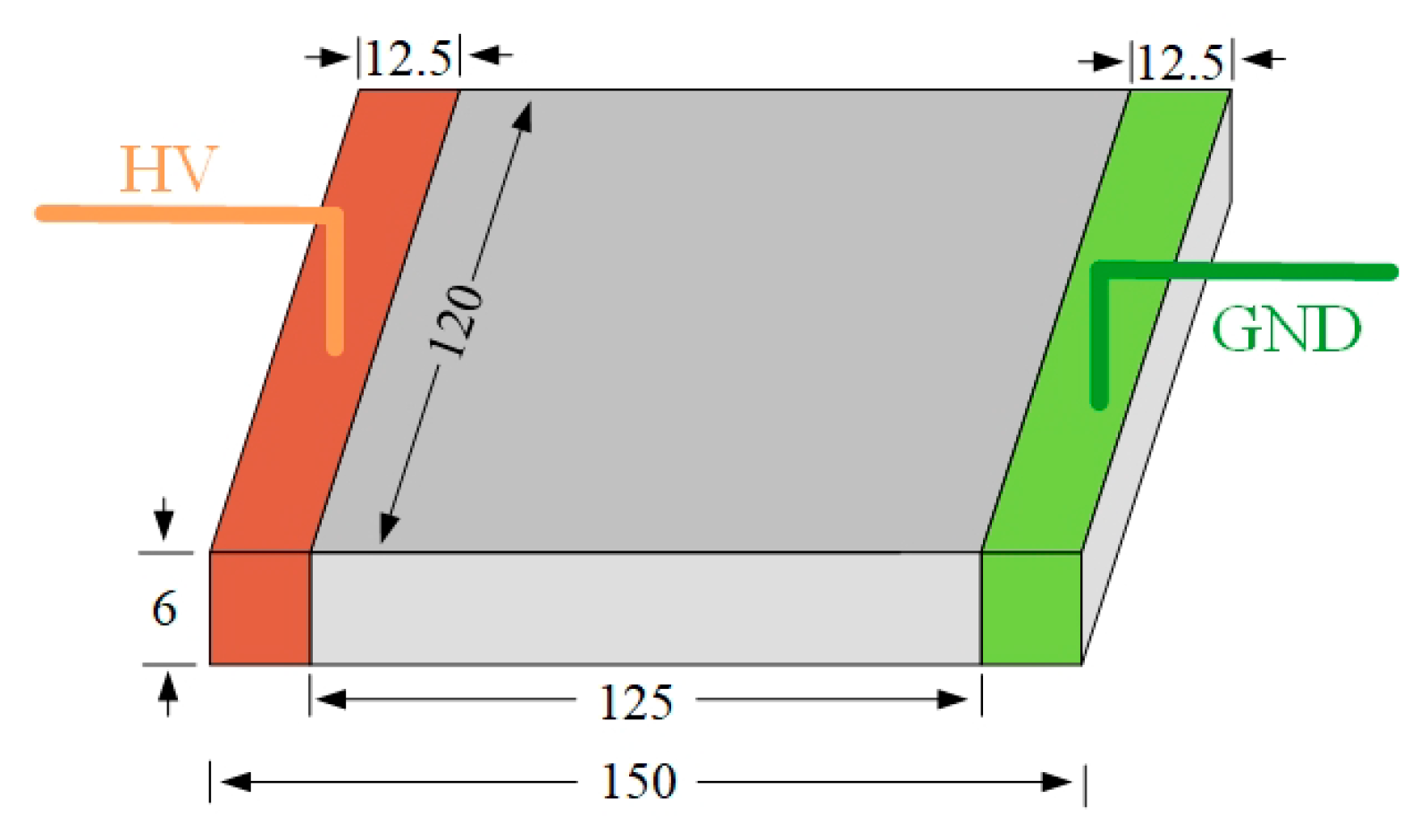
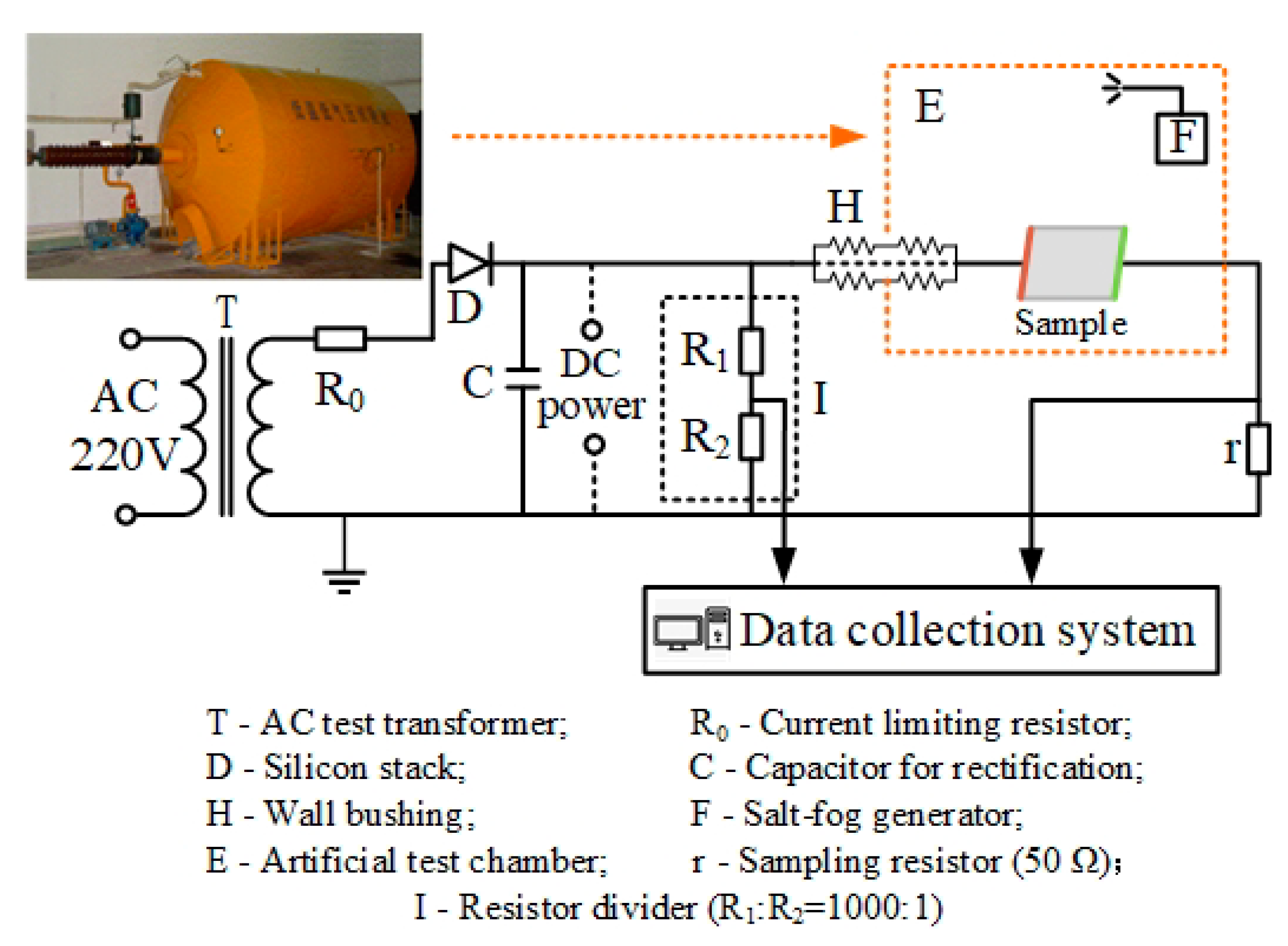
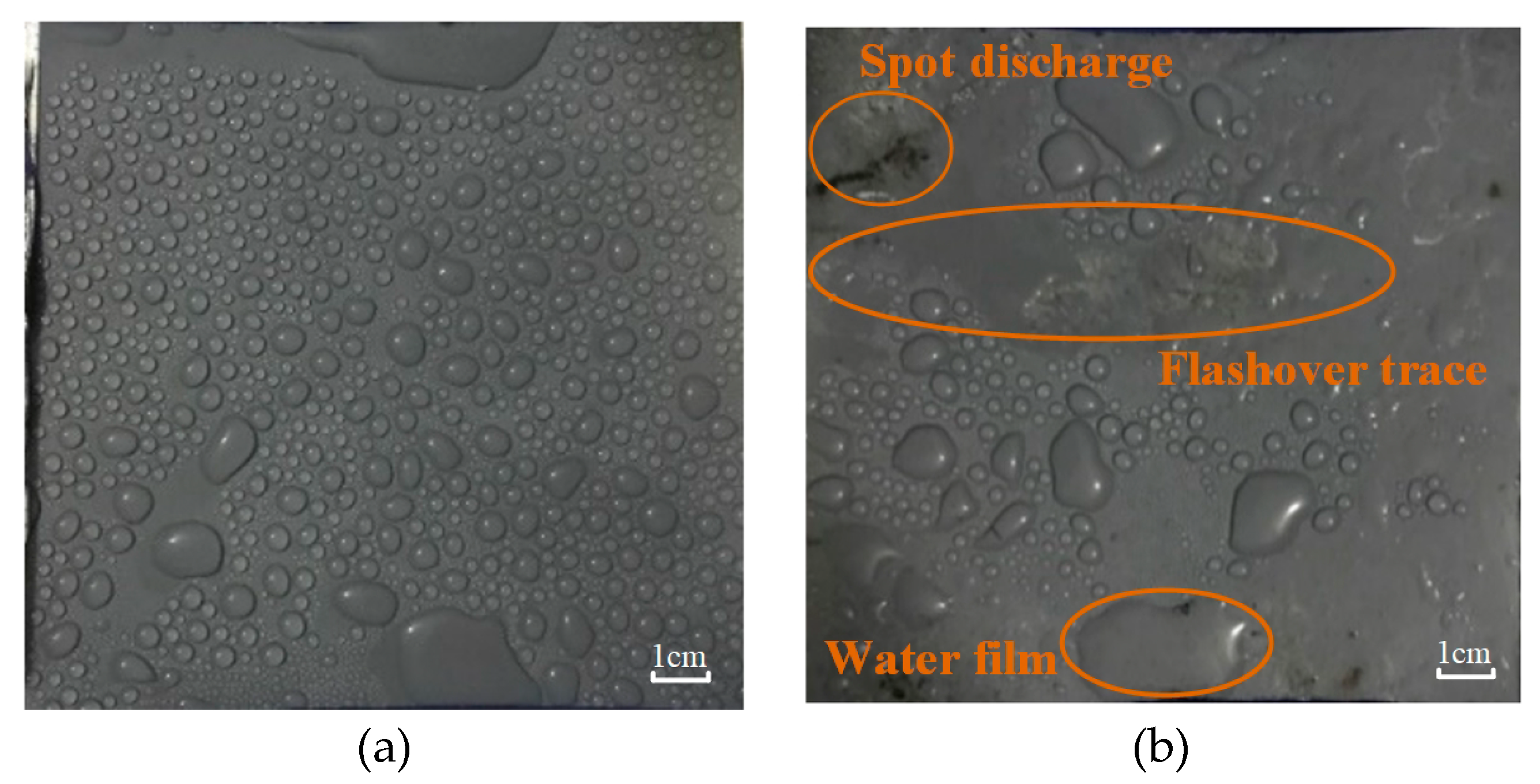
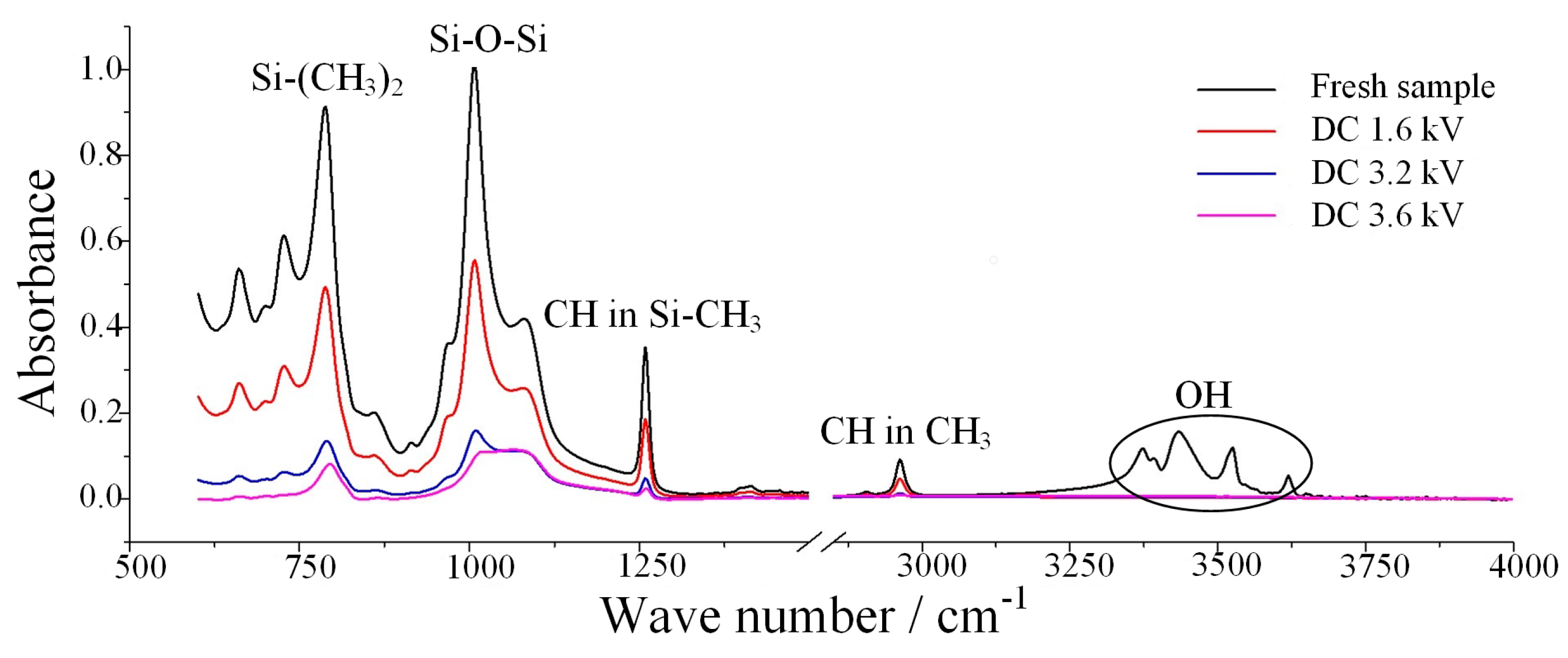
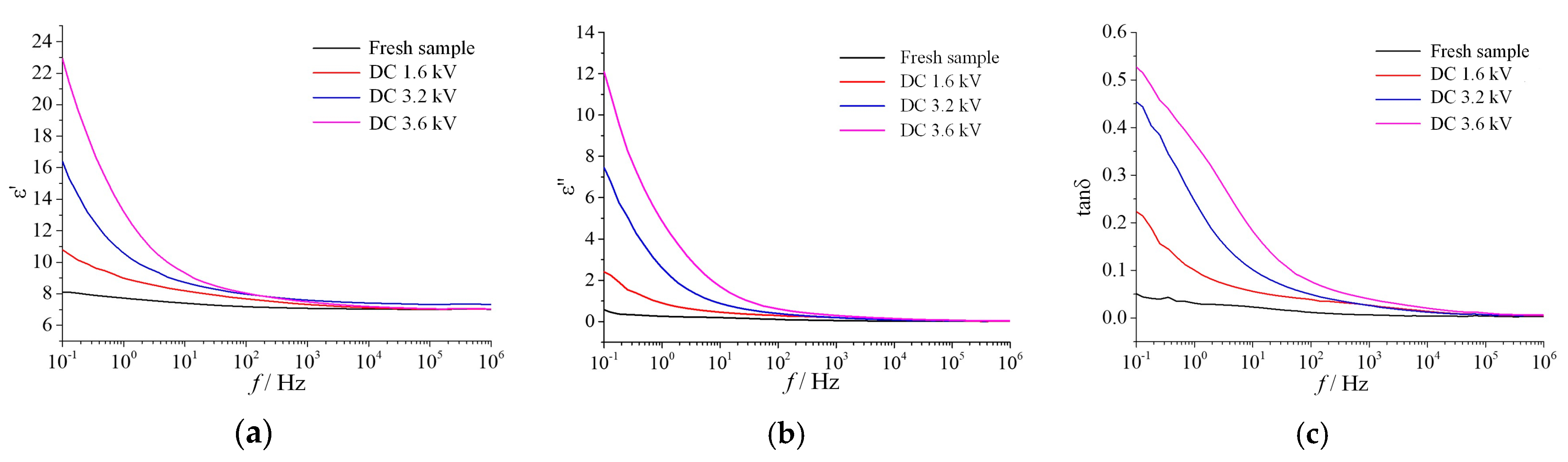
3.1.1. Fog Water Conductivity
The electrical strength of silicone rubber was investigated according to the aforementioned test procedure. The DC flashover voltage results of samples with different fog water conductivity rates and different duration times are shown in
Table 1, the applied voltage was 3.2 kV.
It can be concluded from
Table 1 that the fog water conductivity rate has a significant effect on flashover voltage reduction. For instance, for samples energized for 8 h,
