Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Organovermiculites
2.3. Preparation of PLA/Vermiculite Nanocomposites
2.4. Sample Characterization
3. Results and Discussion
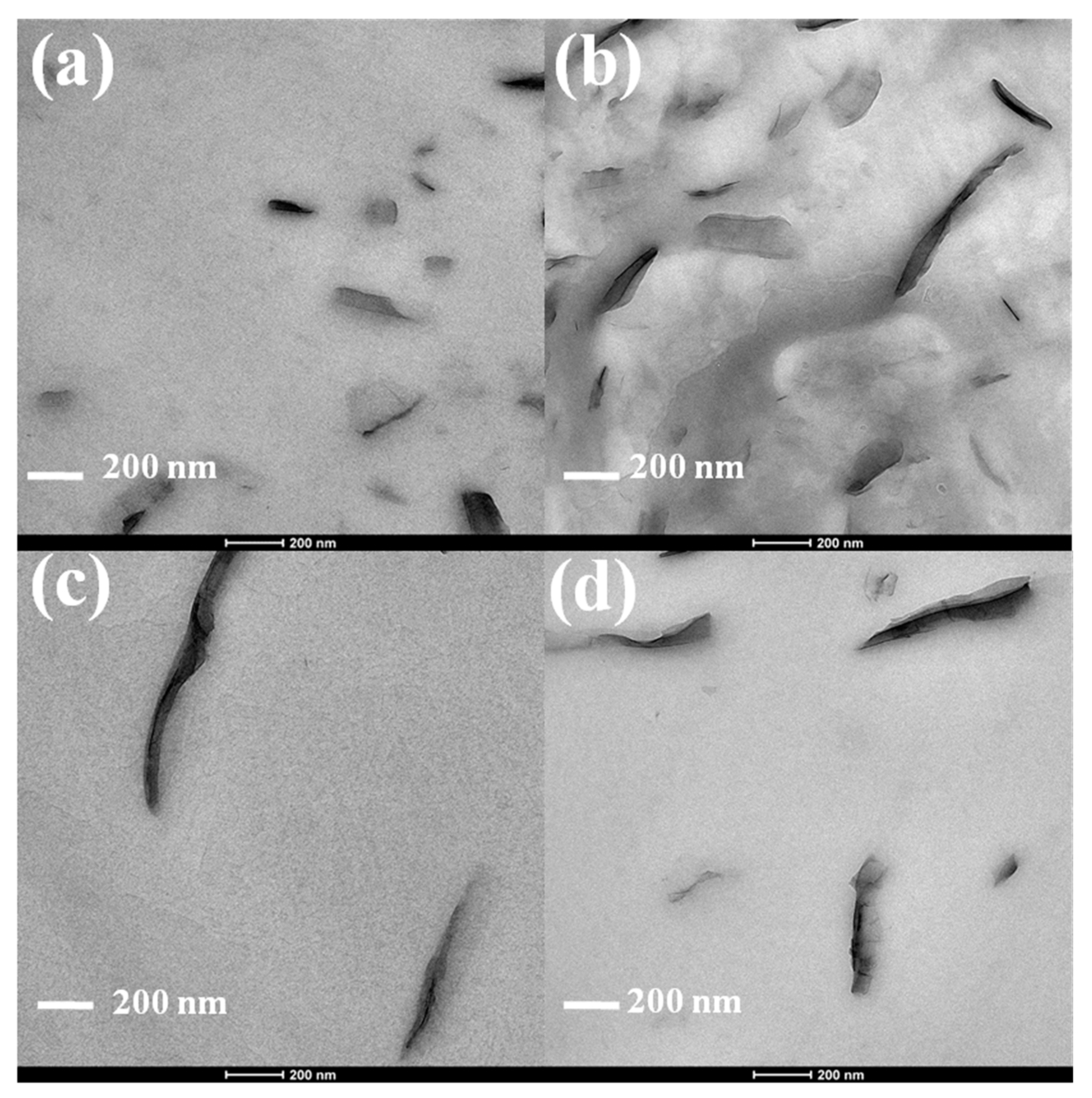
3.1. Characterization of PLA/VMT Nanocomposites
Morphology
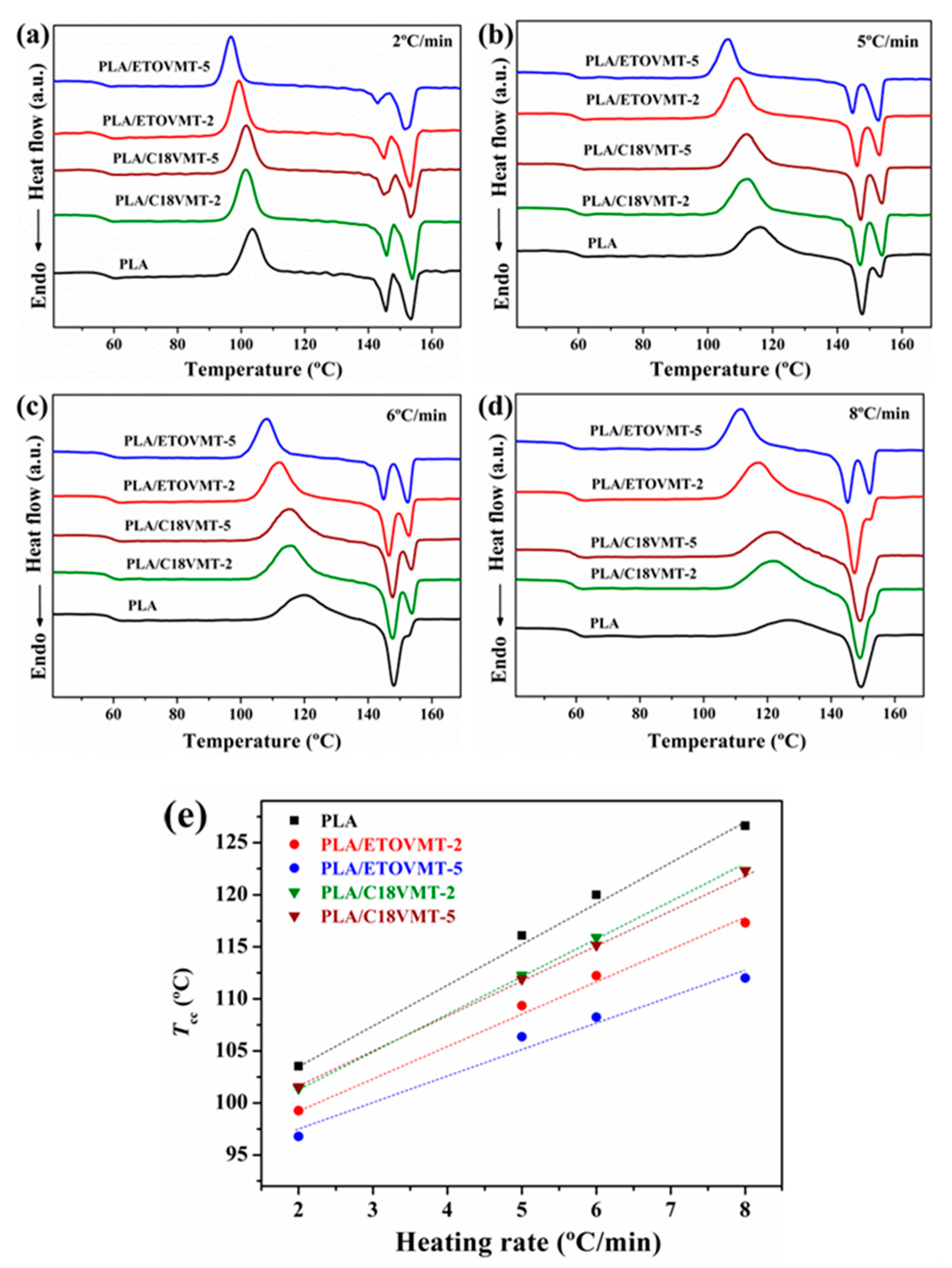
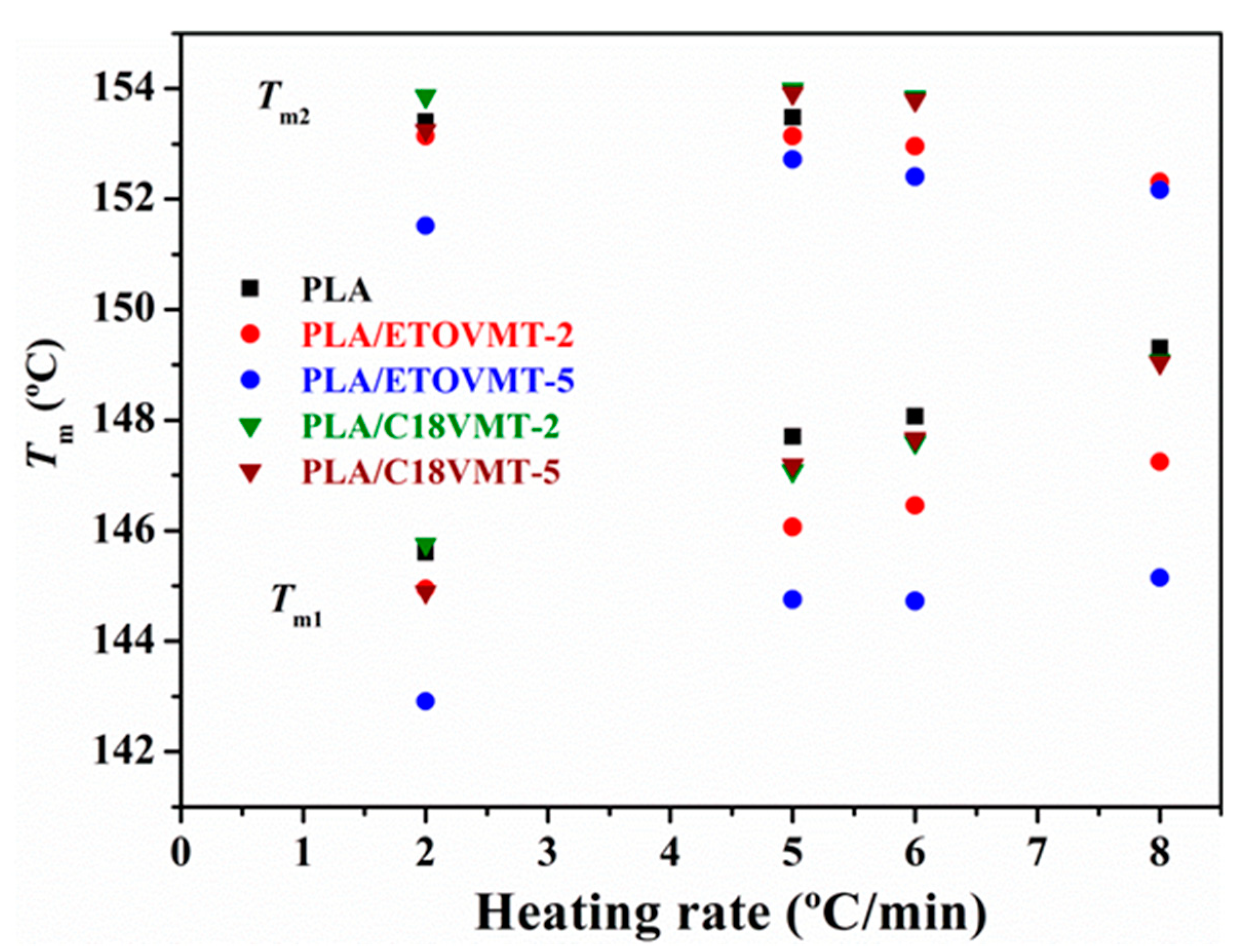
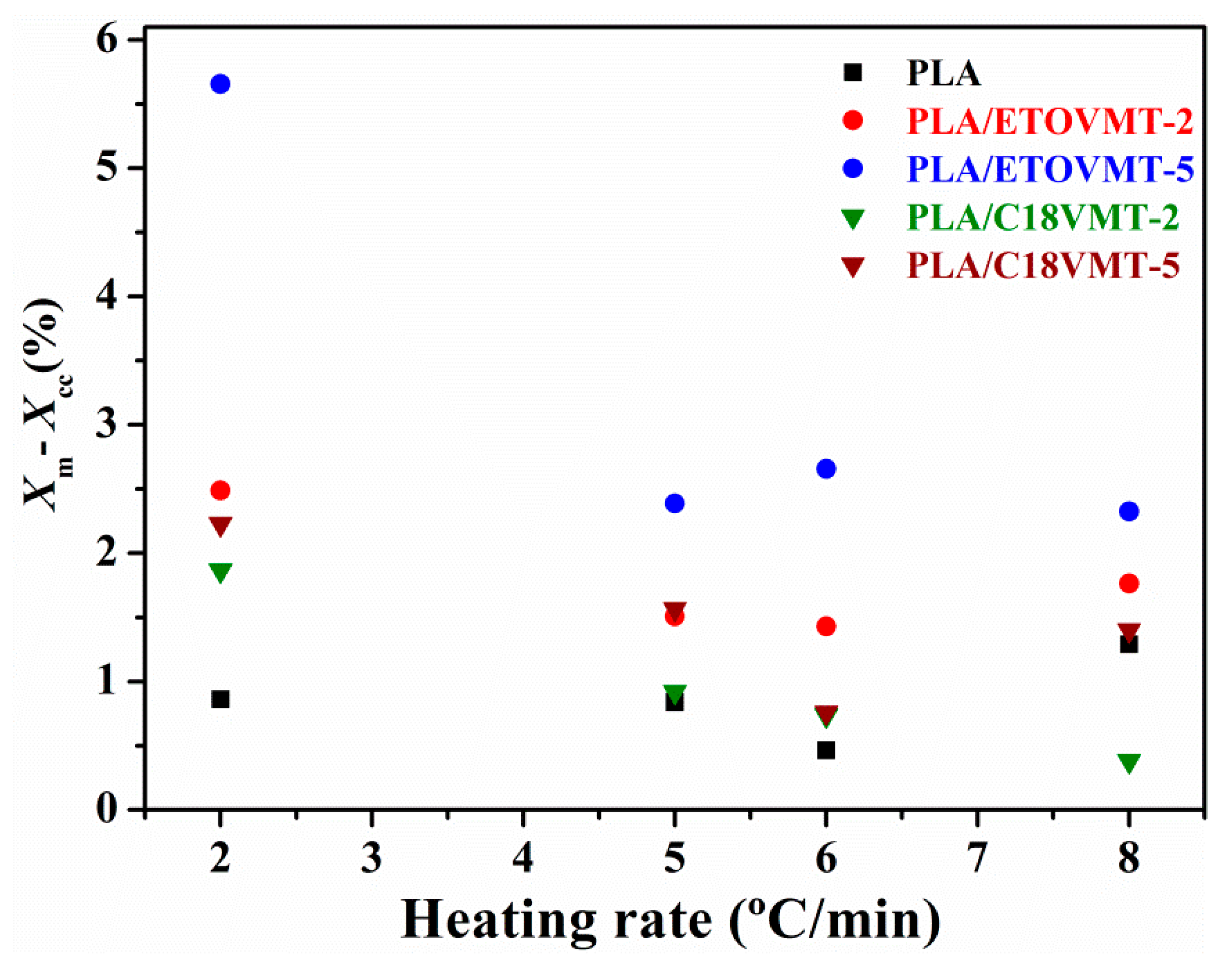
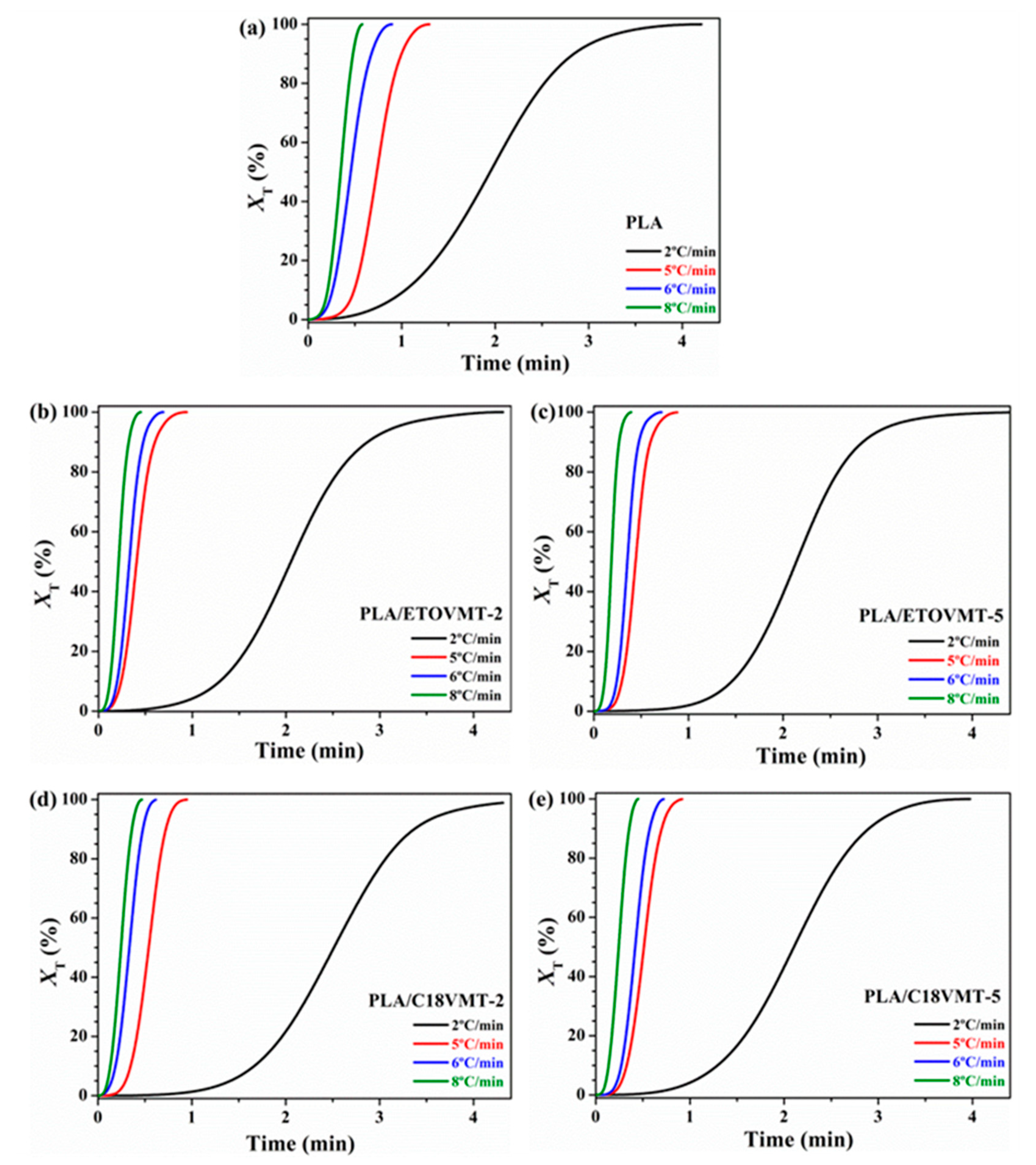
3.2. Non-Isothermal Cold Crystallization and Melting Behavior
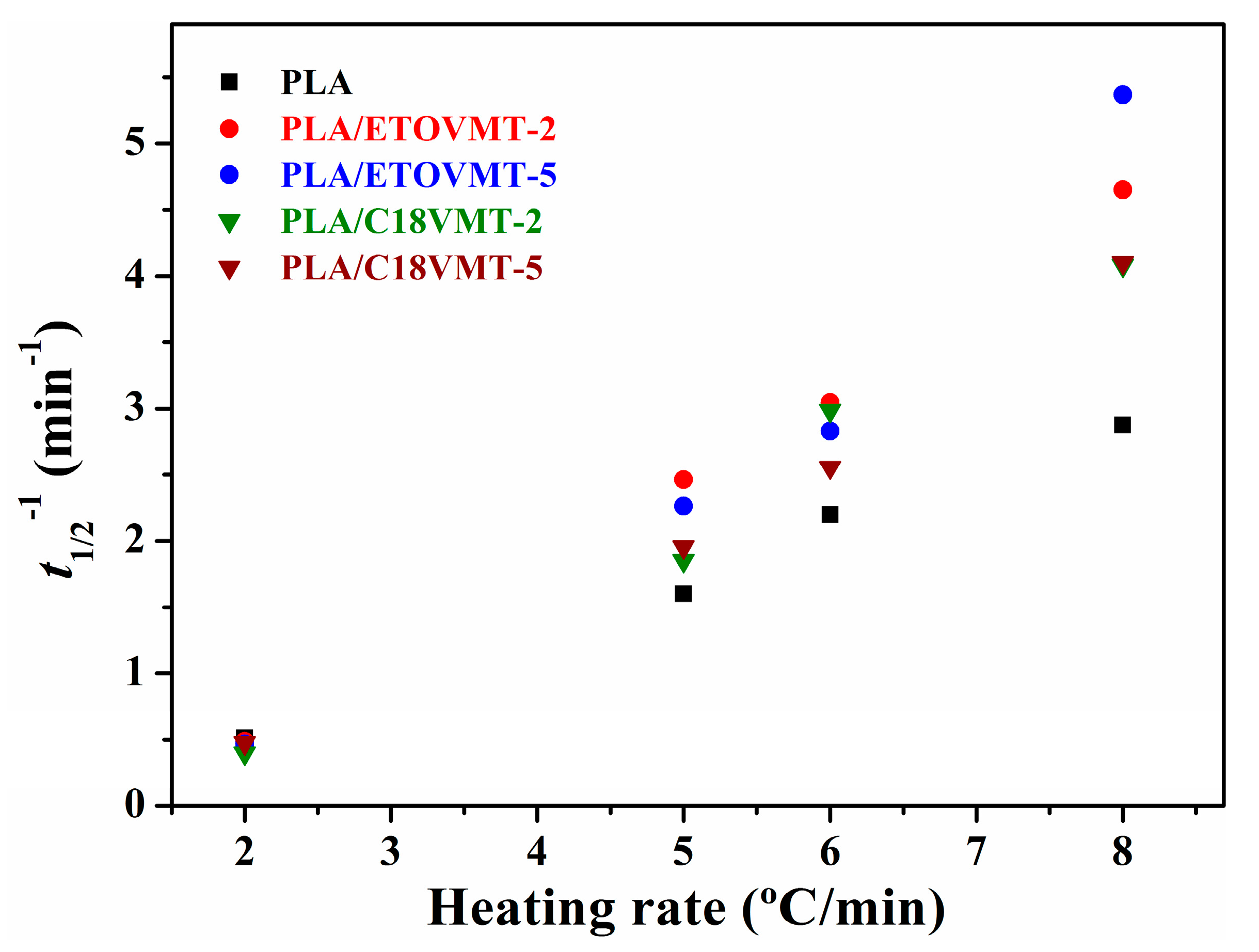
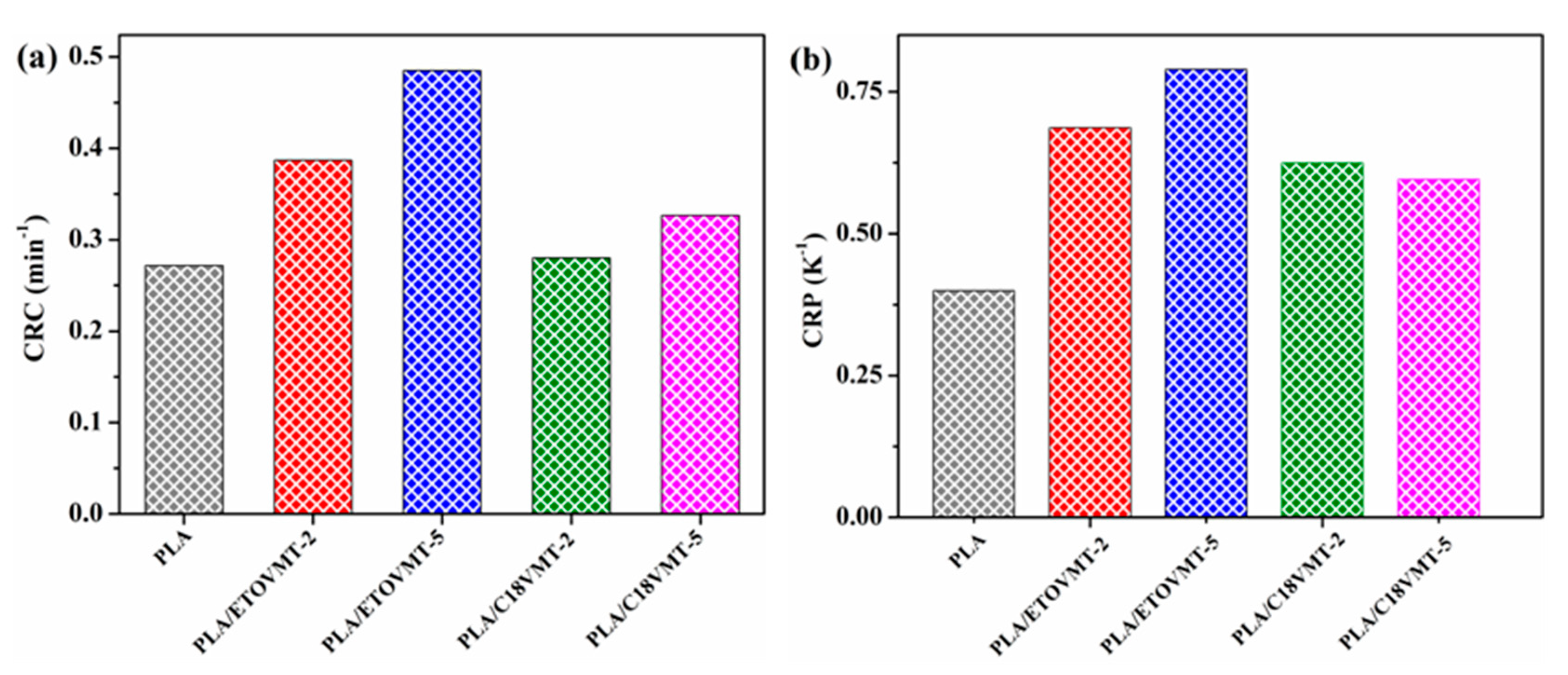
3.3. Non-Isothermal Crystallization Kinetics
3.4. Crystallization Activation Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies: A review. J. Chem. Technol. Biot. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Domenek, S.; Courgneau, C.; Ducruet, V. Characteristics and applications of poly(lactide). In Biopolymers: Biomedical and Environmental Applications; Kalia, S., Averous, L., Eds.; Wiley, New Jersey and Scrivener Publishers: Salem, MA, USA, 2011; Chapter 8; pp. 183–223. [Google Scholar]
- Saeidou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Suprakas, S.R. Polylactide-based bionanocomposites: A promising class of hybrid materials. Acc. Chem. Res. 2012, 45, 1710–1720. [Google Scholar]
- Pandey, J.K.; Kumar, A.P.; Misra, M.; Mohanty, A.K.; Drzal, L.T.; Singh, R.P. Recent advances in biodegradable nanocomposites. J. Nanosci. Nanotechnol. 2005, 5, 497–526. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Okamoto, M. Biodegradable Polylactide and Its Nanocomposites: Opening a New Dimension for Plastics and Composites. Macromol. Rapid Commun. 