Atmospheric Pressure Microwave Plasma Jet for Organic Thin Film Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
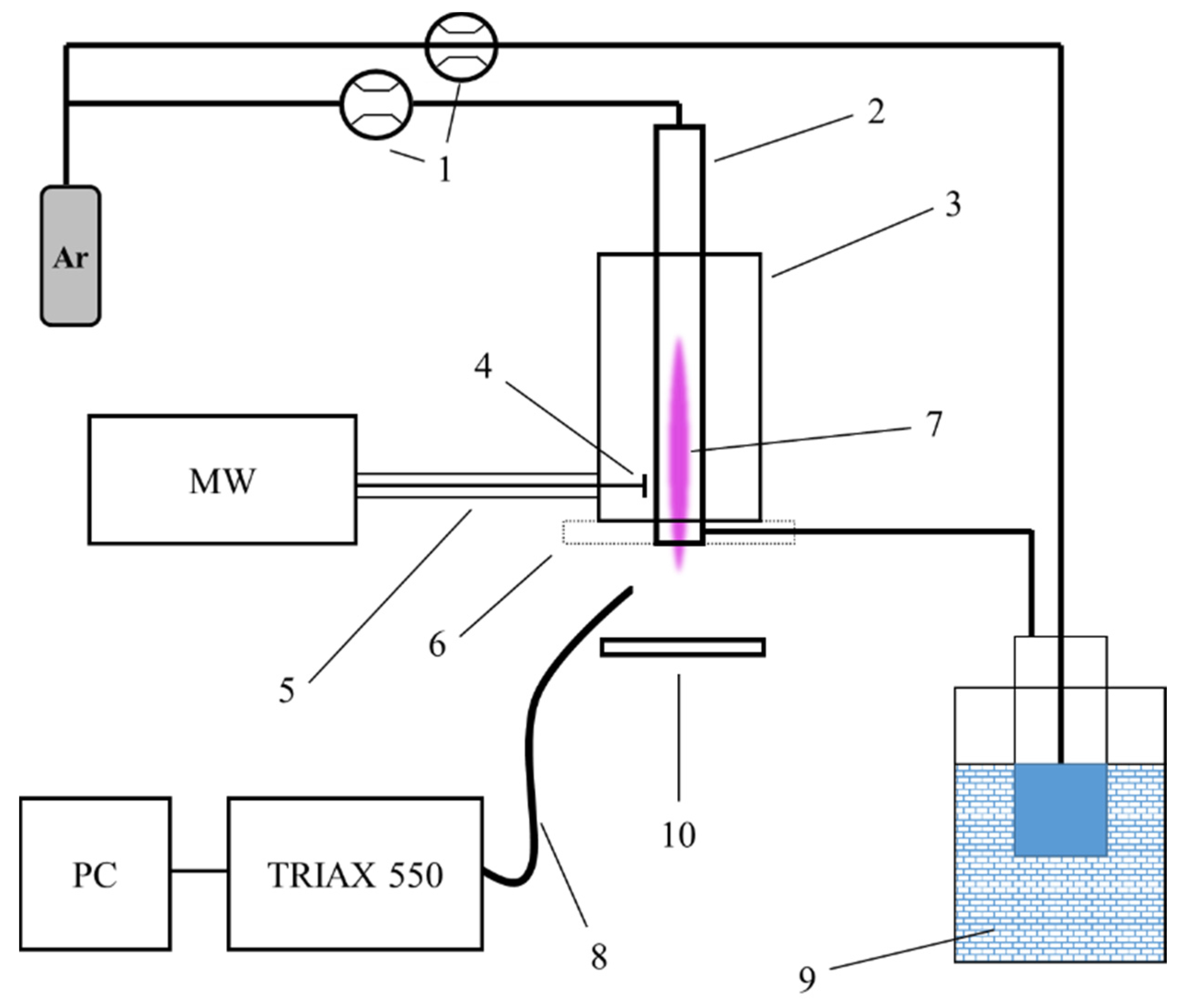
2.2. Experimental Plasma Set-Up
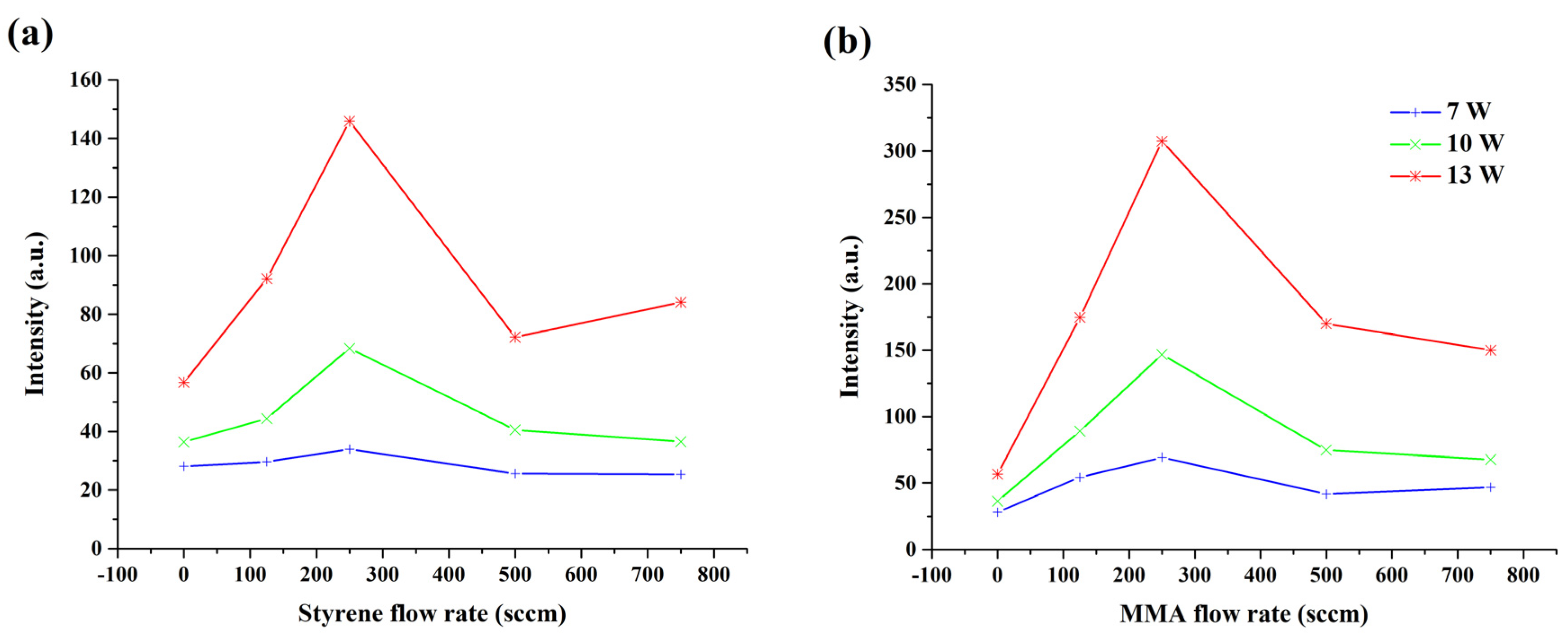
2.3. OES
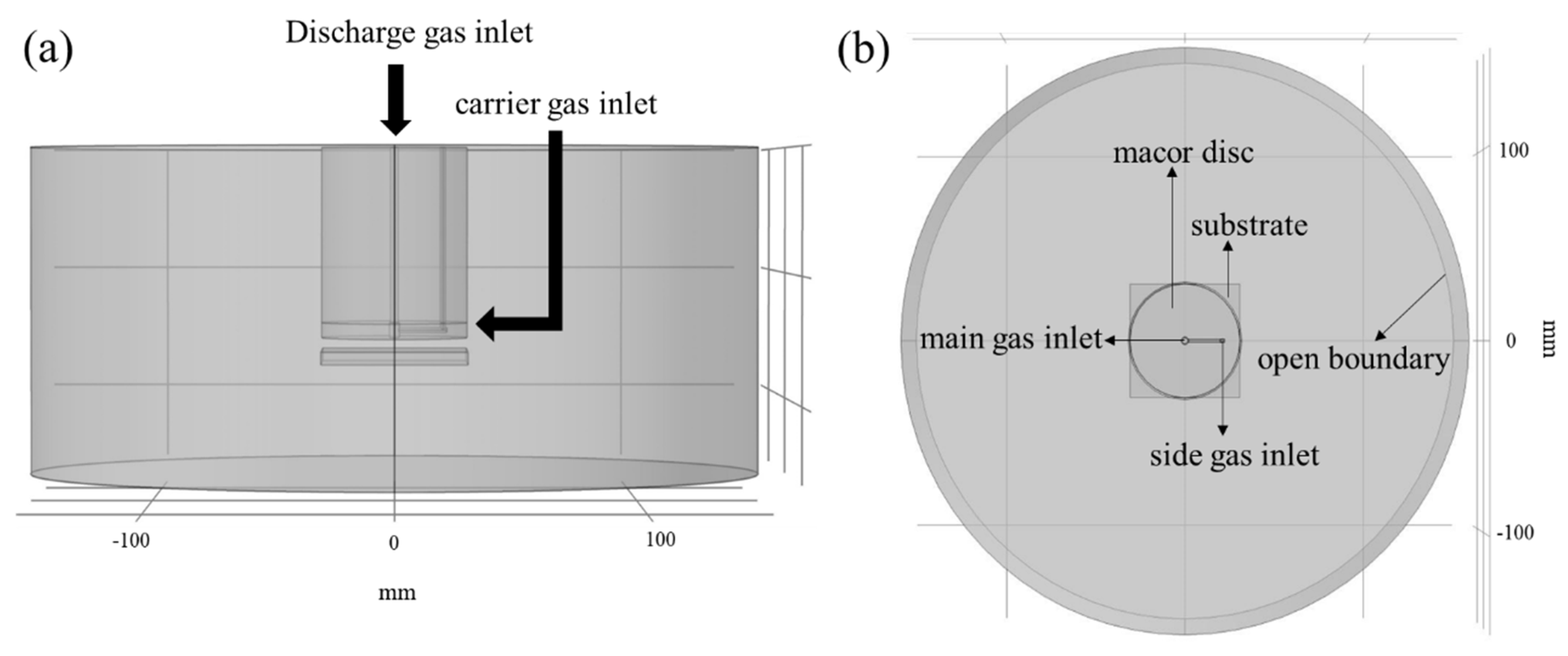
2.4. Computational Fluid Dynamic Simulations
2.5. AFM
2.6. SEM
2.7. Static WCA Analysis
2.8. XPS
3. Results and Discussion
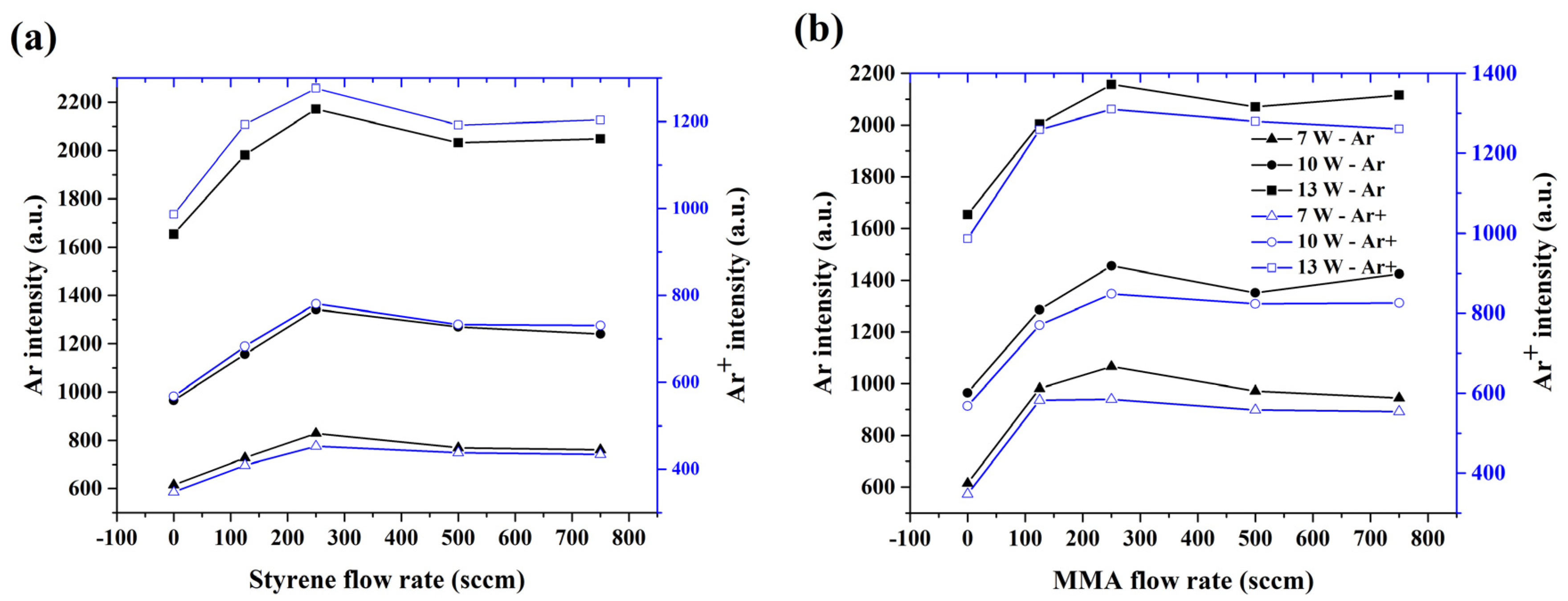
3.1. Discharge Characterization
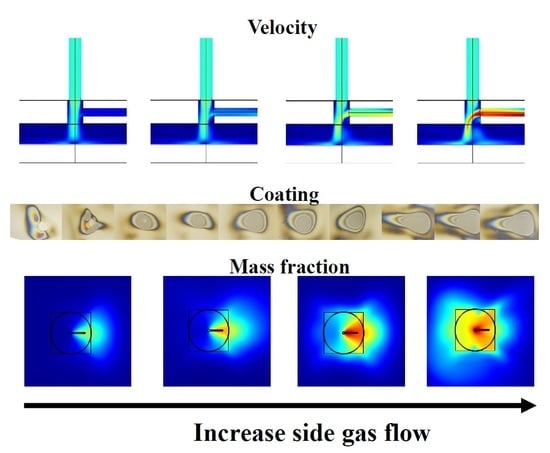
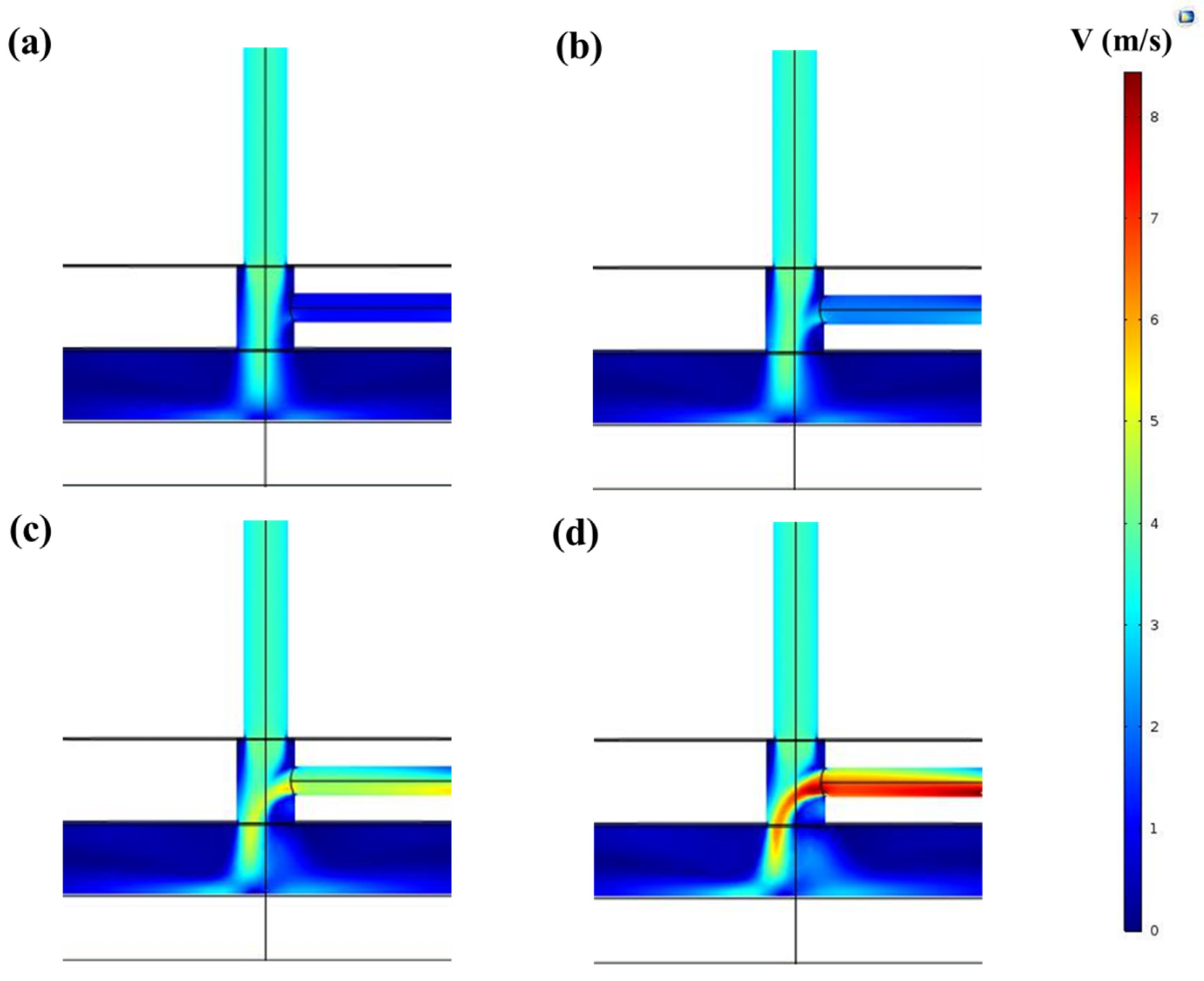
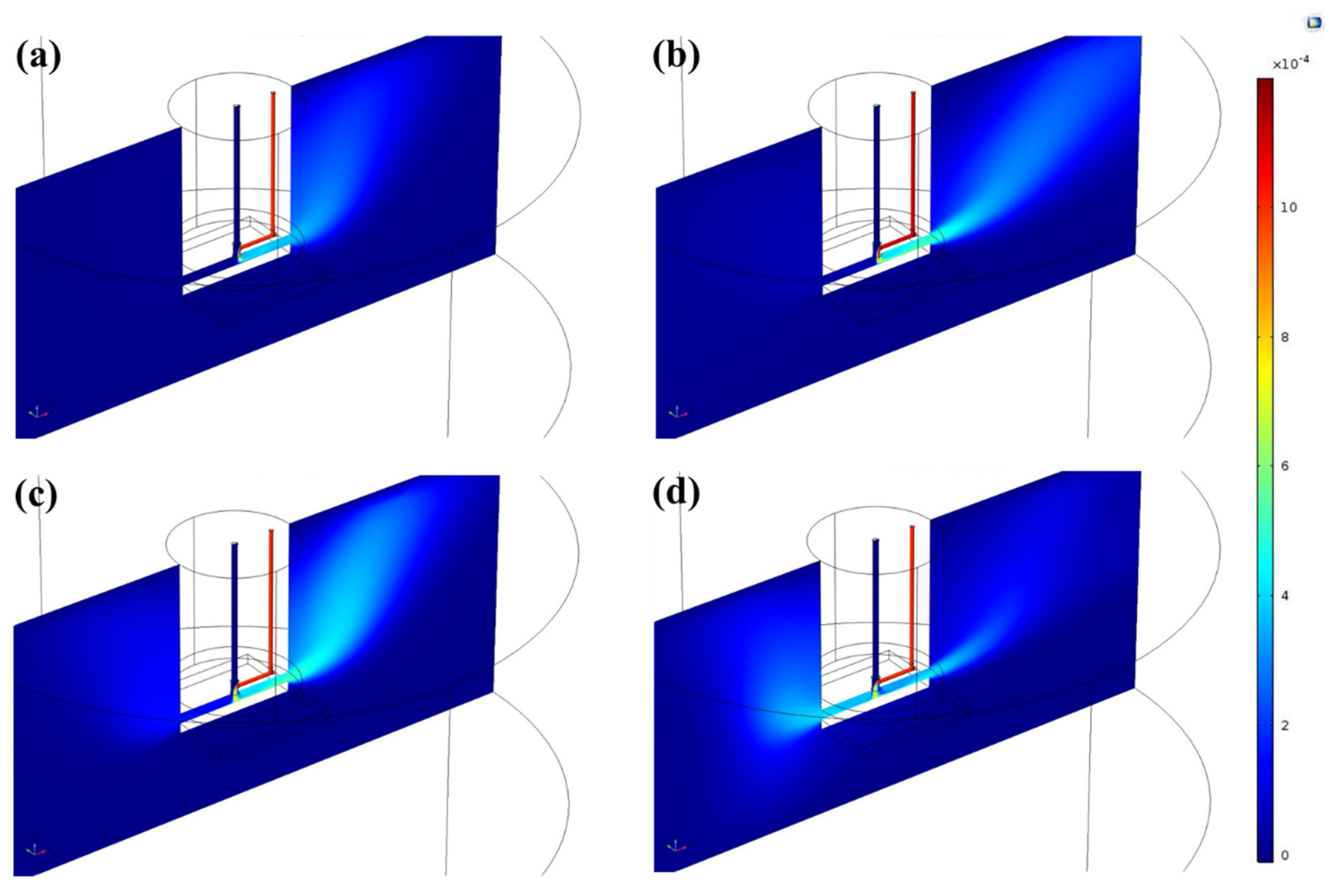
3.2. CFD Simulations
3.3. Physical and Chemical Surface Characterization
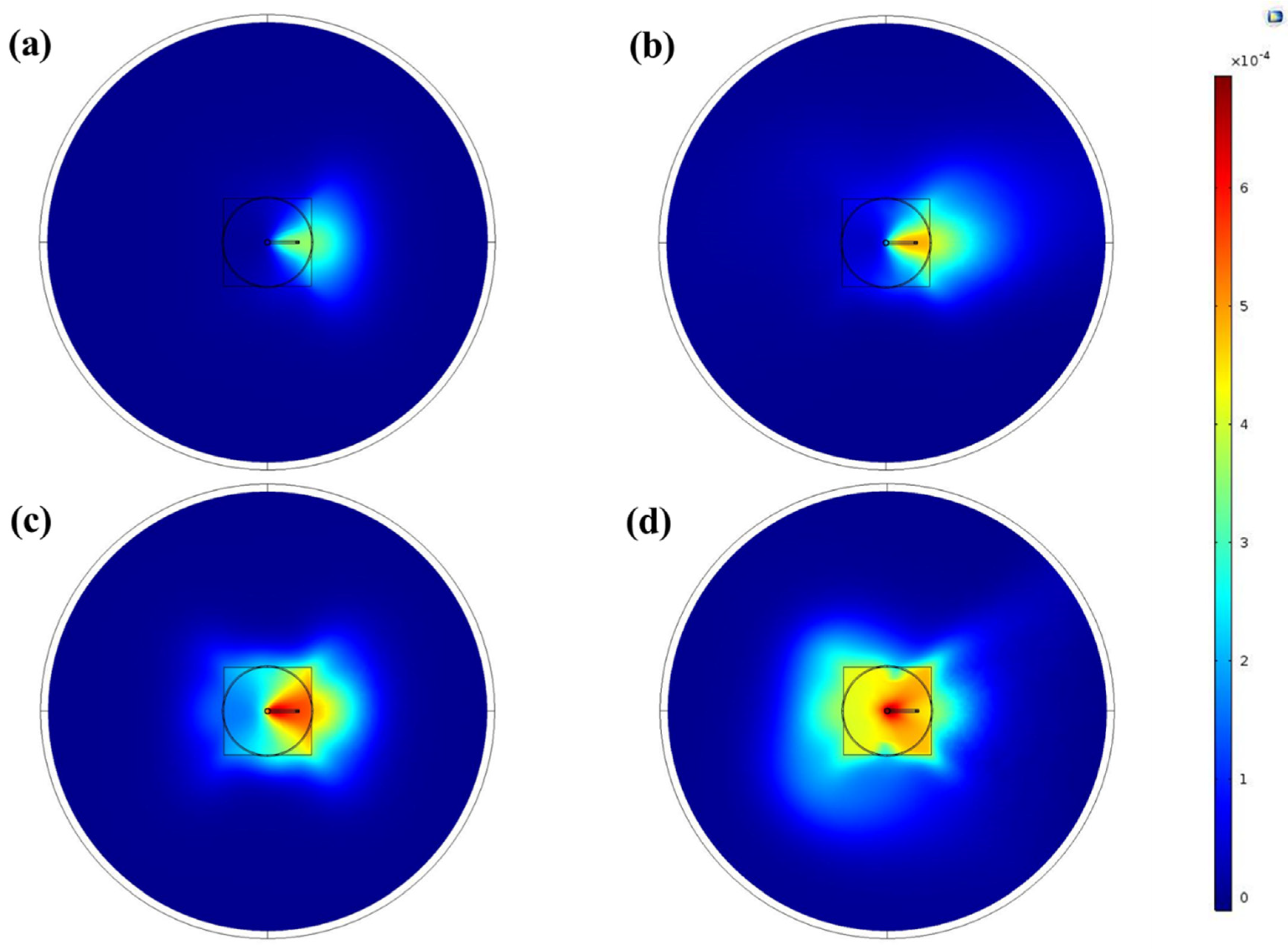
3.3.1. Coating Appearance and Homogeneity
3.3.2. Coating Morphology
3.3.3. Coating Thickness
3.3.4. Coating Roughness
3.3.5. Coating Wettability
3.3.6. Coating Chemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liston, E.; Martinu, L.; Wertheimer, M. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasma jet technology: General features, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Paunovic, M.; Schlesinger, M. Fundamentals of Electrochemical Eeposition; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Sherman, A. Chemical Vapor Deposition for Microelectronics: Principles, Technology, and Applications; Elsevier Science: Park Ridge, NJ, USA, 1987. [Google Scholar]
- Smith, D.L. Thin-Film Deposition: Principles and Practice; McGraw Hill Professional: New York, NY, USA, 1995. [Google Scholar]
