Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Collagenase Hydrogel Preparation
2.2. Cytotoxicity Evaluation
2.3. Release Profile of Collagenase from C/G/GP Hydrogel
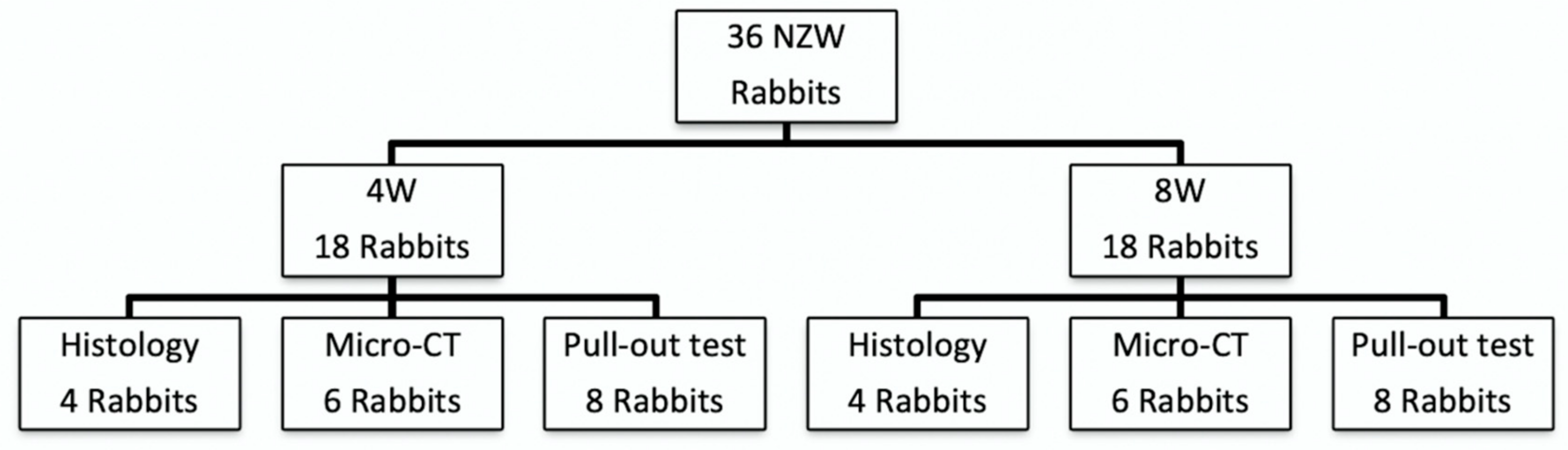
2.4. Animal Study Design
2.5. Surgical Procedure
2.6. Histological Examination
2.7. Micro-Computed Tomography Evaluation
2.8. Biomechanical Testing
2.9. Statistical Analyses
3. Results
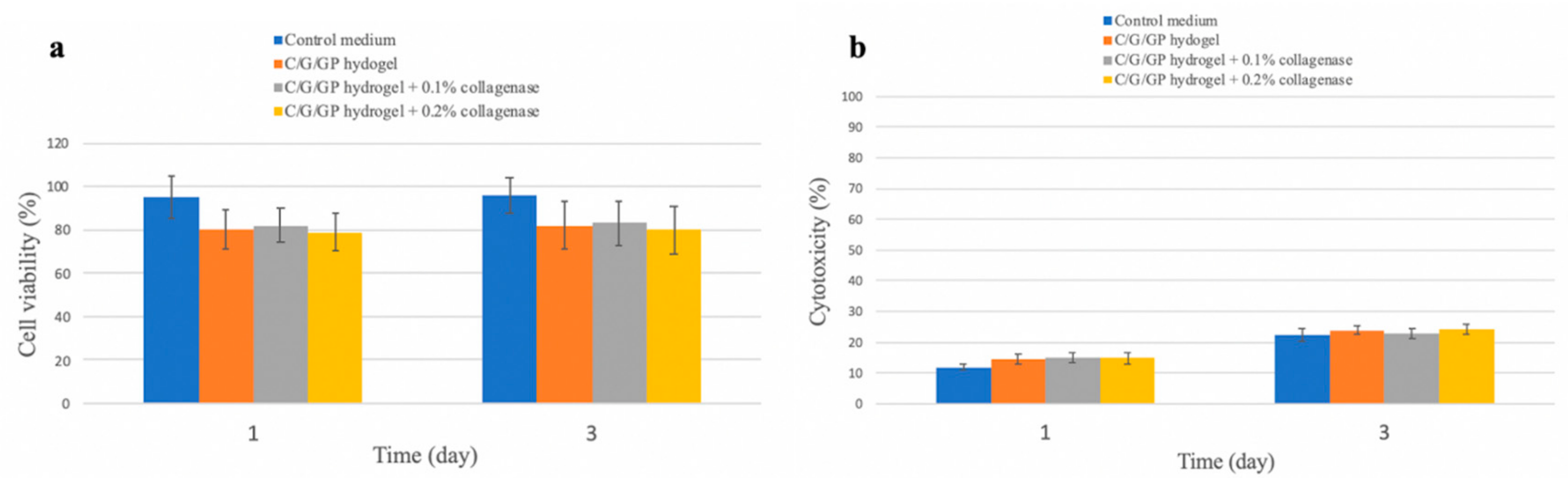
3.1. Cytotoxicity
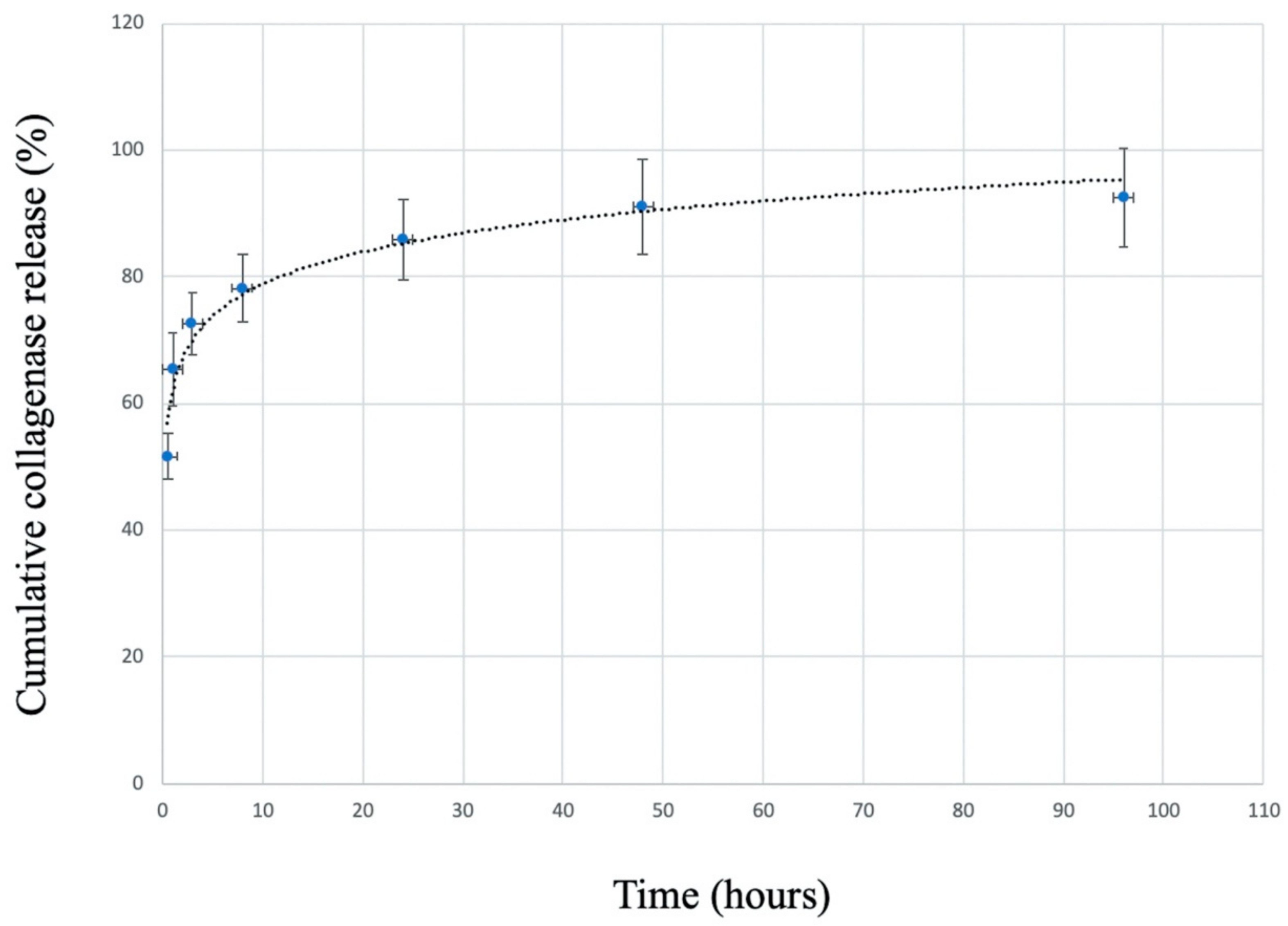
3.2. Collagenase Release Profile
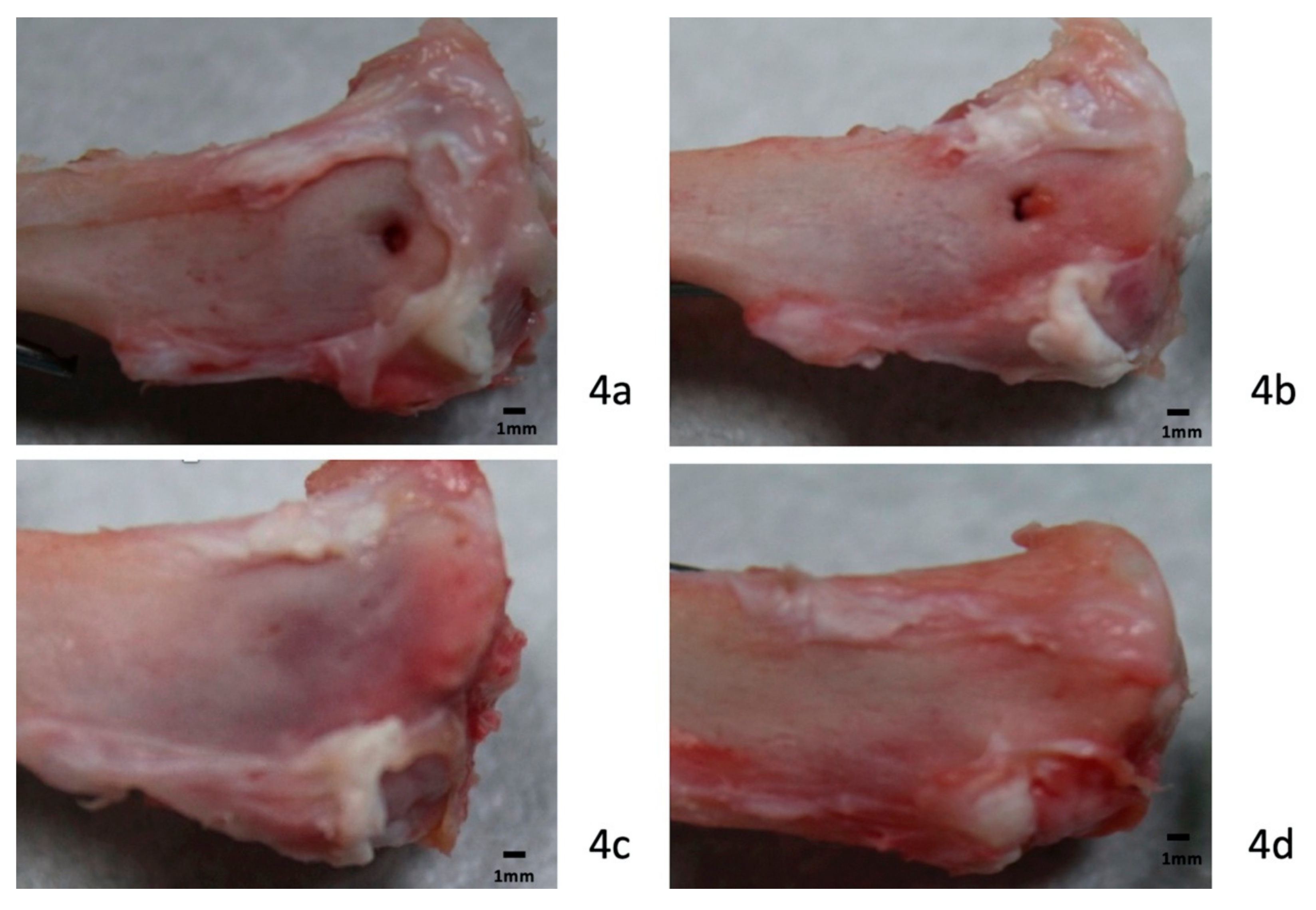
3.3. Gross Observations
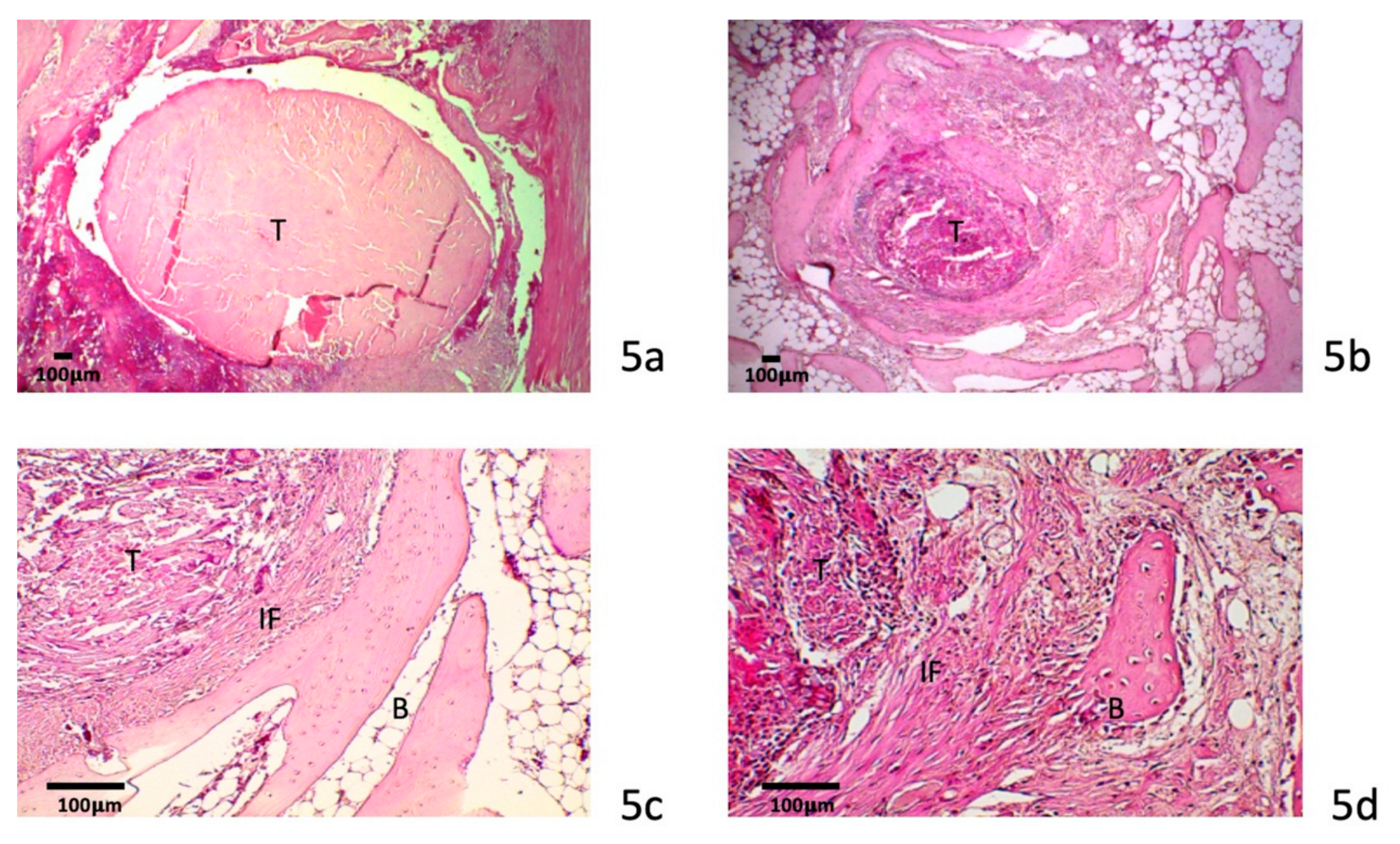
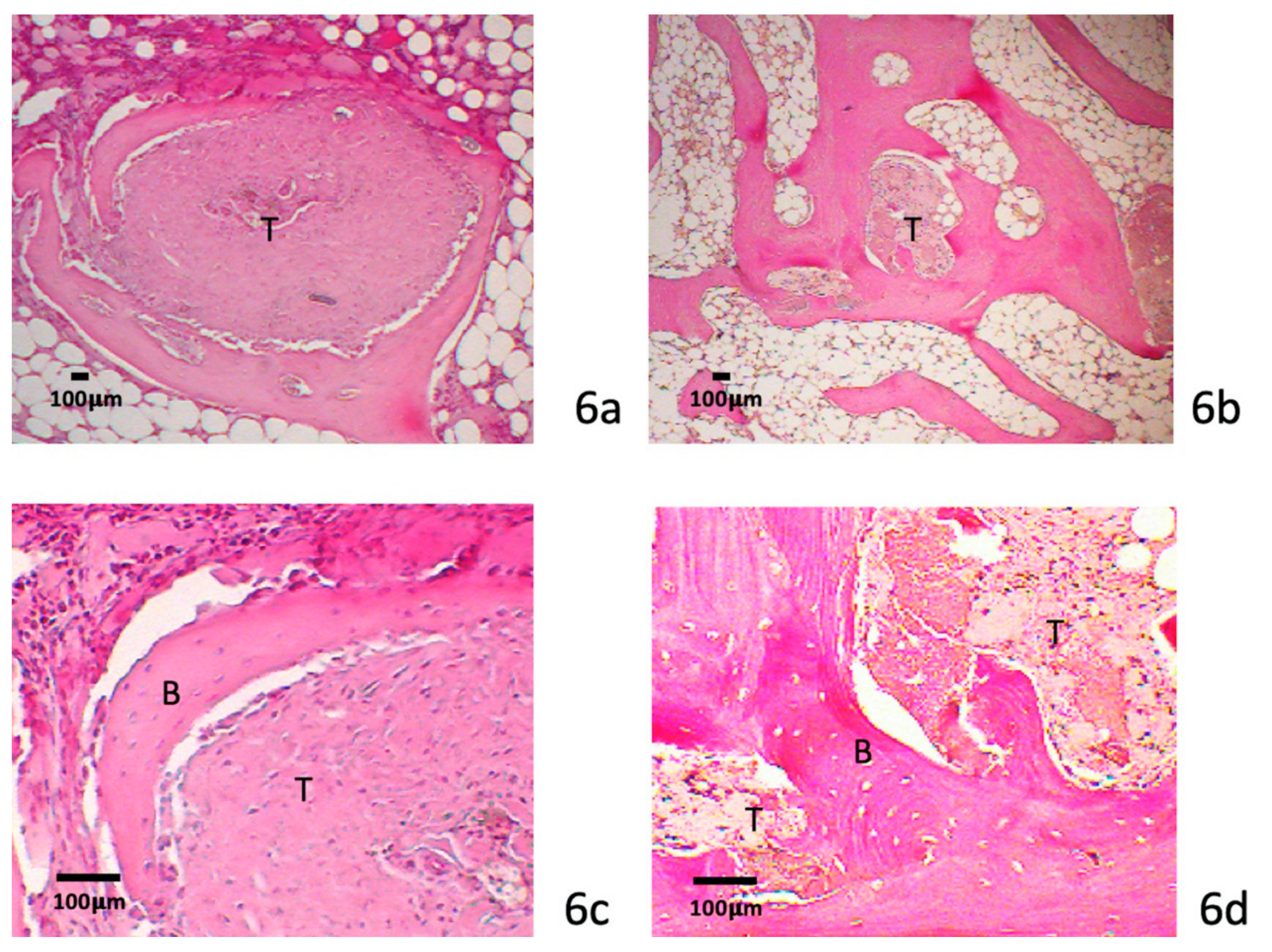
3.4. Histology
3.4.1. Four-Week Specimens
3.4.2. Eight-Week Specimens
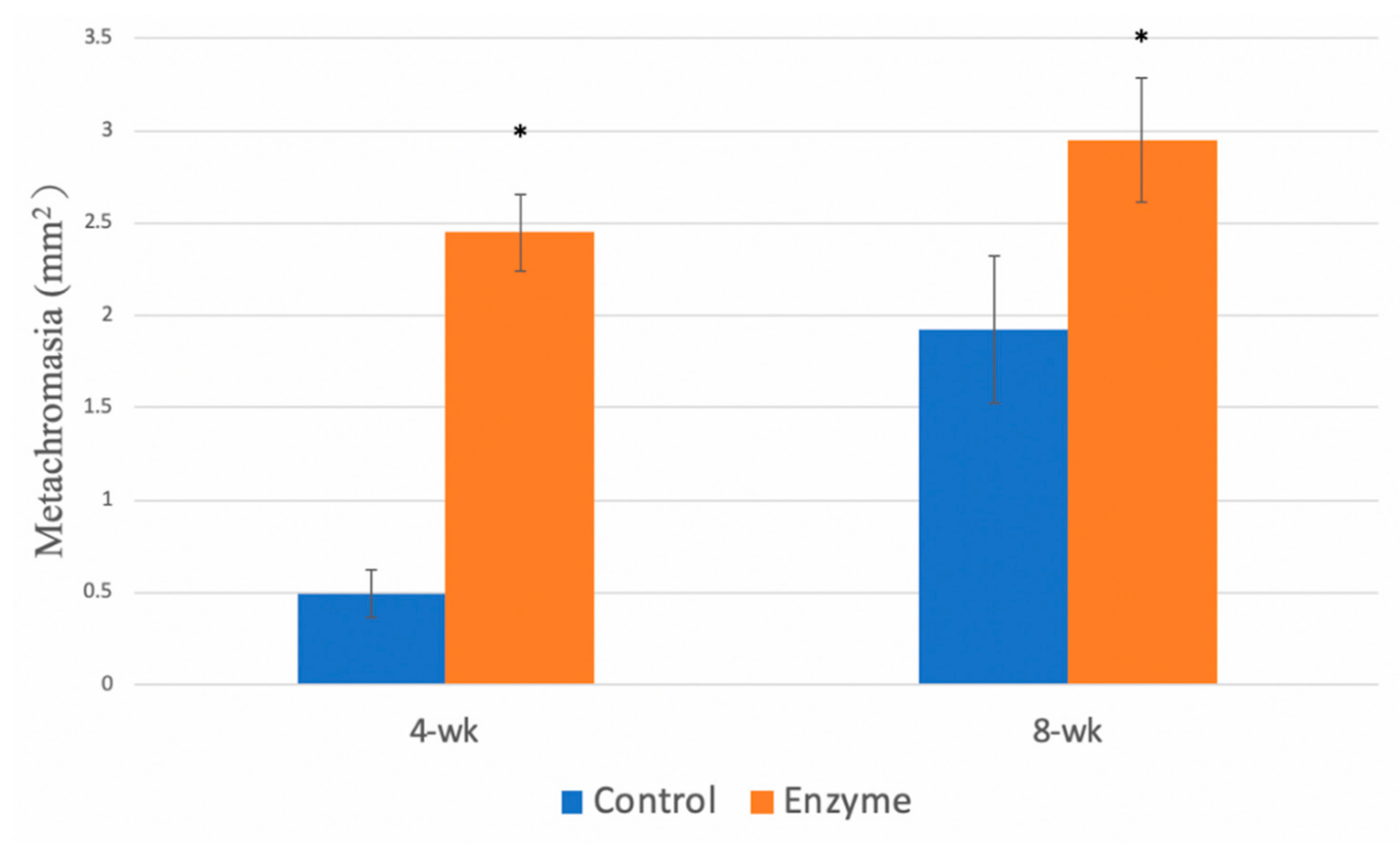
3.4.3. Metachromasia
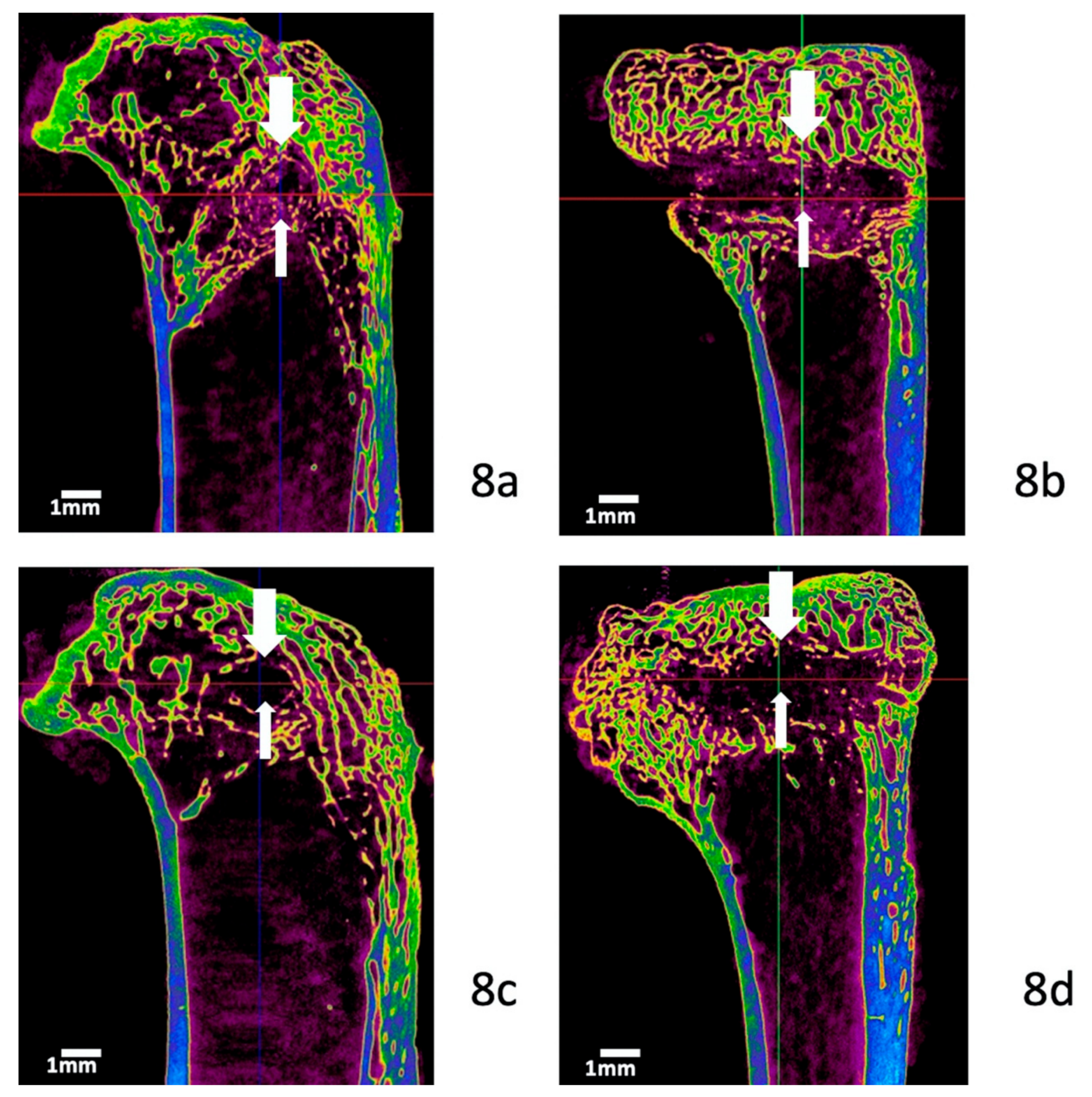
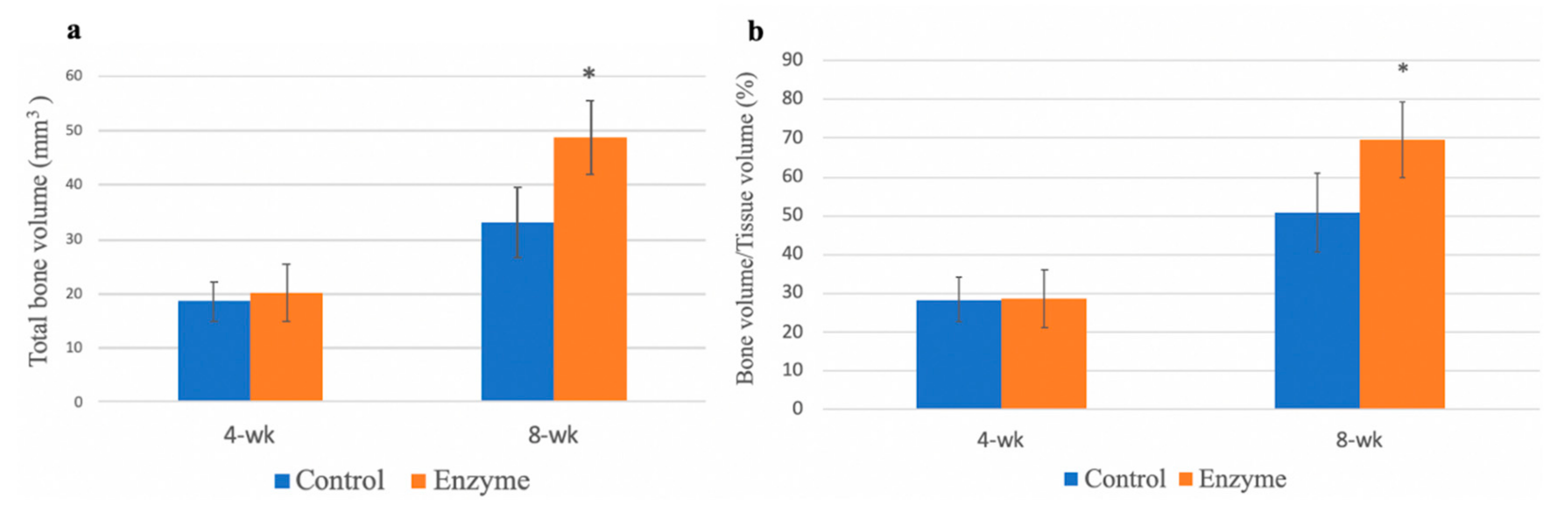
3.5. Micro-Computed Tomography Evaluation
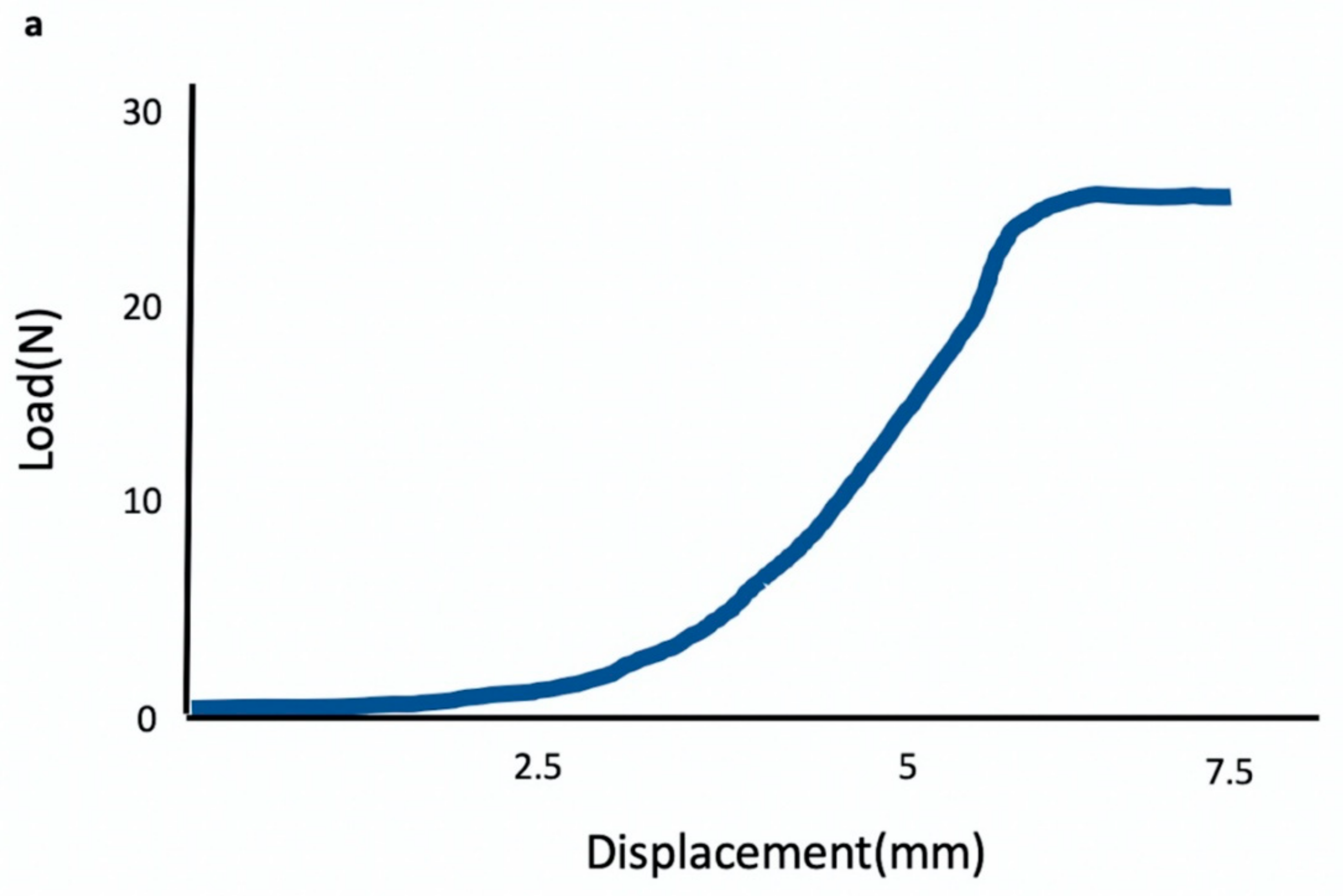
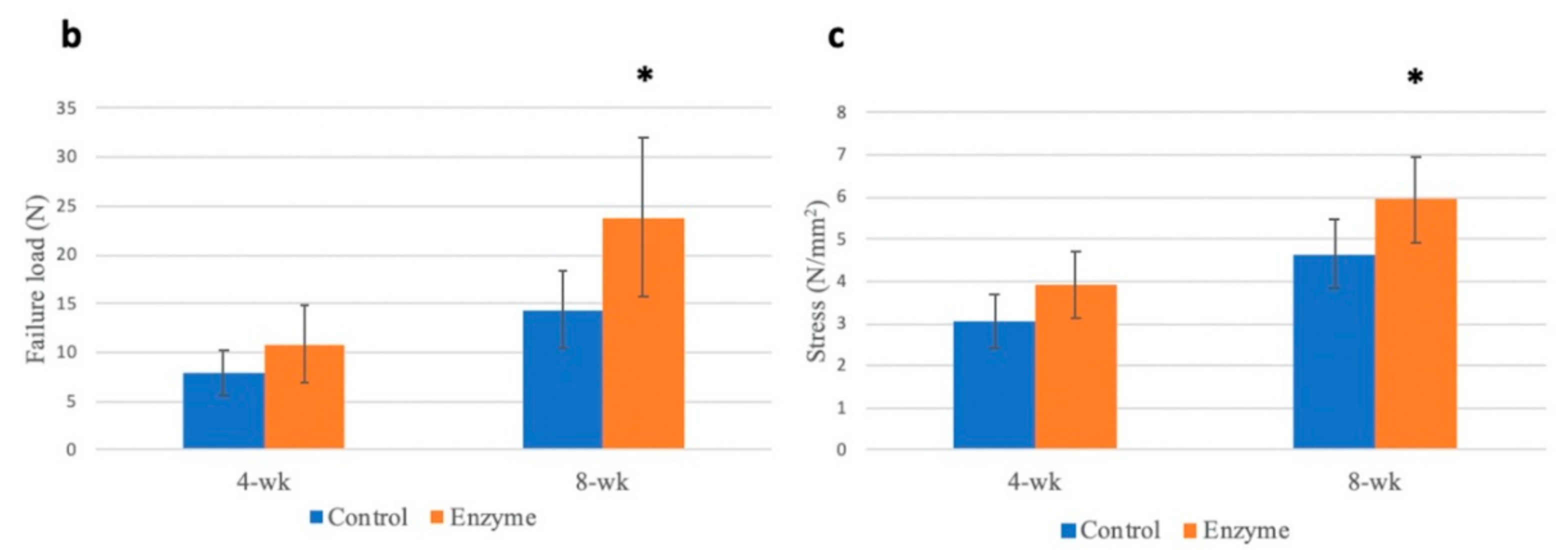
3.6. Biomechanical Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frank, C.B.; Jackson, D.W. Current concepts review—The science of reconstruction of the anterior cruciate ligament. J. Bone Jt. Surg. 1997, 79, 1556–1576. [Google Scholar] [CrossRef] [PubMed]