2003, 24, 815–840. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Ray, S.S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New polylactide/layered silicate nanocomposites. 1. Preparation, characterization, and properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar]
- Krikorian, V.D.; Pochan, J. Unusual crystallization behavior of organoclay reinforced poly(L-lactic acid) nanocomposites. Macromolecules 2004, 37, 6480–6491. [Google Scholar] [CrossRef]
- Krikorian, V.; Pochan, D.J. Poly (L-lactic acid)/layered silicate nanocomposite: Fabrication, characterization, and properties. Chem. Mater. 2003, 15, 4317–4324. [Google Scholar] [CrossRef]
- Pluta, M. Melt compounding of polylactide/organoclay: Structure and properties of nanocomposites. J. Polym. Sci. Pol. Phys. 2006, 44, 3392–3405. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Averous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Day, M.; Nawaby, A.V.; Liao, X. A DSC study of the crystallization behaviour of polylactic acid and its nanocomposites. J. Therm. Anal. Calorim. 2006, 86, 623–629. [Google Scholar] [CrossRef]
- Montava-Jorda, S.; Chacon, V.; Lascano, D.; Sanchez-Nacher, L.; Montanes, N. Manufacturing and characterization of functionalized aliphatic polyester from poly(lactic acid) with halloysite nanotubes. Polymers 2019, 11, 1314. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, Y.; Yang, J.; Li, X.; Yang, X.; Sasikumar, Y.; Zhou, Z.; Chen, M. Fabrication, crystalline behavior, mechanical property and in-vivo degradation of poly(L-lactide) (PLLA)–magnesium oxide whiskers (MgO) nano composites prepared by in-situ polymerization. Polymers 2019, 11, 1123. [Google Scholar] [CrossRef]