- Kumar, V.; Kim, J.H.; Pendyala, C.; Chernomordik, B.; Sunkara, M.K. Gas-phase, bulk production of metal oxide nanowires and nanoparticles using a microwave plasma jet reactor. J. Phys. Chem. C 2008, 112, 17750–17754. [Google Scholar] [CrossRef]
- Li, S.-Z.; Hong, Y.C.; Uhm, H.S.; Li, Z.-K. Synthesis of nanocrystalline iron oxide particles by microwave plasma jet at atmospheric pressure. Jpn. J. Appl. Phys. 2004, 43, 7714. [Google Scholar] [CrossRef]
- Van Vrekhem, S.; Morent, R.; De Geyter, N. Deposition of a PMMA coating with an atmospheric pressure plasma jet. J. Coat. Technol. Res. 2018, 15, 679–690. [Google Scholar] [CrossRef]
- Sankaran, R.M.; Giapis, K.P. Hollow cathode sustained plasma microjets: Characterization and application to diamond deposition. J. Appl. Phys. 2002, 92, 2406–2411. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, J.; Foest, R.; Quade, A.; Ohl, A.; Weltmann, K. Local deposition of SiOx plasma polymer films by a miniaturized atmospheric pressure plasma jet (APPJ). J. Phys. D Appl. Phys. 2008, 41, 194010. [Google Scholar] [CrossRef] [Green Version]
- Carton, O.; Ben Salem, D.; Bhatt, S.; Pulpytel, J.; Arefi-Khonsari, F. Plasma polymerization of acrylic acid by atmospheric pressure nitrogen plasma jet for biomedical applications. Plasma Process. Polym. 2012, 9, 984–993. [Google Scholar] [CrossRef]
- Donegan, M.; Dowling, D.P. Protein adhesion on water stable atmospheric plasma deposited acrylic acid coatings. Surf. Coat. Technol. 2013, 234, 53–59. [Google Scholar] [CrossRef]
- Ben Salem, D.; Carton, O.; Fakhouri, H.; Pulpytel, J.; Arefi-Khonsari, F. Deposition of water stable plasma polymerized acrylic acid/MBA organic coatings by atmospheric pressure air plasma jet. Plasma Process. Polym. 2014, 11, 269–278. [Google Scholar] [CrossRef]
