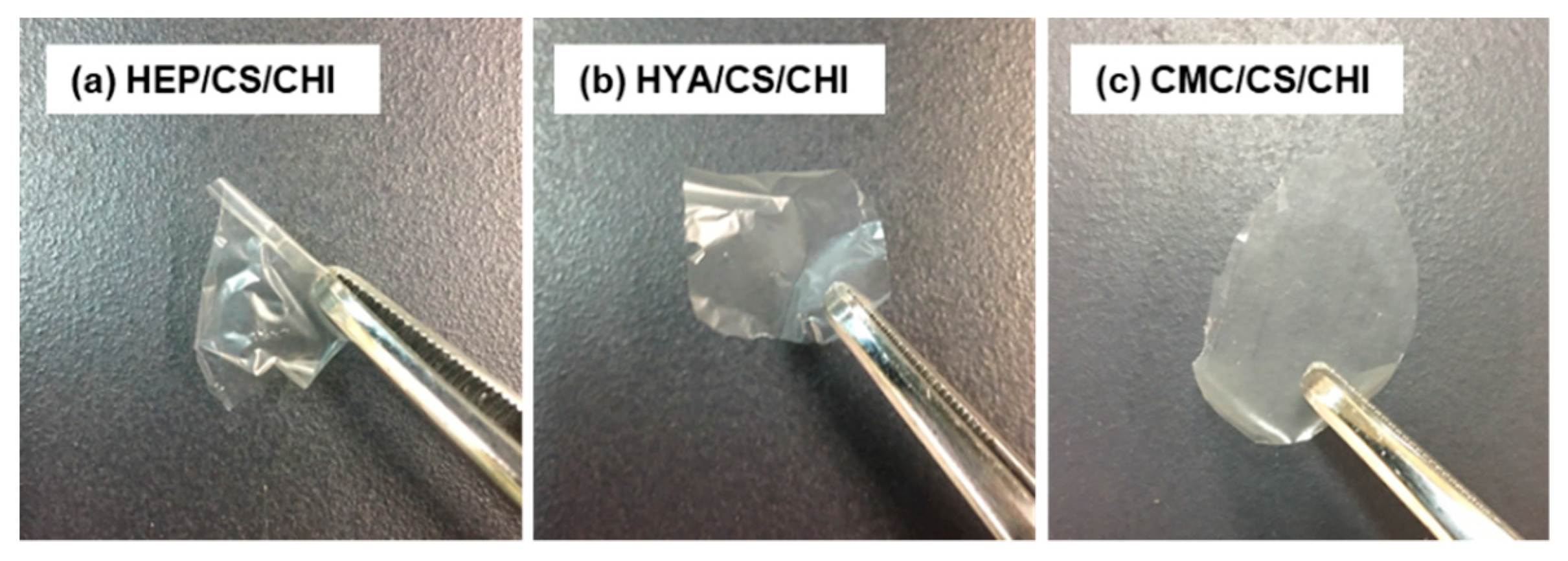
3.4. Preparation and Characterization of Films Composed of Three Kinds of Polysaccharides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oldenkamp, H.F.; Vela Ramirez, J.E.; Peppas, N.A. Re-evaluating the importance of carbohydrates as regenerative biomaterials. Regen. Biomater. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Hashizume, M. Application of polysaccharides as structural materials. Trends Glycosci. Glycotechnol. 2015, 27, 67–79. [Google Scholar] [CrossRef]
- Koido, S.S. Chitin-chitosan: Properties, benefits and risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Rodrigues, M.N.; Oliveira, M.B.; Costa, R.R.; Mano, J.F. Chitosan/chondroitin sulfate membranes produced by polyelectrolyte complexation for cartilage engineering. Biomacromolecules 2016, 17, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Nagako, S.; Kawashima, H.; Yamaguchi, K.; Miyasaka, M. CD44-chondroitin sulfate interactions mediate leukocyte rolling under physiological flow conditions. Immunol. Lett. 2004, 93, 163–170. [Google Scholar] [CrossRef]
- Nandi, A.; Estess, P.; Siegelman, M.H. Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J. Biol. Chem. 2000, 275, 14939–14948. [Google Scholar] [CrossRef]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenes. Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef]
- Hunt, J.A.; Joshi, H.N.; Stella, V.J.; Topp, E.M. Diffusion and Drug release in polymer-films prepared from wester derivatives of hyaluronic-acid. J. Control. Release 1990, 12, 159–169. [Google Scholar] [CrossRef]
- Campoccia, D.; Doherty, P.; Radice, M.; Brun, P.; Abatangelo, G.; Williams, D.F. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials 1998, 19, 2101–2127. [Google Scholar] [CrossRef]
- Gajewiak, J.; Cai, S.; Shu, X.Z.; Prestwich, G.D. Aminooxy pluronics: Synthesis and preparation of glycosaminoglycan adducts. Biomacromolecules 2006, 7, 1781–1789. [Google Scholar] [CrossRef]