- Kalb, B.; Pennings, A.J. General crystallization behavior of poly(L-lactic acid). Polymer 1980, 21, 607–612. [Google Scholar] [CrossRef]
- Mano, J.F.; Wang, Y.M.; Viana, J.C.; Denchev, Z.; Oliveira, M. Cold crystallization of PLLA studied by simultaneous SAXS and WAXS. J. Macromol. Mater. Eng. 2004, 289, 910. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Sakamo, K.; Ono, Y.; Kawahara, W. Melting behavior of poly(L-lactic acid): X-ray and DSC analyses of the melting process. Polymer 2008, 49, 1943–1951. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemicals reactions. In Aktuelle Probleme der Polymer-Physik IV; Aktuelle Probleme der Polymer-Physik; Springer: Munster, Germany, 1973; Volume 251, pp. 980–990. [Google Scholar]
- Tsuji, H.; Takai, H.; Saha, S.K. Isothermal and non-isothermal crystallization behavior of poly(L-lactic acid): Effects of stereocomplex as nucleating agent. Polymer 2006, 47, 3826–3837. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Yeh, J.-T. Crystallization behavior of fully biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. J. Appl. Polym. Sci. 2009, 112, 3754–3763. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Achilias, D.S.; Nanaki, S.; Beslikas, T.; Bikiaris, D. PLA nanocomposites: Effect of filler type on non-isothermal crystallization. Thermochim. Acta 2010, 511, 129–139. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Non-isothermal cold-crystallization behavior and kinetics of poly(L-lactic acid)/WS2 inorganic nanotube nanocomposites. Polymers 2015, 7, 2175–2189. [Google Scholar] [CrossRef]