- Farhat, S.; Gilliam, M.; Rabago-Smith, M.; Baran, C.; Walter, N.; Zand, A. Polymer coatings for biomedical applications using atmospheric pressure plasma. Surf. Coat. Technol. 2014, 241, 123–129. [Google Scholar] [CrossRef]
- Horvath, G.; Moravsky, L.; Krcma, F.; Matejcik, Š. Characterization of a low-cost kilohertz-driven plasma pen operated in Ar gas. IEEE IEEE Trans. Plasma Sci. 2013, 41, 613–619. [Google Scholar] [CrossRef]
- Guaitella, O.; Sobota, A. The impingement of a kHz helium atmospheric pressure plasma jet on a dielectric surface. J. Phys. D Appl. Phys. 2015, 48, 255202. [Google Scholar] [CrossRef]
- Chen, X. Modelling of a radio-frequency plasma torch including a self-consistent electromagnetic field formulation. J. Phys. D Appl. Phys. 1989, 22, 361. [Google Scholar] [CrossRef]
- van der Mullen, J.; Jonkers, J. Fundamental comparison between non-equilibrium aspects of ICP and MIP discharges. Spectrochim. Acta Part B 1999, 54, 1017–1044. [Google Scholar] [CrossRef]
- Krčma, F.; Tsonev, I.; Smejkalová, K.; Truchlá, D.; Kozáková, Z.; Zhekova, M.; Marinova, P.; Bogdanov, T.; Benova, E. Microwave micro torch generated in argon based mixtures for biomedical applications. J. Phys. D Appl. Phys. 2018, 51, 414001. [Google Scholar] [CrossRef]
- Wang, C.; Srivastava, N. OH number densities and plasma jet behavior in atmospheric microwave plasma jets operating with different plasma gases (Ar, Ar/N2, and Ar/O2). Eur. Phys. J. D 2010, 60, 465–477. [Google Scholar] [CrossRef]