- Freedman, K.B.; D’Amato, M.J.; Nedeff, D.D.; Kaz, A.; Bach, B.R. Arthroscopic anterior cruciate ligament reconstruction: A metaanalysis comparing patellar tendon and hamstring tendon autografts. Am. J. Sports Med. 2003, 31, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Poehling-Monaghan, K.L.; Salem, H.; Ross, K.E.; Secrist, E.; Ciccotti, M.C.; Tjoumakaris, F.; Ciccotti, M.G.; Freedman, K.B. Long-term outcomes in anterior cruciate ligament reconstruction: A systematic review of patellar tendon versus hamstring autografts. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef]
- Gifstad, T.; Foss, O.A.; Engebretsen, L.; Lind, M.; Forssblad, M.; Albrektsen, G.; Drogset, J.O. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: A registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am. J. Sports Med. 2014, 42, 2319–2328. [Google Scholar] [CrossRef]
- Samuelsen, B.T.; Webster, K.E.; Johnson, N.R.; Hewett, T.E.; Krych, A.J. Hamstring Autograft versus Patellar Tendon Autograft for ACL Reconstruction: Is There a Difference in Graft Failure Rate? A Meta-analysis of 47,613 Patients. Clin. Orthop. Relat. Res. 2017, 475, 2459–2468. [Google Scholar] [CrossRef]