- Lule, Z.; Kim, J. Nonisothermal crystallization of surface-treated alumina and aluminum nitride-filled polylactic acid hybrid composites. Polymers 2019, 11, 1077. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, D.; Guo, H.; Zhao, J.; Wang, Z.; Hu, H.; Xu, J.; Fu, C. Functionalized boron nitride nanosheets/poly(L-lactide) nanocomposites and their crystallization behavior. Polymers 2019, 11, 440. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchel, J.W. Effects of inorganic fillers on the thermal and mechanical properties of poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Nam, J.Y.; Ray, S.S.; Okamoto, M. Crystallization behavior and morphology of biodegradable polylactide/layered silicate nanocomposite. Macromolecules 2003, 36, 7126–7131. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Wu, L.; Xu, B.; Zhang, Y.; Zhang, M. Nonisothermal cold crystallization behavior and kinetics of polylactide/clay nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1100–1113. [Google Scholar] [CrossRef]
- Ublekov, F.; Baldrian, J.; Kratochvil, J.; Steinhart, M.; Nedkov, E. Influence of clay content on the melting behavior and crystal structure of nonisothermal crystallized poly(L-lactic acid)/nanocomposites. J. Appl. Polym. Sci. 2012, 124, 1643–1648. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Arena, M.; Gennari, M.; Camino, G. Effect of clay type and loading on thermal, mechanical properties and biodegradation of poly(lactic acid) nanocomposites. React. Funct. Polym. 2013, 73, 540–549. [Google Scholar] [CrossRef]
- Ouchiar, S.; Stoclet, G.; Cabaret, C.; Gloaguen, V. Influence of the filler nature on the crystalline structure of polylactide-based nanocomposites: New insights into the nucleating effect. Macromolecules 2016, 49, 2782–2790. [Google Scholar] [CrossRef]