- Tsonev, I.; Atanasov, N.; Atanasova, G.; Krčma, F.; Bogdanov, T. Atmospheric Pressure Microwave Plasma Torch for Biomedical Applications. Plasma Med. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, J.; Hnilica, J.; Šperka, J.; Quade, A.; Kudrle, V.; Foest, R.; Vodák, J.; Zajίčková, L. Tetrakis (trimethylsilyloxy) silane for nanostructured SiO2-like films deposited by PECVD at atmospheric pressure. Surf. Coat. Technol. 2016, 295, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Ting, J.A.S.; Rosario, L.M.D.; Lee, H.V., Jr.; Ramos, H.J.; Tumlos, R.B. Hydrophobic coating on glass surfaces via application of silicone oil and activated using a microwave atmospheric plasma jet. Surf. Coat. Technol. 2014, 259, 7–11. [Google Scholar] [CrossRef]
- Choudhury, A.; Kakati, H.; Pal, A.; Patil, D.; Chutia, J. Synthesis and characterization of plasma polymerized styrene films by rf discharge. Proc. J. Phys. Conf. Ser. 2010, 208, 012104. [Google Scholar] [CrossRef]
- Kinzig, B.; Smardzewski, R. Plasma-polymerized thin coatings from methyl-methacrylate, styrene and tetrafluoroethylene. Surf. Technol. 1981, 14, 3–16. [Google Scholar] [CrossRef]
- Geckeler, K.; Gebhardt, R.; Grünwald, H. Surface modification of polyethylene by plasma grafting with styrene for enhanced biocompatibility. Naturwissenschaften 1997, 84, 150–151. [Google Scholar] [CrossRef]
- Morita, S.; Tamano, J.; Hattori, S.; Ieda, M. Plasma polymerized methyl-methacrylate as an electron-beam resist. J. Appl. Phys. 1980, 51, 3938–3941. [Google Scholar] [CrossRef]