Uf were 21.3 kV, 11.4 kV, 8.7 kV, and 7.4 kV when γ
20 was 100 μS/cm, 1000 μS/cm, 3000 μS/cm, and 5000 μS/cm, respectively, which indicates that the
Uf decreases by 46.48%, 59.15%, and 65.26%. The reason may be that with the increase of fog water conductivity, the deposition of conductive substances on the silicone rubber surface increases, causing the arc to burn more intensely, then resulting in a decrease in the flashover voltage.
Numerous studies have found that the relationship between the DC pollution flashover voltage and
SDD can be expressed as follows [
29,
30,
31]:
where
A is the coefficient related to the insulator material, structure, and power type;
SDD is the salt deposit density (mg/cm
2),
n is the effect coefficient of
SDD on flashover voltage, while the relationship between
SDD and γ
20 can be expressed as [
32]:
where γ
20 is the volume conductivity at the temperature of 20 °C (S/m),
V is the volume of suspension (cm
3),
S is the area of sample surface (cm
2).
For the salt-fog experiment, the volume of salt-fog generated by spray system and the sample surface area are both constant in a certain period of time. Therefore, referring to Equation (4), the relationship between the flashover voltage
Uf and γ
20 in the salt-fog environment can be expressed as follows:
where
B is the coefficient related to the silicone rubber material, structure, and power type; γ
20 is the fog water conductivity (μS/cm);
m is the effect coefficient of γ
20 on flashover voltage.
Fitting the test results in
Table 1 with Equation (6), the fitted curves and the value of parameters
B and
m are shown in
Figure 3 and
Table 2. It can be seen from the fitting results that the DC flashover voltage of SR samples energized in a salt-fog environment for a period of time decreases exponentially with the increase of fog conductivity.