- Sintov, A.; Di-Capua, N.; Rubinstein, A. Cross-linked chondroitin sulphate: Characterization for drug delivery purposes. Biomaterials 1995, 16, 473–478. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikeda, Y. Preparation of cross-linked hyaluronic acid films of low water content. Biomaterials 1997, 18, 189–195. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Organs 2014, 37, 187–205. [Google Scholar] [CrossRef]
- Chen, W.B.; Wang, L.F.; Chen, J.S.; Fan, S.Y. Characterization of polyelectrolyte complexes between chondroitin sulfate and chitosan in the solid state. J. Biomed. Master. Res. 2005, 75A, 128–137. [Google Scholar] [CrossRef]
- Peniche, C.; Fernández, M.; Rodríguez, G.; Parra, J.; Jimenez, J.; Bravo, A.L.; Gómez, D.; San Román, J. Cells supports of chitosan/hyaluronic acid and chondroitin sulphate systems. morphology and biological behavior. J. Mater. Sci. Mater. Med. 2007, 18, 1719–1726. [Google Scholar] [CrossRef]
- Meng, X.; Tian, F.; Yang, J.; He, C.N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef]
- Fu, J.; Ji, J.; Yuan, W.; Shen, J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials 2005, 26, 6684–6692. [Google Scholar] [CrossRef]
- Chua, P.H.; Neoh, K.G.; Kang, E.T.; Wang, W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef]
- Hashizume, M.; Koyabashi, H.; Ohashi, M. Preparation of free-standing films of natural polysaccharides using hot press technique and their surface functionalization with biomimetic apatite. Colloids Surf. B 2011, 88, 534–538. [Google Scholar] [CrossRef]
- Hashizume, M.; Ohash, M.; Kobayashi, H.; Tsuji, Y.; Iijima, K. Free-standing polysaccharide composite films: Improved preparation and physical properties. Colloids Surf. A 2015, 483, 18–24. [Google Scholar] [CrossRef]
- Hashizume, M.; Murata, Y.; Shibata, T.; Iijima, K. Drug loading and release behaviors of freestanding polysaccharide composite films. Polym. J. 2015, 48, 545–555. [Google Scholar] [CrossRef]
- Iijima, K.; Tsuji, Y.; Kuriki, I.; Kakimoto, A.; Nikaido, Y.; Ninomiya, R.; Iyda, T.; Fukai, F.; Hashizume, M. Control of cell adhesion and proliferation utilizing polysaccharide composite film scaffolds. Colloids Surf. B 2017, 160, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2015, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Chemistry: Pure Chemistry, 5th ed.; Chemical Society of Japan: Maruzen, Japan, 1999.