- Zhao, H.; Bian, Y.; Xu, M.; Han, C.; Li, Y.; Dong, Q.; Dong, L. Enhancing the crystallization of poly(l-lactide) using a montmorillonitic substrate favoring nucleation. CrystEngComm 2014, 16, 3896–3905. [Google Scholar] [CrossRef]
- Picard, E.; Espuche, E.; Fulchiron, R. Effect of an organo-modified montmorillonite on PLA crystallization and gas barrier properties. Appl. Clay Sci. 2011, 53, 58–65. [Google Scholar] [CrossRef]
- Di, Y.; Iannace, S.; Di Maio, E.; Nicolais, L. Poly(lactic acid)/organoclay nanocomposites: Thermal, rheological properties and foam processing. J. Polym. Sci. Part B Polym. Phys. 2004, 43, 689–698. [Google Scholar] [CrossRef]
- Fernández, M.J.; Fernández, M.D.; Aranburu, I. Poly(L-lactic acid)/organically modified vermiculite nanocomposites prepared by melt compounding: Effect of clay modification on microstructure and thermal properties. Eur. Polym. J. 2013, 49, 1257–1267. [Google Scholar] [CrossRef]
- Fernández, M.D.; Fernández, M.J. Vermiculite/poly(lactic acid) composites: Effect of nature of vermiculite on hydrolytic degradation in alkaline medium. Appl. Clay Sci. 2017, 143, 29–38. [Google Scholar] [CrossRef]
- Fernández, M.J.; Fernández, M.D.; Aranburu, I. Effect of clay surface modification and organoclay purity on microstructure and thermal properties of poly(l-lactic acid)/vermiculite nanocomposites. Appl. Clay Sci. 2013, 80, 372–381. [Google Scholar] [CrossRef]