- Cooper, R.R.; Misol, S. Tendon and ligament insertion. A light and electron microscopic study. J. Bone Jt. Surg. 1970, 52, 1–20. [Google Scholar] [CrossRef]
- Rodeo, S.A.; Suzuki, K.; Deng, X.-H.; Wozney, J.; Warren, R.F. Use of Recombinant Human Bone Morphogenetic Protein-2 to Enhance Tendon Healing in a Bone Tunnel. Am. J. Sports Med. 1999, 27, 476–488. [Google Scholar] [CrossRef]
- Wong, M.W.N.; Qin, L.; Tai, J.K.O.; Lee, S.K.M.; Leung, K.S.; Chan, K.M. Engineered allogeneic chondrocyte pellet for reconstruction of fibrocartilage zone at bone-tendon junction? A preliminary histological observation. J. Biomed. Mater. Res. 2004, 70B, 362–367. [Google Scholar] [CrossRef]
- Tomita, F.; Yasuda, K.; Mikami, S.; Sakai, T.; Yamazaki, S.; Tohyama, H. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy 2001, 17, 461–476. [Google Scholar] [CrossRef]
- Liao, C.-J.; Lin, Y.-J.; Chiang, H.; Chiang, S.-F.; Wang, Y.-H.; Jiang, C.-C. Injecting partially digested cartilage fragments into a biphasic scaffold to generate osteochondral composites in a nude mice model. J. Biomed. Mater. Res. A 2007, 81, 567–577. