Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies
Abstract
:1. Introduction
2. Amino-Acid Composition of Fish Gelatin
3. Sole-Gel Transition and Rheological Properties of Fish Gelatin Gel
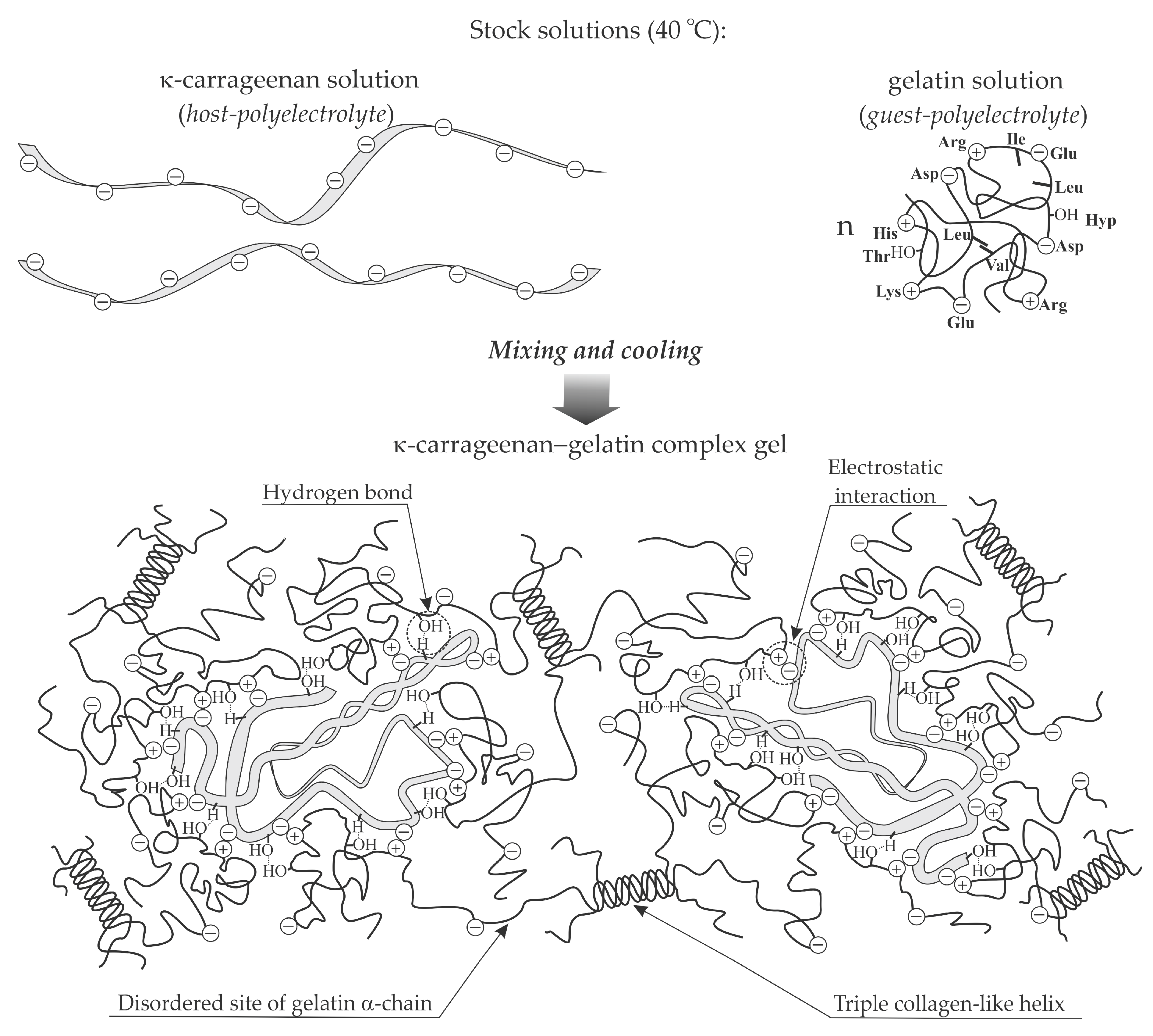
4. Improving the Functional Properties of Fish Gelatin Food Gel
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 142–163. [Google Scholar]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Sultana, S.; Ali, M.M.; Ahamad, M.N.U. Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. Prep. Process. Relig. Cult. Foods 2018, 215–239. [Google Scholar] [CrossRef]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible polymers: An insight into its application in food, biomedicine and cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Sow, L.C.; Kong, K.; Yang, H. Structural Modification of Fish Gelatin by the Addition of Gellan, κ-Carrageenan, and Salts Mimics the Critical Physicochemical Properties of Pork Gelatin. J. Food Sci. 2018, 83, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tu, Z.; Shangguan, X.; Sha, X.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Guillen, M.C.; Turnay, J.; Fernandez-Diaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Akbar, I.; Jaswir, I.; Jamal, P.; Octavianti, F. Fish gelatin nanoparticles and their food applications: A review. Int. Food Res. J. 2017, 24, 255–264. [Google Scholar]
- Bhagwat, P.K.; Dandge, P. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Lv, L.; Huang, Q.; Ding, W.; Xiao, X.; Zhang, H.; Xiong, L. Fish gelatin: The novel potential application. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Wasswa, J.B.; Tang, J.; Gu, X. Utilization of Fish processing By-Products in the Gelatin Industry. Food Rev. Int. 2007, 23, 159–174. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S.; Cathrine, M.S.B.; Kudre, T.G. Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends Food Sci. Technol. 2019, 85, 10–22. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of marine-derived biowaste to develop chitin/fish gelatin products as bioactive carries and moisture scavengers. Sci. Total Environ. 2020, 706, 135747. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; p. 244. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Milovanovic, I. Marine Gelatin from Rest Raw Materials. Appl. Sci. 2018, 8, 2407. [Google Scholar] [CrossRef] [Green Version]
- Haug, I.J.; Draget, K.I.; Smidsrod, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Oliveira, V.D.M.; Assis, C.R.D.; Costa, B.D.A.M.; Neri, R.C.D.A.; Monte, F.T.; Freitas, H.M.S.D.C.V.; França, R.C.P.; Santos, J.; Bezerra, R.D.S.; Porto, A.L.F. Physical, biochemical and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, K.; Terashima, M.; Hozan, D.; Ebato, T.; Nomura, Y.; Ishii, Y.; Shirai, K. Physical Properties of Shark Gelatin Compared with Pig Gelatin. J. Agric. Food Chem. 2000, 48, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- See, S.F.; Hong, P.K.; Ng, K.L.; Wan Aida, W.M.; Babji, A.S. Physicochemical properties of gelatins extracted from skins of different freshwater fish species. Int. Food Res. J. 2010, 17, 809–816. [Google Scholar]