- Bogdanov, T.; Tsonev, I.; Marinova, P.; Benova, E.; Rusanov, K.; Rusanova, M.; Atanassov, I.; Kozáková, Z.; Krčma, F. Microwave plasma torch generated in argon for small berries surface treatment. Appl. Sci. 2018, 8, 1870. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-K.; Huang, J.-C.; Chen, Y.-C.; Lee, M.-T.; Juang, J.-Y. Deposition of highly transparent and conductive Ga-doped zinc oxide films on tilted substrates by atmospheric pressure plasma jet. J. Alloys Compd. 2019, 802, 458–466. [Google Scholar] [CrossRef]
- Belmonte, T.; Henrion, G.; Gries, T. Nonequilibrium atmospheric plasma deposition. J. Therm. Spray Technol. 2011, 20, 744. [Google Scholar] [CrossRef]
- Obrusník, A.; Jelínek, P.; Zajíčková, L. Modelling of the gas flow and plasma co-polymerization of two monomers in an atmospheric-pressure dielectric barrier discharge. Surf. Coat. Technol. 2017, 314, 139–147. [Google Scholar] [CrossRef]
- Yusoff, H.M.; Abrahamson, J.; Shastry, R. Influence of anode surface temperature in a continuously-fed arc discharge depositing carbon nanotubes. In Proceedings of the 2006 International Conference on Nanoscience and Nanotechnology, Brisbane, Australia, 3–7 July 2006. [Google Scholar]
- Juang, J.-Y.; Lin, H.-T.; Liang, C.-T.; Li, P.-R.; Chen, W.-K.; Chen, Y.-Y.; Pan, K.-L. Effect of ambient air flow on resistivity uniformity of transparent Ga-doped ZnO film deposited by atmospheric pressure plasma jet. J. Alloys Compd. 2018, 766, 868–875. [Google Scholar] [CrossRef]
- Onyshchenko, I.; De Geyter, N.; Morent, R. Improvement of the plasma treatment effect on PET with a newly designed atmospheric pressure plasma jet. Plasma Process. Polym. 2017, 14, 1600200. [Google Scholar] [CrossRef]
- COMSOL Multiphysics Reference Guide; COMSOL AB: Stockholm, Sweden, 2011.