- Khanna, Y.P. A barometer of crystallization rates of polymeric materials. Polym. Eng. Sci. 1990, 30, 1615–1619. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, H.; Lou, X.; Ma, D. Crystallization characteristics of polypropylene and low ethylene content polypropylene copolymer with and without nucleating agents. J. Appl. Polym. Sci. 1994, 51, 51–56. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change I. General theory. J. Chem. Phys. 1939, 7, 1103. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. J. Chem. Phys. 1940, 8, 212. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Inst. Stand. Technol. 1956, 57, 217. [Google Scholar] [CrossRef]
- Takhor, R.L. Advances in Nucleation and Crystallization of Glasses; American Ceramics Society: Columbus, OH, USA, 1971; pp. 166–172. [Google Scholar]
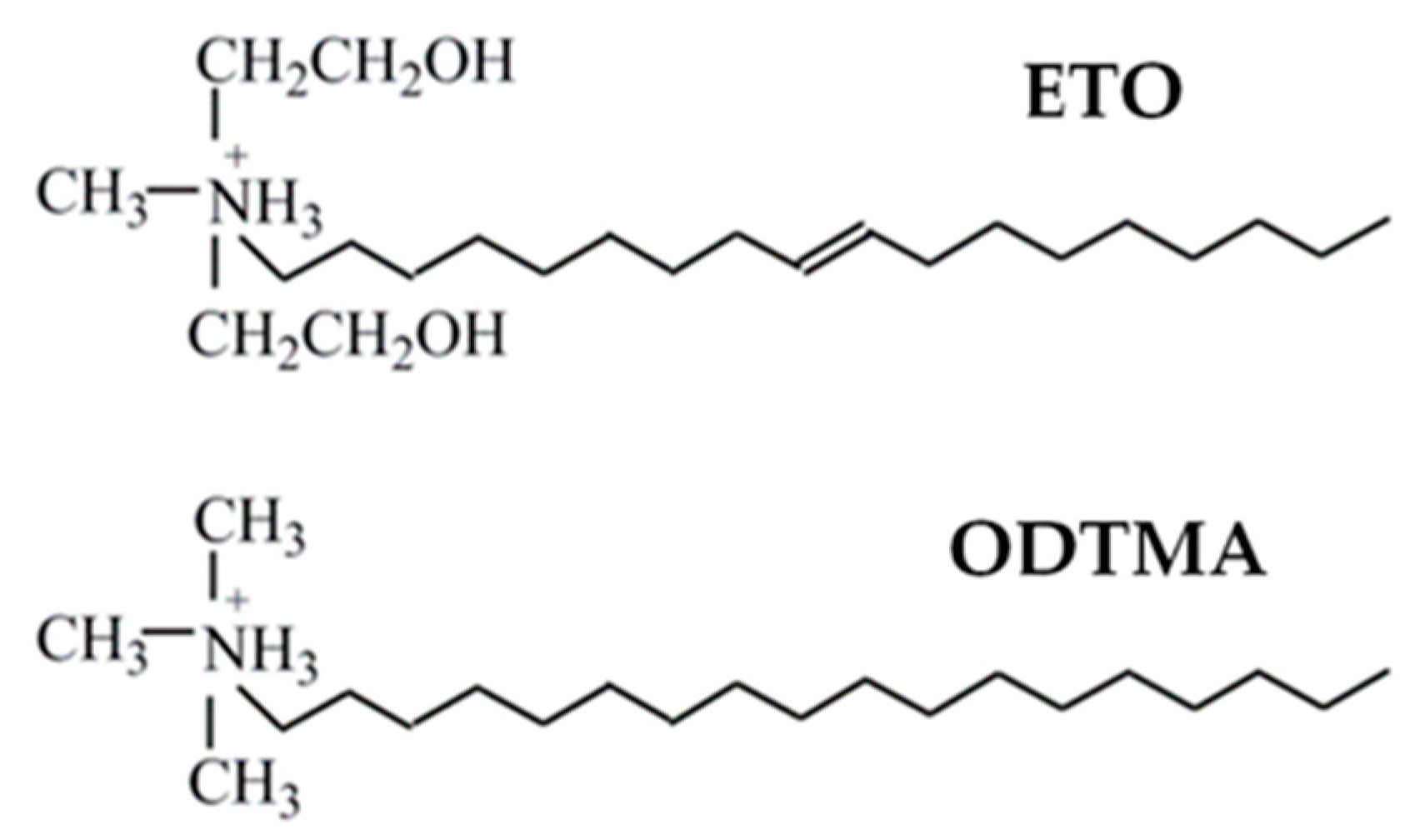


| Clay Code | Organic Modifier | Organic Content (%) | Sample Code | wt % of VMT |
|---|---|---|---|---|
| In Nanocomposites | ||||
| ETOVMT | ETO | 28.1 | PLA/ETOVMT-2 | 2 |
| PLA/ETOVMT-5 | 5 | |||
| C18VMT | ODTMA | 4.5 | PLA/C18VMT-2 | 2 |
| PLA/C18VMT-5 | 5 |
| Sample | ϕ (°C·min−1) | Tg (°C) | Tco (°C) | Tcc (°C) | ∆Hcc (J·g−1) | Xcc (%) | Tm1 (°C) | Tm2 (°C) | ∆Hm (J·g−1) | Xm (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PLA | 2 | 57.9 | 97.3 | 103.5 | 22.4 | 24.1 | 145.6 | 153.4 | 23.2 | 24.9 |
| 5 | 59.9 | 107.0 | 116.1 | 17.4 | 18.7 | 147.7 | 153.5 | 18.2 | 19.6 | |
| 6 | 59.6 | 108.0 | 120.0 | 16.6 | 17.9 | 148.1 | 17.1 | 18.4 | ||
| 8 | 60.6 | 110.2 | 126.6 | 10.1 | 10.8 | 149.3 | 11.3 | 12.1 | ||
| PLA/ETOVMT-2 | 2 | 57.4 | 94.7 | 99.2 | 22.9 | 24.5 | 144.9 | 153.1 | 24.3 | 26.6 |
| 5 | 58.9 | 102.8 | 109.3 | 16.3 | 17.2 | 146.1 | 153.1 | 17.0 | 18.7 | |