Table 3 shows the variation of discharge characteristics in different salt-fog conductivity conditions when the applied voltage was 3.2 kV and the duration time was 8 h.
From the leakage current data, it can be seen that during a complete aging test, the maximum pulse (Imax), and discharge amount (Q) of the leakage current on the sample surface increased significantly with the increase of fog water conductivity. For example, when γ20 was 1000 μS/cm, during the test, Imax was 0.989 mA and Q was 0.136 C, while when γ20 increased to 5000 μS/cm, Imax was 7.836 mA and Q was 5.793 C, which increased by 6.92 times and 41.60 times, respectively, indicating that the salt-fog environment with higher fog water conductivity leads to a more intense discharge.
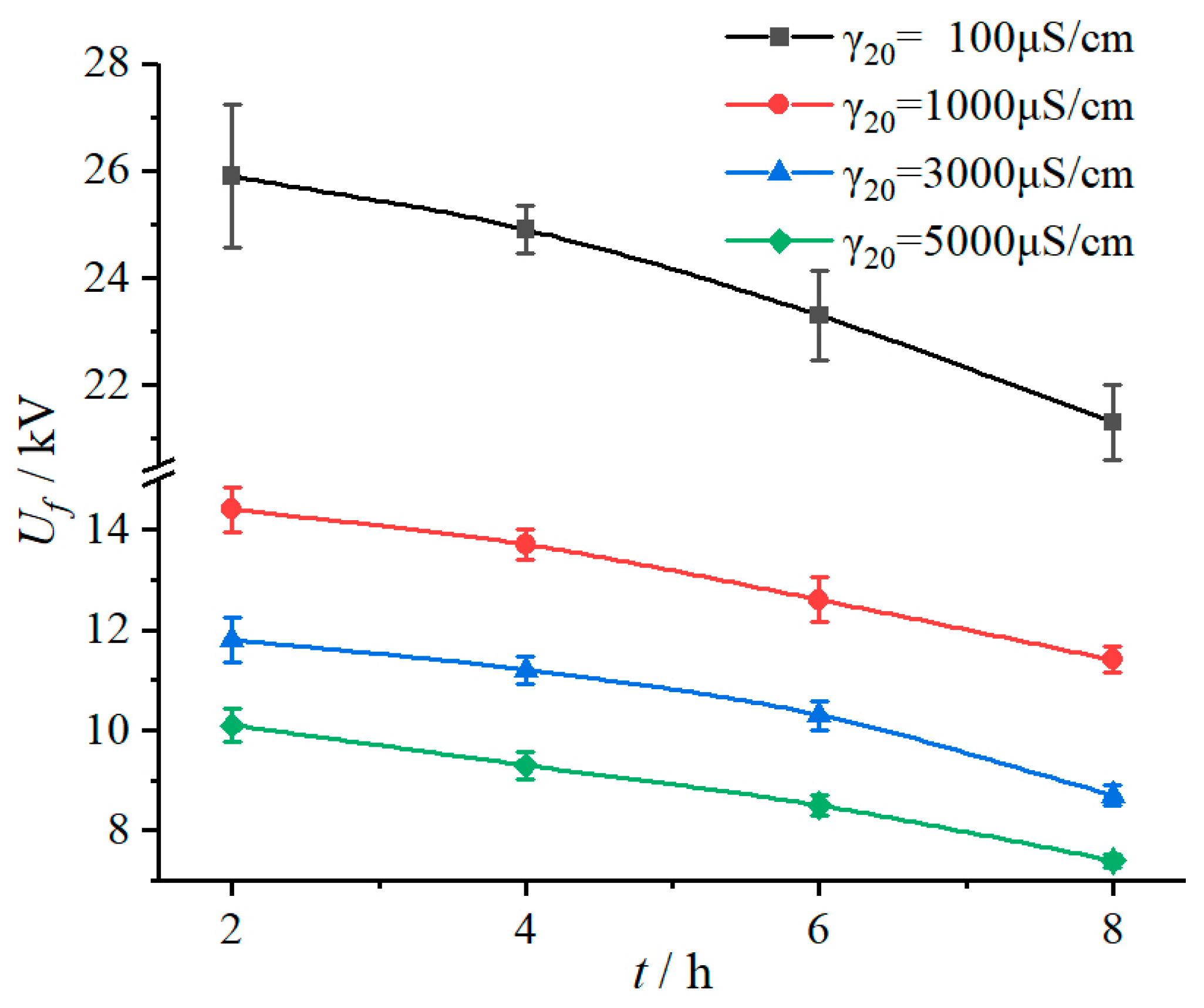
3.1.2. Duration Time of Applied Voltage
According to the test results in
Table 1, under the same fog water conductivity environment, flashover voltage (
Uf) declined with increase of duration time of applied voltage (
t), as shown in
Figure 4. In addition, it can be seen from the fitting results in
Table 2 that with the extension of duration time, the influence coefficient characteristic exponent m increased. For example, when the duration time increased from 2 h to 4 h, 6 h, and 8 h, the
m value increased by 2.87%, 5.41%, and 11.58%, respectively, which gives a sign that the fog water conductivity has a greater influence on flashover voltage drop with the duration time increases.