- Kramida, A.; Ralchenko, Y.; Reader, J. Team 2018 NIST Atomic Spectra Database (ver. 5.5. 3); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Gilmore, F.R.; Laher, R.R.; Espy, P.J. Franck–Condon factors, r-centroids, electronic transition moments, and Einstein coefficients for many nitrogen and oxygen band systems. J. Phys. Chem. Ref. Data 1992, 21, 1005–1107. [Google Scholar] [CrossRef] [Green Version]
- Cools, P.; Sainz-García, E.; Geyter, N.D.; Nikiforov, A.; Blajan, M.; Shimizu, K.; Alba-Elías, F.; Leys, C.; Morent, R. Influence of DBD Inlet Geometry on the Homogeneity of Plasma-Polymerized Acrylic Acid Films: The Use of a Microplasma–Electrode Inlet Configuration. Plasma Process. Polym. 2015, 12, 1153–1163. [Google Scholar] [CrossRef]
- Yasuda, H. Glow discharge polymerization. J. Polym. Sci. Macromol. Rev. 1981, 16, 199–293. [Google Scholar] [CrossRef]
- Thompson, L.; Mayhan, K. The plasma polymerization of vinyl monomers. II. A detailed study of the plasma polymerization of styrene. J. Appl. Polym. Sci. 1972, 16, 2317–2341. [Google Scholar] [CrossRef]
- Mackie, N.M.; Castner, D.G.; Fisher, E.R. Characterization of pulsed-plasma-polymerized aromatic films. Langmuir 1998, 14, 1227–1235. [Google Scholar] [CrossRef]
- Liguori, A.; Pollicino, A.; Stancampiano, A.; Tarterini, F.; Focarete, M.L.; Colombo, V.; Gherardi, M. Deposition of Plasma-Polymerized Polyacrylic Acid Coatings by a Non-Equilibrium Atmospheric Pressure Nanopulsed Plasma Jet. Plasma Process. Polym. 2016, 13, 375–386. [Google Scholar] [CrossRef]
- Dong, B.; Manolache, S.; Somers, E.B.; Lee Wong, A.C.; Denes, F.S. Generation of antifouling layers on stainless steel surfaces by plasma-enhanced crosslinking of polyethylene glycol. J. Appl. Polym. Sci. 2005, 97, 485–497. [Google Scholar] [CrossRef]
- France, R.M.; Short, R.D. Plasma treatment of polymers: The effects of energy transfer from an argon plasma on the surface chemistry of polystyrene, and polypropylene. A high-energy resolution X-ray photoelectron spectroscopy study. Langmuir 1998, 14, 4827–4835. [Google Scholar] [CrossRef]