- Gilsenan, P.M.; Ross-Murphy, S.B. Rheological characterization of gelatin gels from mammalian and marine sources. Food Hydrocoll. 2000, 14, 191–195. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Ultraviolet irradiation improves gel strength of fish gelatin. Food Chem. 2009, 113, 1160–1164. [Google Scholar] [CrossRef]
- Da Silva, R.S.G.; Pinto, L.A.A. Physical Cross-linkers: Alternatives to Improve the Mechanical Properties of Fish Gelatin. Food Eng. Rev. 2012, 4, 165–170. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gomez-Guillen, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Montero, P. Extraction of gelatin from fish skins by high pressure treatment. Food Hydrocoll. 2005, 19, 923–928. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejska, I.; Kaczorowski, K.; Piotrowska, B.; Sadowska, M. Modification of the properties of gelatin from skins of Baltic cod (Gadus morhua) with transglutaminase. Food Chem. 2004, 86, 203–209. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lai, K.; Fan, Y.; Liu, Y.; Huang, Y. Effects of sucrose, glucose and fructose on the large deformation behaviors of fish skin gelatin gels. Food Hydrocoll. 2020, 101, 105537. [Google Scholar] [CrossRef]
- Araghi, M.; Moslehi, Z.; Nafchi, A.M.; Mostahsan, A.; Salamat, N.; Garmakhany, A.D. Cold water fish gelatin modification by a natural phenolic cross-linker (ferulic acid and caffeic acid). Food Sci. Nutr. 2015, 3, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrod, O. Physical behavior of fish gelatin—κ-carrageenan mixtures. Carbohyd. Polym. 2004, 56, 11–19. [Google Scholar] [CrossRef]
- Pranoto, Y.; Lee, C.M.; Park, N.J. Characterization of fish gelatin films added with gellan and κ-carrageenan. LWT Food Sci. Technol. 2007, 40, 766–774. [Google Scholar] [CrossRef]
- Razzak, M.A.; Kim, M.; Chung, D. Elucidation of aqueous interactions between fish gelatin and sodium alginate. Carbohydr. Polym. 2016, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Phawaphuthanon, N.; Yu, D.; Ngamnikom, P.; Shin, I.-S.; Chung, D. Effect of fish gelatin-sodium alginate interactions on foam formation and stability. Food Hydrocoll. 2019, 88, 119–126. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pajak, A.; Kolodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta A 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Anvari, M.; Joyner, H.S. Effect of fish gelatin-gum arabic interactions on structural and functional properties of concentrated emulsions. Food Res. Int. 2017, 102, 1–7. [Google Scholar] [CrossRef]
