Efficient Biofilms Eradication by Enzymatic-Cocktail of Pancreatic Protease Type-I and Bacterial α-Amylase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.2. Biofilm Formation and Inhibition Assay
2.3. Biofilm Prevention Assay
2.4. Antibacterial Assay
2.5. Statistical Analysis
3. Results and Discussion
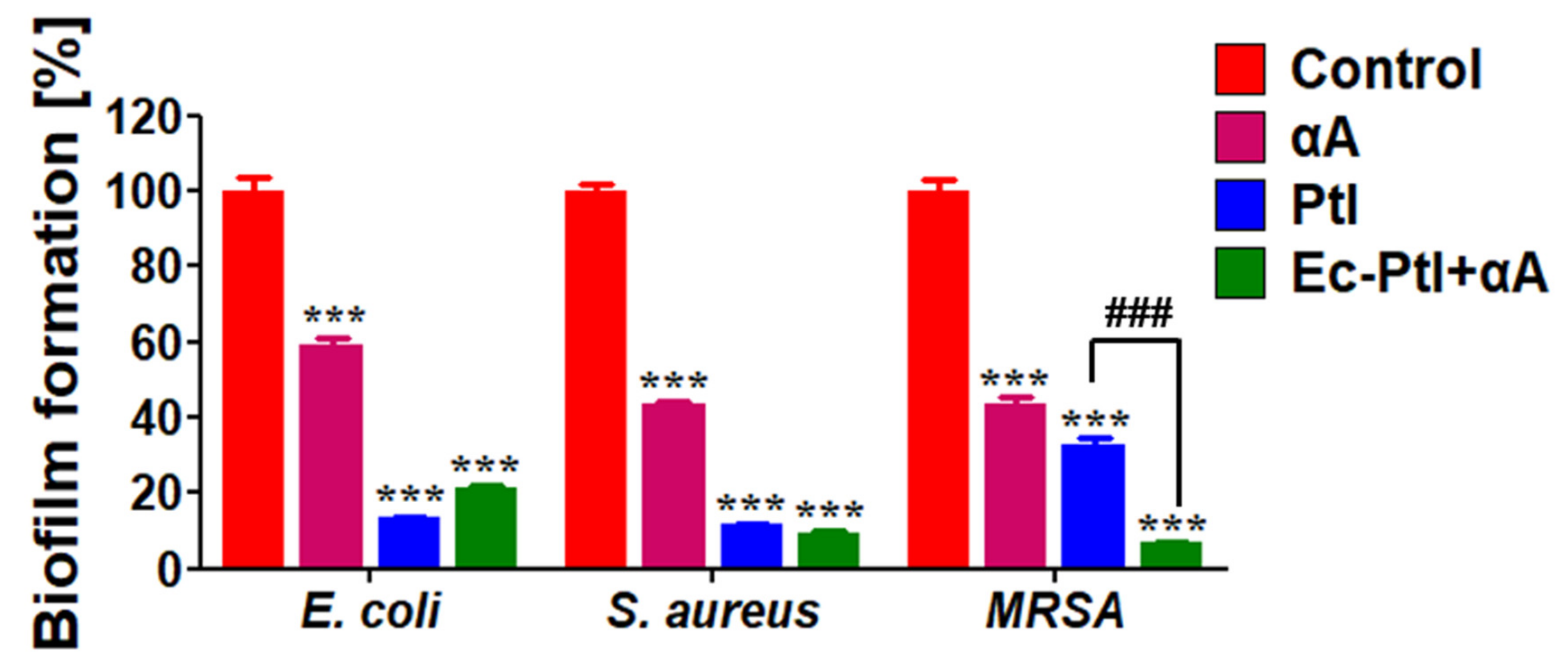
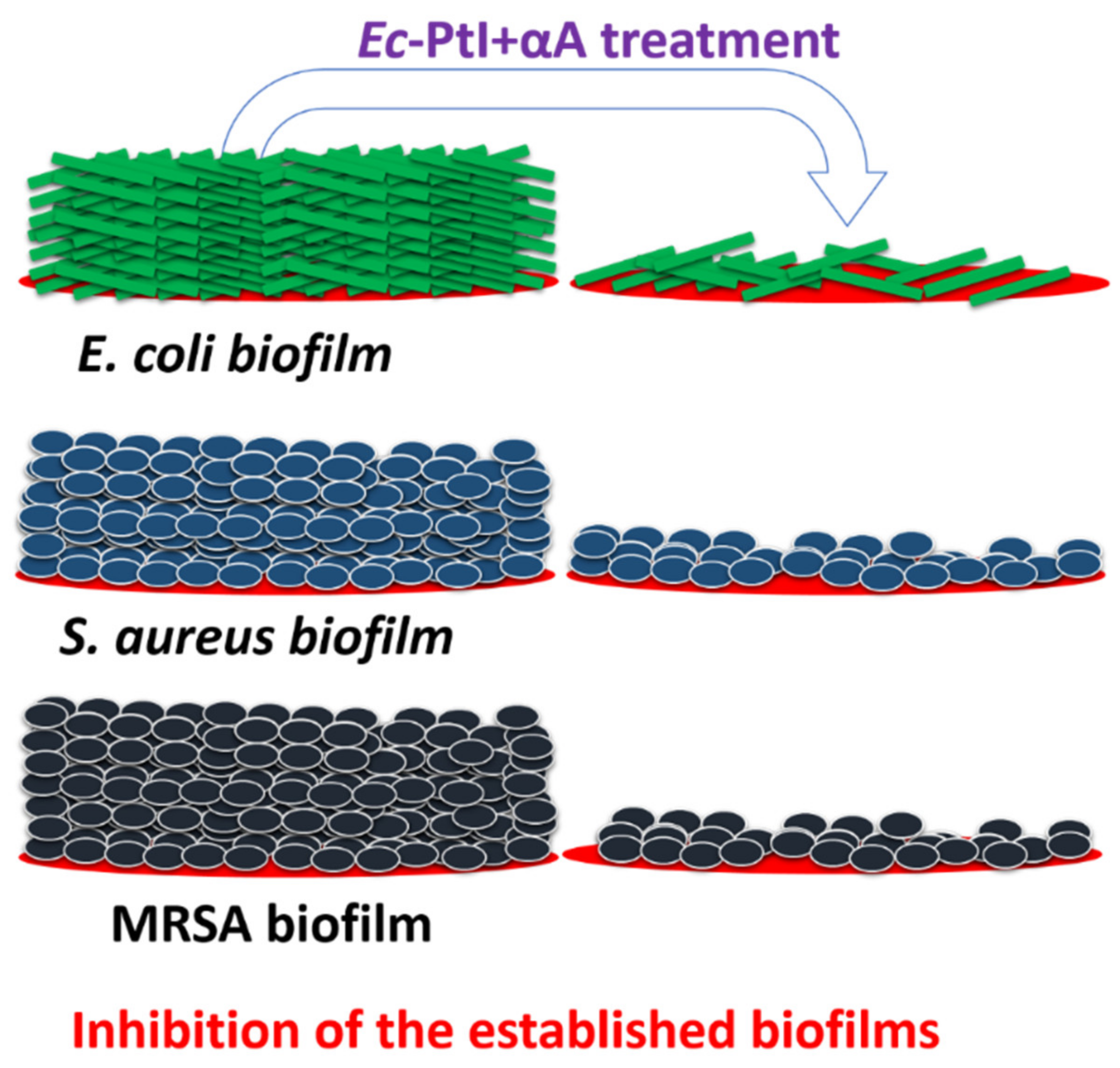