- Noeske, M.; Degenhardt, J.; Strudthoff, S.; Lommatzsch, U. Plasma jet treatment of five polymers at atmospheric pressure: Surface modifications and the relevance for adhesion. Int. J. Adhes. Adhes. 2004, 24, 171–177. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: Hoboken, NJ, USA, 1992. [Google Scholar]
- Casserly, T.B.; Gleason, K.K. Effect of substrate temperature on the plasma polymerization of poly (methyl methacrylate). Chem. Vap. Depos. 2006, 12, 59–66. [Google Scholar] [CrossRef]
- Merche, D.; Poleunis, C.; Bertrand, P.; Sferrazza, M.; Reniers, F. Synthesis of polystyrene thin films by means of an atmospheric-pressure plasma torch and a dielectric barrier discharge. IEEE Trans. Plasma Sci. 2009, 37, 951–960. [Google Scholar] [CrossRef]

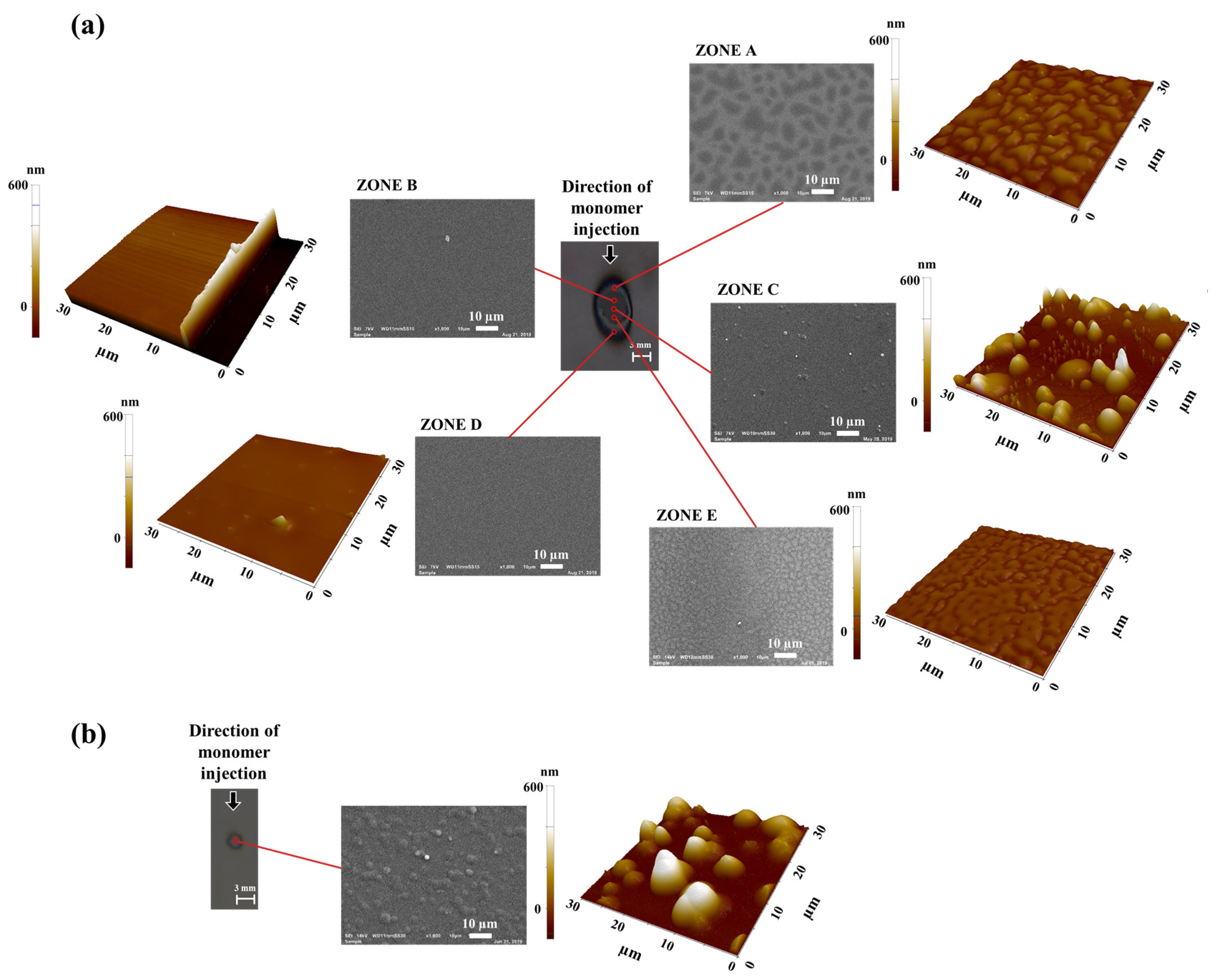
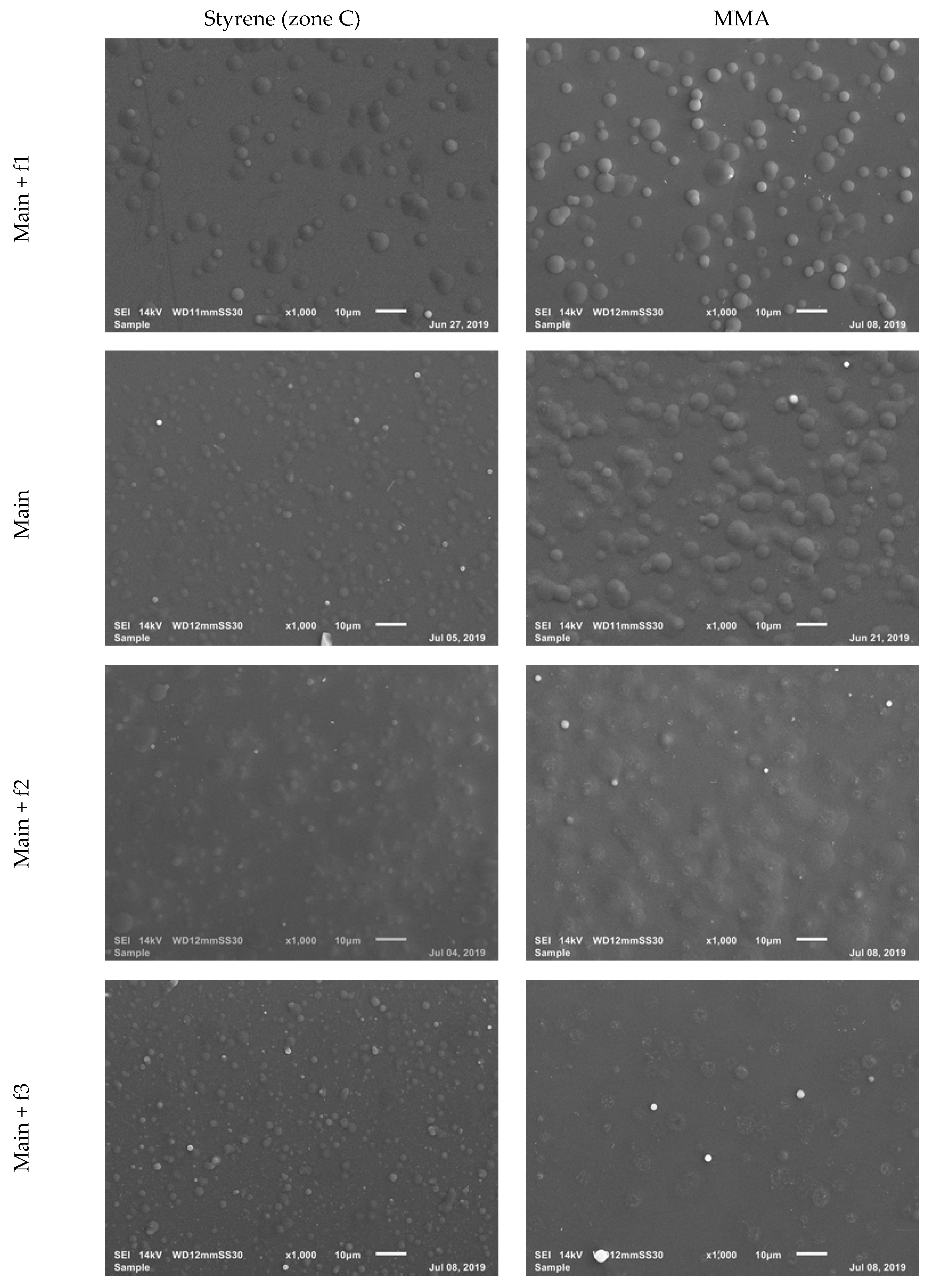
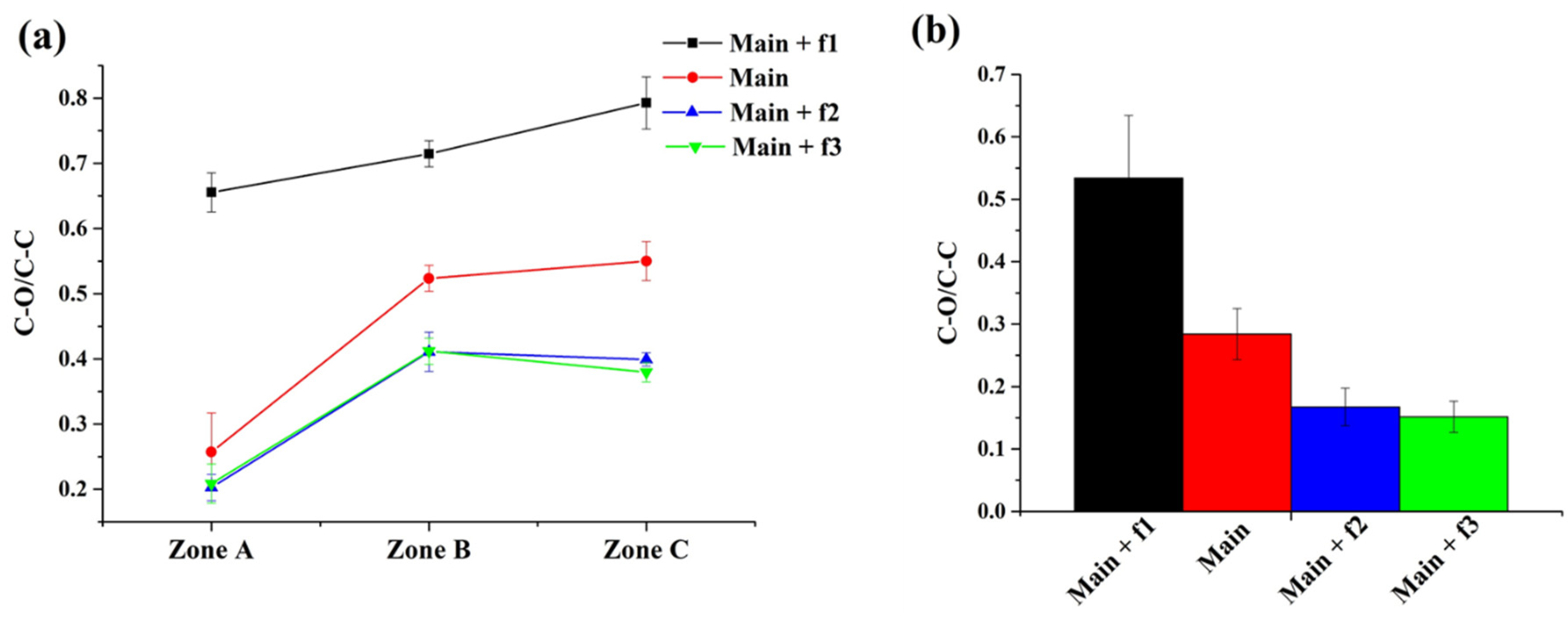
| Sample | Power (W) | Time (s) | Distance to Substrate (mm) | Carrier Gas Flow Rate (sccm) |
|---|---|---|---|---|
| Main | 10 | 3 | 5 | 250 |
| Main + t1 | 10 | 1 | 5 | 250 |
| Main + t2 | 10 | 5 | 5 | 250 |
| Main + p1 | 7 | 3 | 5 | 250 |
| Main + p2 | 13 | 3 | 5 | 250 |
| Main + f1 | 10 | 3 | 5 | 125 |
| Main + f2 | 10 | 3 | 5 | 500 |
| Main + f3 | 10 | 3 | 5 | 750 |
| Main + d1 | 10 | 3 | 3 | 250 |
| Main + d2 | 10 | 3 | 7 | 250 |
| Band/Line | Transition | Wavelength (nm) | Integration Time (s) |
|---|---|---|---|
| OH | A-X, band 0-0 | 305.0–310.0 | 0.1 |
| N2 2nd positive | C-B, bands 0-0, 2-4, 1-3, 0-2 | 337.1, 370.9, 375.4, 380.4 | 0.1 |
| N | 4P3/2-4P5/2 | 824.2 | 1 |
| Ar+ | 2D3/2-2D3/2 | 404.3 | 1 |
| Ar | 2[3/2]-2[3/2]° | 714.7 | 0.1 |
| C | 1P°-1S | 247.9 | 0.1 |
| CH | 2Δ-2Π, band 0-0 | 431.3 | 1 |
| O | 5P-5S° | 777.0 | 0.01 |
| Condition | Main | Main + t1 | Main + t2 | Main + p1 | Main + p2 | Main + f1 | Main + f2 | Main + f3 | Main + d1 | Main + d2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Thickness (nm) | 229 ± 8 | 58 ± 3 | 486 ± 12 | 210 ± 5 | 217 ± 6 | 110 ± 5 | 220 ± 4 | 190 ± 10 | 140 ± 6 | 248 ± 8 |
| Condition/Roughness (nm) | Main | Main + t1 | Main + t2 | Main + p1 | Main + p2 | Main + f1 | Main + f2 | Main + f3 | Main + d1 | Main + d2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Styrene (zone A) | 22 ± 2 | 23 ± 3 | 38 ± 5 | 17 ± 2 | 29 ± 4 | 22 ± 3 | 41 ± 2 | 45 ± 4 | 19 ± 2 | 20 ± 3 |