- Chung, D. Fish gelatin: Molecular interactions and applications. In Biopolymer-Based Formulations. Biomedical and Food Applications; Pal, K., Banerjee, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–85. [Google Scholar] [CrossRef]
- Cheng, L.H.; Lim, B.L.; Chow, K.H.; Chong, S.M.; Chang, Y.C. Using fish gelatin and pectin to make a low-fat spread. Food Hydrocoll. 2008, 22, 1637–1640. [Google Scholar] [CrossRef]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA, 1964; p. 478. [Google Scholar]
- Johnston-Banks, F.A. Gelatin. In Food Gels; Harris, P., Ed.; Springer: Dordrecht, The Netherlands, 1990; pp. 233–289. [Google Scholar]
- Gomez-Guillen, M.C.; Lopez-Caballero, M.E.; Aleman, A.; Lopez de Lacey, A.; Gimenez, B.; Montero, P. Antioxidant and antimicrobial peptide fractions from squid and tuna skin gelatin. In Sea By-Products as Real Material: New Ways of Application; Le Bihan, E., Ed.; Transworld Research Network: Trivandrum, Kerala, India, 2010; pp. 89–115. [Google Scholar]
- Liu, D.; Zhou, P.; Li, T.; Regenstein, J.M. Comparison of acid-soluble collagens from the skins and scales of four carp species. Food Hydrocoll. 2014, 41, 290–297. [Google Scholar] [CrossRef]
- Al-Saidi, G.S.; Al-Alawi, A.; Rahman, M.S.; Guizani, N. Fourier transform infrared (FTIR) spectroscopic study of extracted gelatin from shaari (Lithrinus microdon) skin: Effects of extraction conditions. Int. Food Res. J. 2012, 19, 1167–1173. [Google Scholar]
- Zhang, T.; Sun, R.; Ding, M.; Li, L.; Tao, N.; Wang, X.; Zhong, J. Commercial cold-water fish skin gelatin and bovine bone gelatin: Structural, functional, and emulsion stability differences. LWT Food Sci. Technol. 2020, 125, 109207. [Google Scholar] [CrossRef]
- Djabourov, M.; Papon, P. Influence of thermal treatment on the structure and stability of gelatin gels. Polymer 1983, 24, 537–541. [Google Scholar] [CrossRef]
- Ledward, D.A. Gelation of gelatin. In Functional Properties of Food Macromolecules; Mitchell, J.R., Ledward, D.A., Eds.; Elsevier Applied Science Publishers: London, UK, 1986; pp. 171–201. [Google Scholar]
- Gimenez, B.; Gomez-Guillen, M.C.; Montero, P. Storage of dried fish skins on quality characteristics of extracted gelatin. Food Hydrocoll. 2005, 19, 958–963. [Google Scholar] [CrossRef] [Green Version]
- Da Trindade Alfaro, A.; Balbinot, E.; Weber, C.I.; Tonial, I.B. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Structure and rheology of gelatin gels—Recent progress. Polymer 1992, 33, 2622–2627. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Structure and Rheology of Gelatin Gels. Imaging Sci. J. 1997, 45, 205–209. [Google Scholar] [CrossRef]
- Gilsenan, P.M.; Ross-Murphy, S.B. Shear creep of gelatin gels from mammalian and piscine collagens. Int. J. Biol. Macromol. 2001, 29, 53–61. [Google Scholar] [CrossRef]