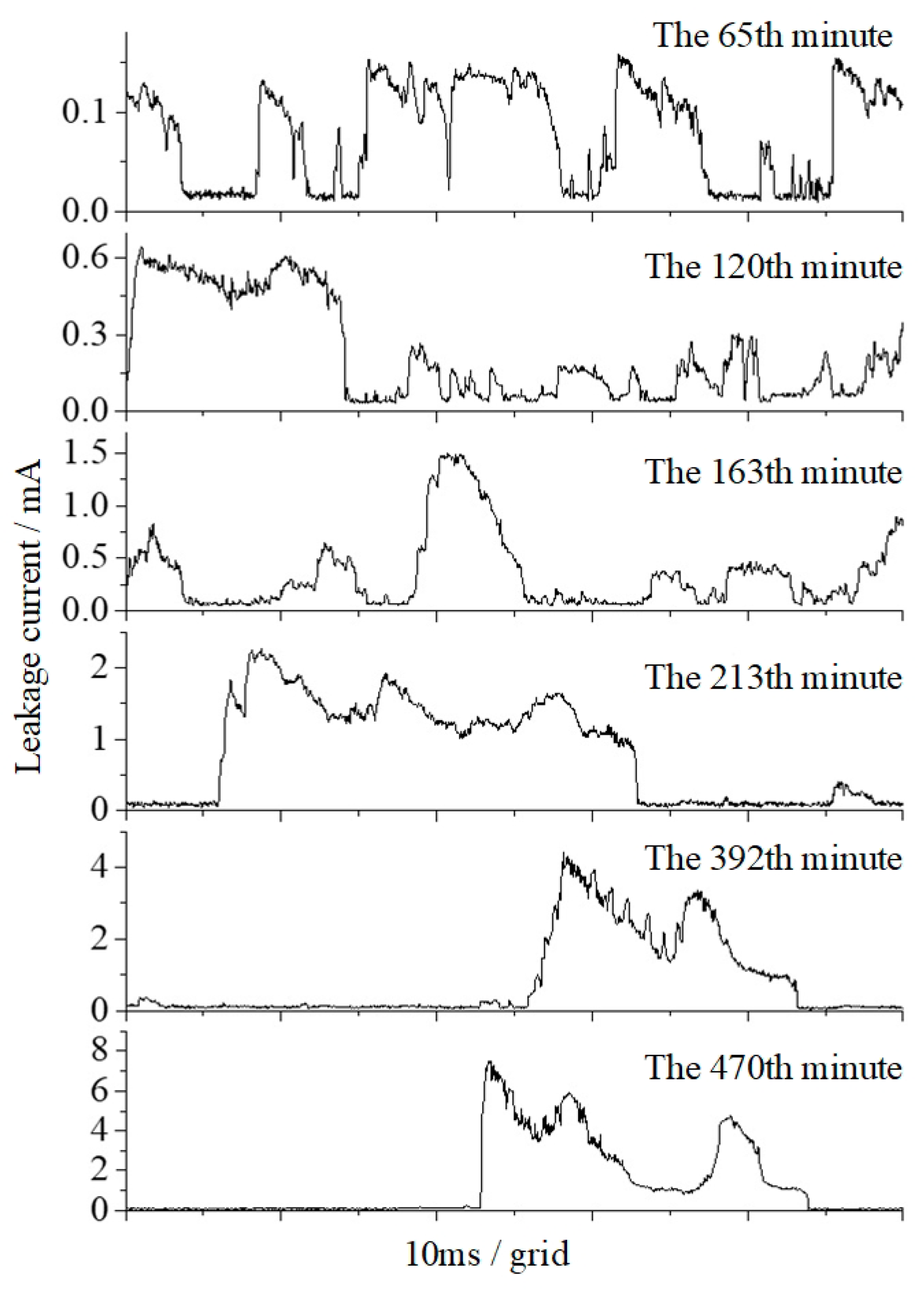
Table 4 shows the specific values of the leakage current characteristics of different time periods during the 8 h test in the salt-fog condition, γ
20 is 5000 μS/cm and
Ua is 3.2 kV. The current waveform also indicates that the discharge degree was gradually getting stronger, as shown in
Figure 5. To be specific, in the first two hours of the test, the maximum pulse amplitude and accumulated discharge amount were 0.682 mA and 0.476 C, which increased to 7.836 mA and 2.646 C in the last two hours, increasing by 10.49 times and 4.56 times, respectively.