- Kikuchi, Y.; Noda, A. Polyelectrolyte complexes of heparin with chitosan. J. Appl. Polym. Sci. 1976, 20, 2561–2563. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Infrared Spectroscopy of aqueous carboxylic acids: Comparison between different acids and their salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Liu, F.; Zhang, Z.J. Heparin/chitosan nanoparticle carriers prepared by polyelectrolyte complexation. Biomed. Mater. Res. A 2007, 83, 806–812. [Google Scholar] [CrossRef]
- Yeh, M.K.; Cheng, K.M.; Hu, C.S.; Huang, Y.C.; Young, J.J. Novel protein-loaded chondroitin sulfate-chitosan nanoparticles: Preparation and characterization. Acta Biomater. 2011, 7, 3804–3812. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Kaihara, S.; Suzuki, Y.; Fujimoto, K. In situ synthesis of polysaccharide nanoparticles via polyion complex of carboxymethyl cellulose and chitosan. Colloid Surf. B 2011, 85, 343–348. [Google Scholar] [CrossRef]
- Müller, M.; Kessler, B.; Richter, S. Preparation of monomodal polyelectrolyte complex nanoparticles of PDADMAC/poly(maleic acid-alt-alpha-methylstyrene) by consecutive centrifugation. Langmuir 2005, 21, 7044–7051. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Keivani, F.; Mohammadi, M. Encapsulation of food ingredients by solid lipid nanoparticles (SLNs). In Lipid-Based Nanostructures for Food Encapsulation Purposes, 1st ed.; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 179–216. [Google Scholar] [CrossRef]
- Insua, I.; Wilkinson, A.; Fernandez-Trillo, F. Polyion complex (PIC) particles: Preparation and biomedical applications. Eur. Polym. J. 2016, 81, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; Cummings, C.; Brass, A.; Chen, Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem. J. 1991, 274, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and characterization of low methoxyl pectin/gelatin/carboxymethyl cellulose absorbent hydrogel film for wound dressing applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hashida, M.; Harashima, H. Hyaluronic acid controls the uptake pathway and intracellular trafficking of an octaarginine-modified gene vector in CD44 positive- and CD44 negative-cells. Biomaterials 2015, 52, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ma, Y.; Yang, Y.; Zhang, L.; Han, H.; Chen, J. CD44 promotes cell proliferation in non-small cell lung cancer. Oncol. Lett. 2018, 15, 5627–5633. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 2. [Google Scholar] [CrossRef]

| Composition | Diameter/nm | PDI/- | ζ-Potential/mV |
|---|---|---|---|
| HEP/CHI | 680.9 ± 23.5 | 0.264 | 40.15 ± 1.55 |
| CS/CHI | 491.5 ± 43.6 | 0.199 | 26.47 ± 0.23 |
| HYA/CHI | 791.4 ± 95.0 | 0.303 | 0.08 ± 1.05 |
| CMC/CHI | 519.4 ± 11.9 | 0.257 | 21.97 ± 2.14 |
| Film | Thickness/μm |
|---|---|
| HEP/CHI | 4.38 ± 0.44 |
| CS/CHI | 5.35 ± 1.53 |
| HYA/CHI | 1.76 ± 0.32 |
| CMC/CHI | 5.65 ± 1.52 |
| Film | Equilibrium Swelling Ratio/% |
|---|---|
| HEP/CHI | 149 ± 22.6 |
| CS/CHI | 138 ± 30.5 |
| HYA/CHI | 725 ± 110.9 |
| CMC/CHI | 412 ± 31.5 |
| Film | Tensile Strength/MPa | Young’s Modulus/MPa |
|---|---|---|
| HEP/CHI | 11.8 ± 0.4 | 154.5 ± 76.2 |
| CS/CHI | 121.6 ± 56.0 | 877.9 ± 254.2 |
| HYA/CHI | 296.5 ± 69.0 | 6662.2 ± 1615.3 |
| CMC/CHI | 14.1 ± 4.0 | 687.3 ± 223.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, M.; Iijima, K. Fabrication and Characterization of Polysaccharide Composite Films from Polyion Complex Particles. Polymers 2020, 12, 435. https://doi.org/10.3390/polym12020435
Yamazaki M, Iijima K. Fabrication and Characterization of Polysaccharide Composite Films from Polyion Complex Particles. Polymers. 2020; 12(2):435. https://doi.org/10.3390/polym12020435
Chicago/Turabian StyleYamazaki, Makoto, and Kazutoshi Iijima. 2020. "Fabrication and Characterization of Polysaccharide Composite Films from Polyion Complex Particles" Polymers 12, no. 2: 435. https://doi.org/10.3390/polym12020435
APA StyleYamazaki, M., & Iijima, K. (2020). Fabrication and Characterization of Polysaccharide Composite Films from Polyion Complex Particles. Polymers, 12(2), 435. https://doi.org/10.3390/polym12020435