- Lai, J.Y.; Lin, P.K.; Hsiue, G.H.; Cheng, H.Y.; Huang, S.J.; Li, Y.T. Low Bloom strength gelatin as a carrier for potential use in retinal sheet encapsulation and transplantation. Biomacromolecules 2009, 10, 310–319. [Google Scholar] [CrossRef]
- Lai, J.Y. The Role of Bloom Index of Gelatin on the Interactions with Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2009, 10, 3442–3456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, S.F.; Luo, L.J.; Lai, J.Y.; Ma, D.H.K. On the Importance of Bloom Number of Gelatin to the Development of Biodegradable in Situ Gelling Copolymers for Intracameral Drug Delivery. Int. J. Pharm. 2016, 511, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Calderon, P.; Lopez, D.; Matiacevich, D.L.S.; Osorio, F.; Enrione, J. State diagram of salmon (Salmo salar) gelatin films. J. Sci. Food Agric. 2011, 91, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Calderon, P.; Flores, E.; Gonzales-Munoz, A.; Perczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Enrione, J.; Char, C.; Perczynska, M.; Padilla, C.; Gonzales-Munoz, A.; Olguin, Y.; Quinzio, C.; Iturriaga, L.; Diaz-Calderon, P. Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight. Polymers 2020, 12, 1587. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Laneuville, S.I. Protein + Polysaccharide Coacervates and Complexes: From Scientific Background to their Application as Functional Ingredients in Food Products. In Modern Biopolymer Science; Kasapis, S., Norton, I.T., Eds.; Elsevier: London, UK, 2009; pp. 327–363. [Google Scholar]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Voron’ko, N.G. Polyelectrolyte Polysaccharide–Gelatin Complexes: Rheology and Structure. Polymers 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, L.; Zhang, K.; Fang, Y.; Nishinari, K.; Phillips, G.O. Mapping the Complex Phase Behaviors of Aqueous Mixtures of κ-Carrageenan and Type B Gelatin. J. Phys. Chem. B 2015, 119, 9982–9992. [Google Scholar] [CrossRef]
- Voron’ko, N.G.; Derkach, S.R.; Vovk, M.A.; Tolstoy, P.M. Complexation of κ-carrageenan with gelatin in the aqueous phase analysed by 1H NMR kinetics and relaxation. Carbohyd. Polym. 2017, 169, 117–126. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kolotova, A.V.; Voron’ko, N.G.; Malkin, A.Y. Modification of the rheological properties of fish gelatin with sodium alginate. Polymers 2020, in press. [Google Scholar]
- Yannas, I.V. Collagen and Gelatin in the Solid State. Polym. Rev. 1972, 7, 40–106. [Google Scholar]
- Brodsky, B.; Shah, N.K. The triple-helix motif in proteins. FASEB J. 1995, 9, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Voron’ko, N.G.; Derkach, S.R.; Kuchina, Y.A.; Sokolan, N.I. The chitosan–gelatin (bio)polyelectrolyte complexes formation in an acidic medium. Carbohyd. Polym. 2016, 138, 265–272. [Google Scholar] [CrossRef] [PubMed]

| Source | Cold Water Fish Skin | Warm Water Fish Skin | Pork Skin [2] | Calf Skin [21] | ||||