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gao, W.; Hu, H.; Ma, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J. Control. Release 2014, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Ahmadi, R.; de Bruijn, J.D. Biocompatibility and gelation of chitosan-glycerol phosphate hydrogels. J. Biomed. Mater. Res. A 2008, 86, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Yang, S.-H.; Su, W.-Y.; Chen, Y.-C.; Yang, K.-C.; Cheng, W.T.-K.; Wu, S.-C.; Lin, F.-H. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration: An in vitro study. Tissue Eng. Part A 2010, 16, 695–703. [Google Scholar] [CrossRef]
- Gulotta, L.V.; Kovacevic, D.; Ying, L.; Ehteshami, J.R.; Montgomery, S.; Rodeo, S.A. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am. J. Sports Med. 2008, 36, 1290–1297. [Google Scholar] [CrossRef]
- Goradia, V.K.; Rochat, M.C.; Kida, M.; Grana, W.A. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am. J. Sports Med. 2000, 28, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Grana, W.A.; Egle, D.M.; Mahnken, R.; Goodhart, C.W. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am. J. Sports Med. 1994, 22, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Kawamura, S.; Ying, L.; Rodeo, S.A. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009, 5, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Stannard, J.P.; Pfeiffer, F.M.; Kuroki, K.; Bozynski, C.C.; Cook, J.L. Suspensory