| 6 | 59.5 | 104.7 | 112.2 | 16.2 | 17.1 | 146.5 | 152.9 | 16.9 | 18.5 | |
| 8 | 59.1 | 107.6 | 117.3 | 16.1 | 16.9 | 147.2 | 152.3 | 17.1 | 18.7 | |
| PLA/ETOVMT-5 | 2 | 57.0 | 92.7 | 96. 8 | 18.6 | 19.0 | 142.9 | 151.5 | 21.8 | 24.7 |
| 5 | 58.7 | 100.6 | 106.4 | 13.5 | 13.8 | 144.7 | 152.7 | 14.3 | 16.2 | |
| 6 | 58.3 | 101.9 | 108.2 | 13.9 | 14.2 | 144.7 | 152.4 | 14.9 | 16.9 | |
| 8 | 58.4 | 104.3 | 112.0 | 13.7 | 13.9 | 145.1 | 152.2 | 14.4 | 16.3 | |
| PLA/C18VMT-2 | 2 | 57.9 | 96.3 | 101.1 | 24. 8 | 26.1 | 145.7 | 153.9 | 26.5 | 27.9 |
| 5 | 58.8 | 104.0 | 112.3 | 16.4 | 17.3 | 147.1 | 153.9 | 17.3 | 18.2 | |
| 6 | 58.7 | 107.0 | 115.9 | 15.5 | 16.3 | 147.6 | 153.8 | 16.2 | 17.1 | |
| 8 | 58.9 | 110.1 | 122.3 | 15.1 | 15.9 | 149.1 | 15.5 | 16.3 | ||
| PLA C18VMT-5 | 2 | 57.4 | 96.6 | 101.6 | 20.7 | 21.1 | 144. 9 | 153.2 | 22.8 | 23.3 |
| 5 | 59.3 | 104.3 | 111.9 | 15.1 | 15.5 | 147.2 | 153.9 | 16.7 | 17.0 | |
| 6 | 59.8 | 107.0 | 115.2 | 14.9 | 15.2 | 147.7 | 153.8 | 15.6 | 16.0 | |
| 8 | 58.4 | 110.1 | 122.3 | 15.1 | 12.5 | 149.0 | 13.6 | 13.91 |
| Sample | Xt (%) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
|---|---|---|---|---|---|---|---|---|---|---|
| PLA | α | 0.84 | 0.81 | 0.80 | 0.79 | 0.79 | 0.79 | 0.78 | 0.78 | 0.78 |
| F(T) | 2.10 | 2.57 | 2.89 | 3.15 | 3.38 | 3.61 | 3.84 | 4.12 | 4.51 | |
| r2 | 0.9889 | 0.9935 | 0.9953 | 0.9964 | 0.9969 | 0.9976 | 0.9983 | 0.9989 | 0.9995 | |
| PLA/ETOVMT-2 | α | 0.58 | 0.59 | 0. 60 | 0.60 | 0.61 | 0.61 | 0.61 | 0.62 | 0.62 |
| F(T) | 2.30 | 2.57 | 2.76 | 2.91 | 3.05 | 3.19 | 3.34 | 3.53 | 3.81 | |
| r2 | 0.9969 | 0.9968 | 0.9965 | 0.9965 | 0.9963 | 0.9962 | 0.9964 | 0.9963 | 0.9967 | |