|---|---|---|---|---|---|---|---|---|
| Cod [10] | Hake [10] | Alaska Pollock [2] | Tilapia [1,2] | Tuna [46] | Black Carp [19,47] | |||
| Amino Acid Composition (Residues Per 1000 Total Amino Acid Residues) | ||||||||
| Glycine (Gly) | 344 | 331 | 358 | 347 | 336 | 314 | 330 | 313 |
| Basic groups | 99 | 97 | 91 | 86 | 90 | 88 | 86 | 101 |
| Lysine (Lys) | 29 | 28 | 26 | 25 | 25 | 29 | 27 | 34 |
| Hydroxylysine(Hyl) | 6 | 5 | 6 | 8 | 6 | 2 | 6 | 11 |
| Histidine (His) | 8 | 10 | 8 | 6 | 7 | 4 | 4 | 5 |
| Arginine (Arg) | 56 | 54 | 51 | 47 | 52 | 53 | 49 | 51 |
| Carboxylic groups | 130 | 123 | 125 | 117 | 115 | 126 | 118 | 116 |
| Aspartic acid (Asp) | 52 | 49 | 51 | 48 | 44 | 48 | 46 | 45 |
| Glutamic acid (Glu) | 78 | 74 | 74 | 69 | 71 | 78 | 72 | 71 |
| Hydroxylic groups | 142 | 134 | 146 | 140 | 150 | 131 | 147 | 144 |
| Serine (Ser) | 64 | 49 | 63 | 35 | 48 | 37 | 35 | 37 |
| Threonine (Thr) | 25 | 22 | 25 | 24 | 21 | 25 | 18 | 18 |
| Hydroxyproline (Hyp) | 50 | 59 | 55 | 79 | 78 | 69 | 91 | 86 |
| Tyrosine (Tyr) | 3 | 4 | 3 | 2 | 3 | 0 | 3 | 3 |
| Hydrophobic groups | 286 | 314 | 280 | 309 | 321 | 336 | 322 | 326 |
| Alanine (Ala) | 96 | 119 | 108 | 122 | 119 | 119 | 112 | 114 |
| Valine (Val) | 18 | 19 | 18 | 15 | 28 | 22 | 26 | 22 |
| Leucine (Leu) | 22 | 23 | 20 | 23 | 21 | 22 | 24 | 25 |
| Isoleucine (Ile) | 11 | 9 | 11 | 8 | 7 | 12 | 10 | 11 |
| Proline (Pro) | 106 | 114 | 95 | 119 | 117 | 133 | 132 | 135 |
| Phenylalanine (Phe) | 16 | 15 | 12 | 13 | 13 | 14 | 14 | 13 |
| Methionine (Met) | 17 | 15 | 16 | 9 | 16 | 14 | 4 | 6 |
| Gelatin | Gelling Temperature, °C | Melting Temperature, °C | References |
|---|---|---|---|
| Cold water fish gelatin | 4–8 | 16–18 | [1] |
| 7–11 | 11–19 | [2] | |
| 4–5 | 12–13 | [5] | |
| 4–12 | <17 | [9] | |
| 4–10 | 13–16 | [21] | |
| 16–21 | [34] | ||
| 7–9 | 18–20 | [52] | |
| 12 | 14–21 | [53] | |
| Warm water fish gelatin | 21–22 | 28–29 | [1] |
| 15–20 | 20–27 | [2] | |
| 18–19 | 24–29 | [9] | |
| 22 | [24] | ||
| 22–29 | [34] | ||
| 19–22 | 24–25 | [53] | |
| Mammalian gelatin | 26–27 | 33–34 | [1] |
| 20–25 | 28–31 | [2,34] | |
| 29 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. https://doi.org/10.3390/polym12123051
Derkach SR, Voron’ko NG, Kuchina YA, Kolotova DS. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers. 2020; 12(12):3051. https://doi.org/10.3390/polym12123051
Chicago/Turabian StyleDerkach, Svetlana R., Nikolay G. Voron’ko, Yuliya A. Kuchina, and Daria S. Kolotova. 2020. "Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies" Polymers 12, no. 12: 3051. https://doi.org/10.3390/polym12123051
APA StyleDerkach, S. R., Voron’ko, N. G., Kuchina, Y. A., & Kolotova, D. S. (2020). Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers, 12(12), 3051. https://doi.org/10.3390/polym12123051