Versus Interference Screw Fixation for Arthroscopic Anterior Cruciate Ligament Reconstruction in a Translational Large-Animal Model. Arthroscopy 2016, 32, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Yasuda, K.; Tomita, F.; Tohyama, H.; Minami, A. The effect of transforming growth factor-β1 on intraosseous healing of flexor tendon autograft replacement of anterior cruciate ligament in dogs. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 1034–1041. [Google Scholar] [CrossRef]
- Lovric, V.; Chen, D.; Yu, Y.; Oliver, R.A.; Genin, F.; Walsh, W.R. Effects of Demineralized Bone Matrix on Tendon-Bone Healing in an Intra-articular Rodent Model. Am. J. Sports Med. 2012, 40, 2365–2374. [Google Scholar] [CrossRef]
- Mutsuzaki, H.; Fujie, H.; Nakajima, H.; Fukagawa, M.; Nomura, S.; Sakane, M. Effect of Calcium Phosphate–Hybridized Tendon Graft in Anatomic Single-Bundle ACL Reconstruction in Goats. Orthop. J. Sports Med. 2016, 4. [Google Scholar] [CrossRef]
- Yoon, J.P.; Chung, S.W.; Jung, J.W.; Lee, Y.S.; Kim, K.I.; Park, G.Y.; Kim, H.M.; Choi, J.H. Is a Local Administration of Parathyroid Hormone Effective to Tendon-to-Bone Healing in a Rat Rotator Cuff Repair Model? J. Orthop. Res. 2019, 38, 82–91. [Google Scholar] [CrossRef]