3.1.3. Applied Voltage
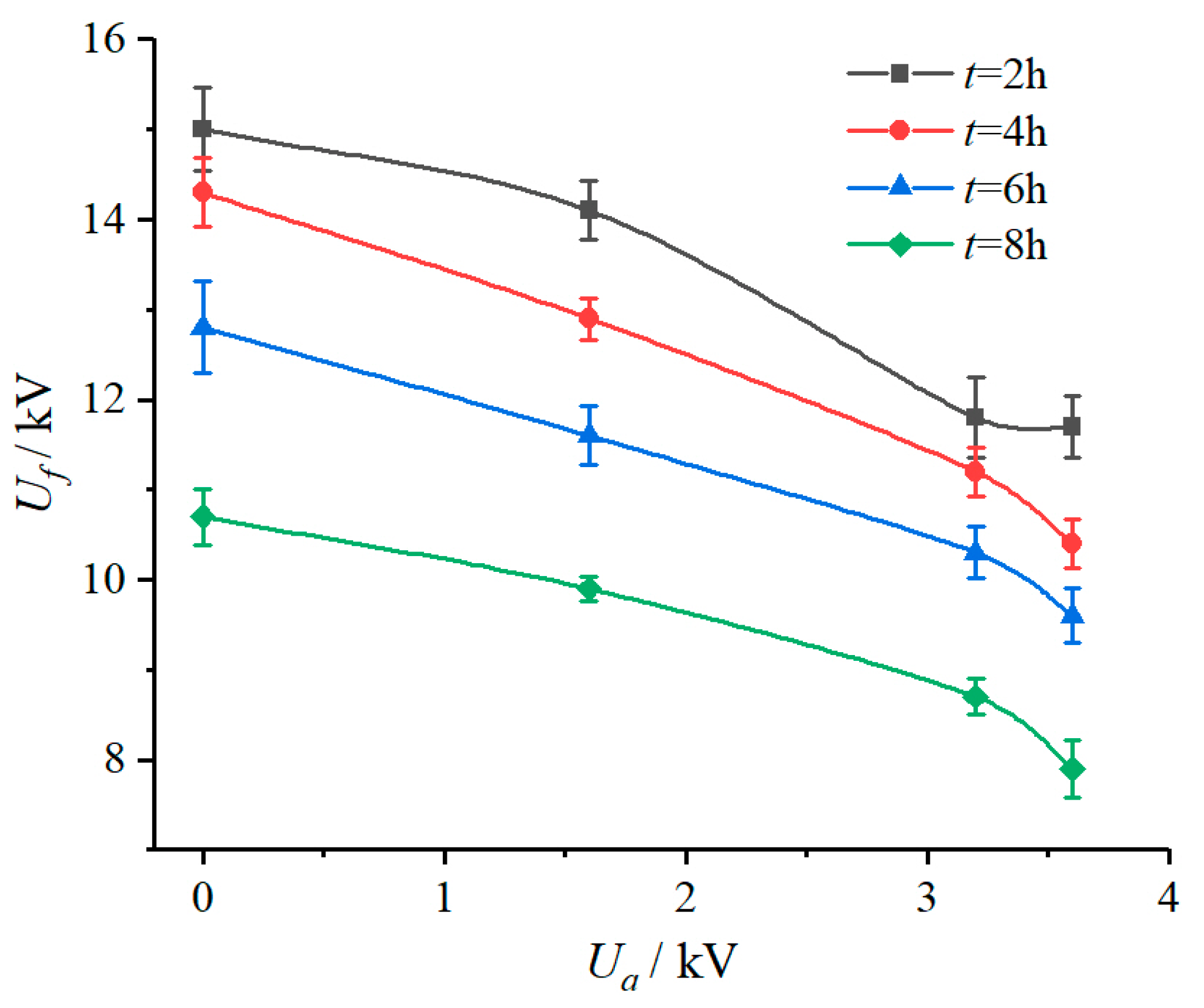
The DC flashover voltage test results of the samples under different applied voltage conditions are shown in
Table 5 and
Figure 6. It can be seen that whether the sample was energized in the salt-fog environment and the applied voltage had an influence on the DC flashover voltage. To be specific, in the salt-fog environment with γ
20 of 3000 μS/cm, compared with 10.7 kV of the unenergized sample, the
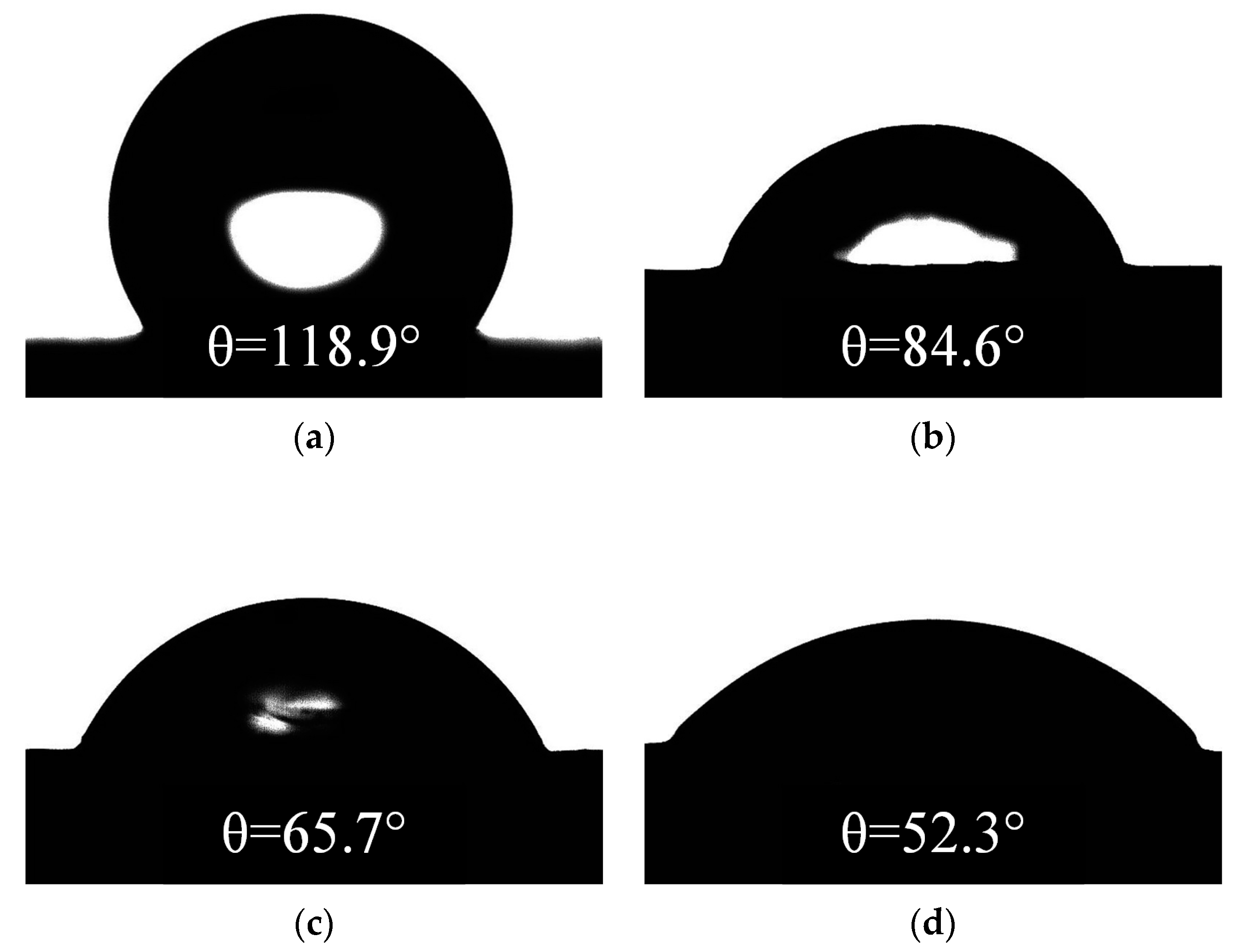
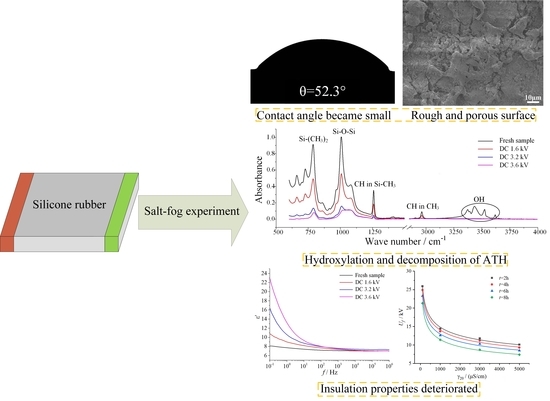
Uf of samples energized with 1.6 kV, 3.2 kV, and 3.6 kV were 9.9 kV, 8.7 kV, and 7.9 kV, reducing by 7.48%, 18.69%, and 26.17%, respectively. This indicates that in the salt-fog environment, the aging of SR was aggravated by the increase of electric field stress, the hydrophobicity was reduced or even lost, and the insulation performance deteriorated, resulting in a decrease of electrical strength.
Similarly, the leakage current flowing through the surface of the sample increased as the applied voltage increased, as shown in
Table 6. In the salt-fog condition with a 3000 μS/cm fog water conductivity rate, the maximum leakage current of the sample with a voltage of 3.6 kV increased by 3.20 times more than that with 1.6 kV, and the cumulative discharge increased by 5.15 times, indicating a more severe discharge under a higher applied voltage.