| Styrene (zone B) | 20 ± 2 | 14 ± 2 | 30 ± 4 | 18 ± 3 | 25 ± 5 | 15 ± 3 | 29 ± 6 | 32 ± 6 | 19 ± 3 | 20 ± 3 |
| Styrene (zone C) | 128 ± 6 | 63 ± 7 | 173 ±5 | 75 ± 8 | 149 ± 10 | 64 ± 4 | 79 ± 5 | 102 ± 8 | 125 ± 5 | 136 ± 8 |
| MMA | 137 ± 7 | 110 ± 10 | 156 ± 11 | 122 ± 10 | 185 ± 9 | 236 ± 12 | 110 ± 9 | 54 ± 5 | 122 ± 6 | 136 ± 8 |
| Condition | Zone A | Zone B | Zone C | MMA |
|---|---|---|---|---|
| Main | 45.0 ± 2.0 | 34.2 ± 1.8 | 28.2 ± 3.1 | 26.5 ± 3.1 |
| Main + t1 | 41.3 ± 3.5 | 31.0 ± 1.5 | 22.3 ± 2.3 | 23.7 ± 3.5 |
| Main + t2 | 45.7 ± 2.1 | 36.5 ± 2.6 | 31.1 ± 2.7 | 30.8 ± 2.8 |
| Main + p1 | 35.3 ± 3.2 | 20.5 ± 2.4 | 18.0 ± 3.3 | 24.4 ± 3.1 |
| Main + p2 | 50.5 ± 3.5 | 36.3 ± 2.4 | 32.4 ± 3.6 | 35.4 ± 2.9 |
| Main + f1 | 18.0 ± 1.9 | 12.1 ± 1.8 | 8.1 ± 2.1 | 10.8 ± 1.9 |
| Main + f2 | 55.4 ± 2.5 | 45.8 ± 1.2 | 30.0 ± 2.2 | 37.6 ± 2.0 |
| Main + f3 | 61.6 ± 2.4 | 52.9 ± 1.0 | 35.2 ± 1.7 | 62.0 ± 1.7 |
| Main + d1 | 45.0 ± 3.6 | 36.1 ± 2.0 | 23.0 ± 3.0 | 28.5 ± 3.2 |
| Main + d2 | 42.5 ± 2.5 | 33.6 ± 1.5 | 27.3 ± 2.3 | 38.0 ± 2.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narimisa, M.; Krčma, F.; Onyshchenko, Y.; Kozáková, Z.; Morent, R.; De Geyter, N. Atmospheric Pressure Microwave Plasma Jet for Organic Thin Film Deposition. Polymers 2020, 12, 354. https://doi.org/10.3390/polym12020354
Narimisa M, Krčma F, Onyshchenko Y, Kozáková Z, Morent R, De Geyter N. Atmospheric Pressure Microwave Plasma Jet for Organic Thin Film Deposition. Polymers. 2020; 12(2):354. https://doi.org/10.3390/polym12020354
Chicago/Turabian StyleNarimisa, Mehrnoush, František Krčma, Yuliia Onyshchenko, Zdenka Kozáková, Rino Morent, and Nathalie De Geyter. 2020. "Atmospheric Pressure Microwave Plasma Jet for Organic Thin Film Deposition" Polymers 12, no. 2: 354. https://doi.org/10.3390/polym12020354
APA StyleNarimisa, M., Krčma, F., Onyshchenko, Y., Kozáková, Z., Morent, R., & De Geyter, N. (2020). Atmospheric Pressure Microwave Plasma Jet for Organic Thin Film Deposition. Polymers, 12(2), 354. https://doi.org/10.3390/polym12020354