- Talhouk, R.S.; Bissell, M.J.; Werb, Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 1992, 118, 1271–1282. [Google Scholar] [CrossRef]
- Bramono, D.S.; Richmond, J.C.; Weitzel, P.P.; Kaplan, D.L.; Altman, G.H. Matrix metalloproteinases and their clinical applications in orthopaedics. Clin. Orthop. Relat. Res. 2004, 428, 272–285. [Google Scholar] [CrossRef]
- De Mos, M.; van El, B.; DeGroot, J.; Jahr, H.; van Schie, H.T.M.; van Arkel, E.R.; Tol, H.; Heijboer, R.; van Osch, G.J.V.M.; Verhaar, J.A.N. Achilles tendinosis: Changes in biochemical composition and collagen turnover rate. Am. J. Sports Med. 2007, 35, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.C.; Corps, A.N.; Pennington, C.J.; Clark, I.M.; Edwards, D.R.; Bradley, M.M.; Hazleman, B.L.; Riley, G.P. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006, 54, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Lavagnino, M.; Arnoczky, S.P.; Egerbacher, M.; Gardner, K.L.; Burns, M.E. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J. Biomech. 2006, 39, 2355–2362. [Google Scholar] [CrossRef]
- September, A.V.; Cook, J.; Handley, C.J.; van der Merwe, L.; Schwellnus, M.P.; Collins, M. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br. J. Sports Med. 2009, 43, 357–365. [Google Scholar] [CrossRef]