| PLA/ETOVMT-5 | α | 0.55 | 0.59 | 0.60 | 0.57 | 0.58 | 0.58 | 0.61 | 0.62 | 0.59 |
| F(T) | 2.53 | 2.57 | 2.76 | 3.02 | 3.14 | 3.25 | 3.34 | 3.53 | 3.73 | |
| r2 | 0.9906 | 0.9968 | 0.9965 | 0.9959 | 0.9967 | 0.9971 | 0.9964 | 0.9963 | 0.9982 | |
| PLA/C18VMT-2 | α | 0.58 | 0.59 | 0.60 | 0.57 | 0.58 | 0.58 | 0.61 | 0.62 | 0.60 |
| F(T) | 2.69 | 2.57 | 2.75 | 3.26 | 3.41 | 3.55 | 3.34 | 3.53 | 4.11 | |
| r2 | 1 | 0.9968 | 0.9965 | 0.9934 | 0.9937 | 0.9935 | 0.9964 | 0.9963 | 0.9933 | |
| PLA/C18VMT-5 | α | 0.58 | 0.65 | 0.59 | 0.64 | 0.65 | 0.65 | 0.61 | 0.62 | 0.66 |
| F(T) | 2.69 | 2.70 | 2.76 | 3.08 | 3.24 | 3.40 | 3.34 | 3.53 | 4.02 | |
| r2 | 1 | 1 | 0.9965 | 0.9996 | 0.9998 | 0.9999 | 0.9964 | 0.9963 | 0.9997 |
| ∆E (kJ·mol−1) | ||
|---|---|---|
| Sample | Kissinger | Takhor |
| PLA | −69.7 | −76.3 |
| PLA/ETOVMT-2 | −87.8 | −94.2 |
| PLA/ETOVMT-5 | −102.5 | −108.8 |
| PLA/C18VMT-2 | −76.5 | −82.9 |
| PLA/C18VMT-5 | −110.1 | −83.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, M.J.; Fernández, M.D. Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites. Polymers 2020, 12, 364. https://doi.org/10.3390/polym12020364
Fernández MJ, Fernández MD. Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites. Polymers. 2020; 12(2):364. https://doi.org/10.3390/polym12020364
Chicago/Turabian StyleFernández, M. Jesús, and M. Dolores Fernández. 2020. "Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites" Polymers 12, no. 2: 364. https://doi.org/10.3390/polym12020364
APA StyleFernández, M. J., & Fernández, M. D. (2020). Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites. Polymers, 12(2), 364. https://doi.org/10.3390/polym12020364