- Treviño, E.A.; McFaline-Figueroa, J.; Guldberg, R.E.; Platt, M.O.; Temenoff, J.S. Full-thickness rotator cuff tear in rat results in distinct temporal expression of multiple proteases in tendon, muscle, and cartilage. J. Orthop. Res. 2018, 37, 490–502. [Google Scholar] [CrossRef]
- Bedi, A.; Kovacevic, D.; Hettrich, C.; Gulotta, L.V.; Ehteshami, J.R.; Warren, R.F.; Rodeo, S.A. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J. Shoulder Elb. Surg. 2010, 19, 384–391. [Google Scholar] [CrossRef]
- Haslauer, C.M.; Proffen, B.L.; Johnson, V.M.; Murray, M.M. Expression of modulators of extracellular matrix structure after anterior cruciate ligament injury. Wound Repair Regen 2014, 22, 103–110. [Google Scholar] [CrossRef]
- Demirag, B.; Sarisozen, B.; Ozer, O.; Kaplan, T.; Ozturk, C. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J. Bone Jt. Surg. 2005, 87, 2401–2410. [Google Scholar]
- Paiva, K.B.S.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 561, 74–87. [Google Scholar] [CrossRef]
- Kylmaoja, E.; Nakamura, M.; Tuukkanen, J. Osteoclasts and Remodeling Based Bone Formation. Curr. Stem. Cell Res. Ther. 2016, 11, 626–633. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wang, C.-J.; Yang, K.D.; Kuo, Y.-R.; Huang, H.-C.; Huang, Y.-T.; Sun, Y.-C.; Wang, F.-S. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-beta1 and IGF-I expression. J. Orthop. Res. 2004, 22, 854–861. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-M.; Lin, Y.-C.; Chen, C.-Y.; Hsieh, Y.-Y.; Liaw, C.-K.; Huang, S.-W.; Tsuang, Y.-H.; Chen, C.-H.; Lin, F.-H. Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model. Polymers 2020, 12, 436. https://doi.org/10.3390/polym12020436
Huang Y-M, Lin Y-C, Chen C-Y, Hsieh Y-Y, Liaw C-K, Huang S-W, Tsuang Y-H, Chen C-H, Lin F-H. Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model. Polymers. 2020; 12(2):436. https://doi.org/10.3390/polym12020436
Chicago/Turabian StyleHuang, Yu-Min, Yi-Cheng Lin, Chih-Yu Chen, Yueh-Ying Hsieh, Chen-Kun Liaw, Shu-Wei Huang, Yang-Hwei Tsuang, Chih-Hwa Chen, and Feng-Huei Lin. 2020. "Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model" Polymers 12, no. 2: 436. https://doi.org/10.3390/polym12020436
APA StyleHuang, Y.-M., Lin, Y.-C., Chen, C.-Y., Hsieh, Y.-Y., Liaw, C.-K., Huang, S.-W., Tsuang, Y.-H., Chen, C.-H., & Lin, F.-H. (2020). Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model. Polymers, 12(2), 436. https://doi.org/10.3390/polym12020436