3.1. Biofilm Inhibition Study
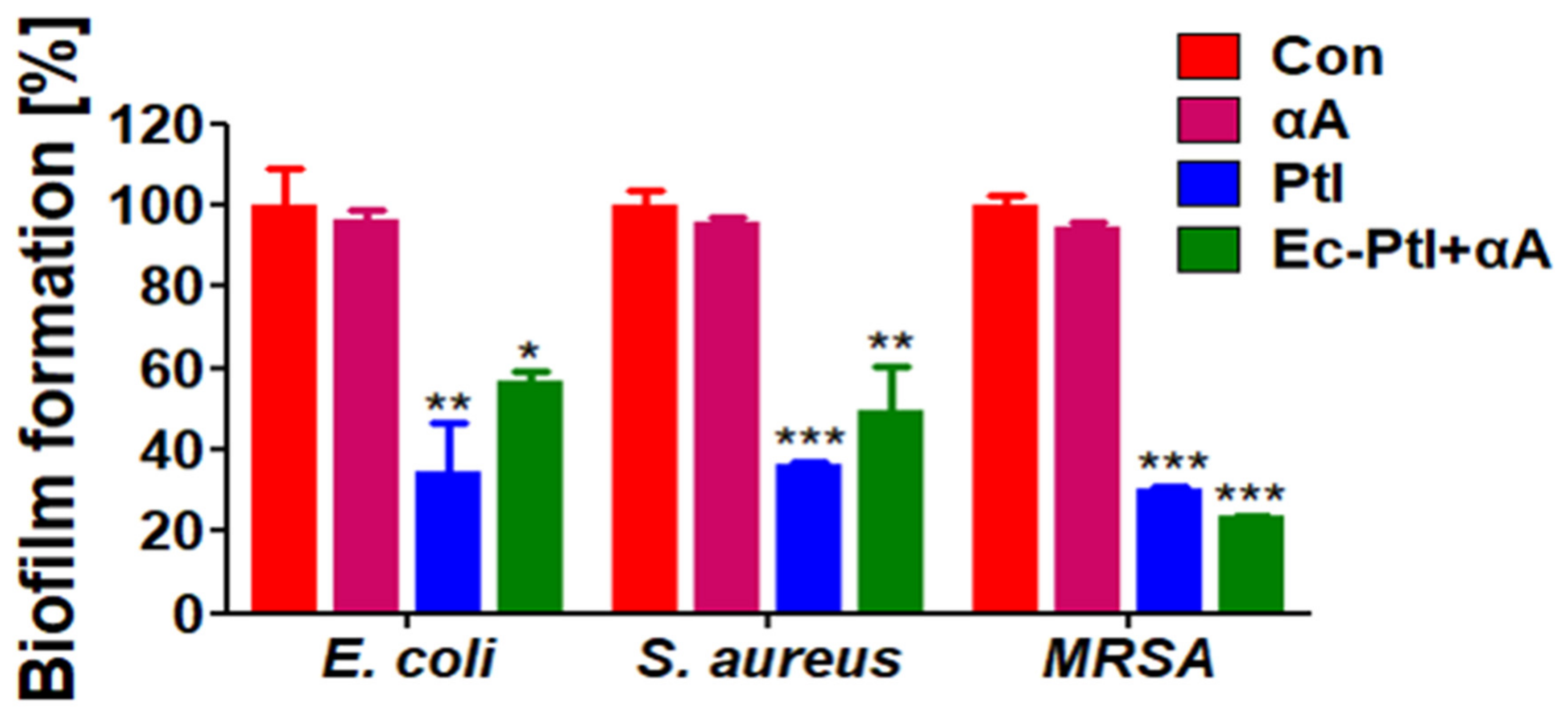
3.2. Prevention of Biofilm Formation
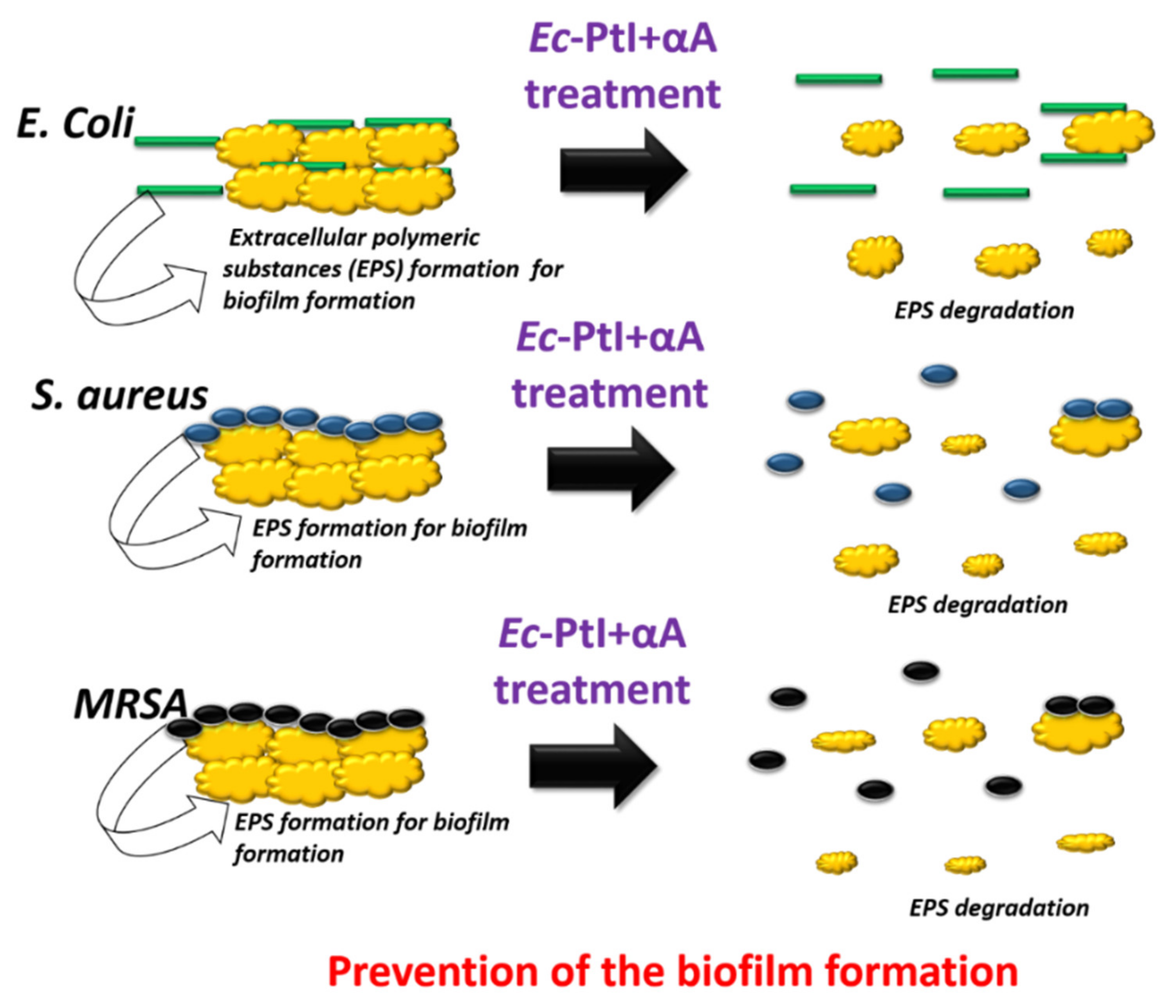
3.3. Proposed Mechanisms for the Anti-Biofilm Effect
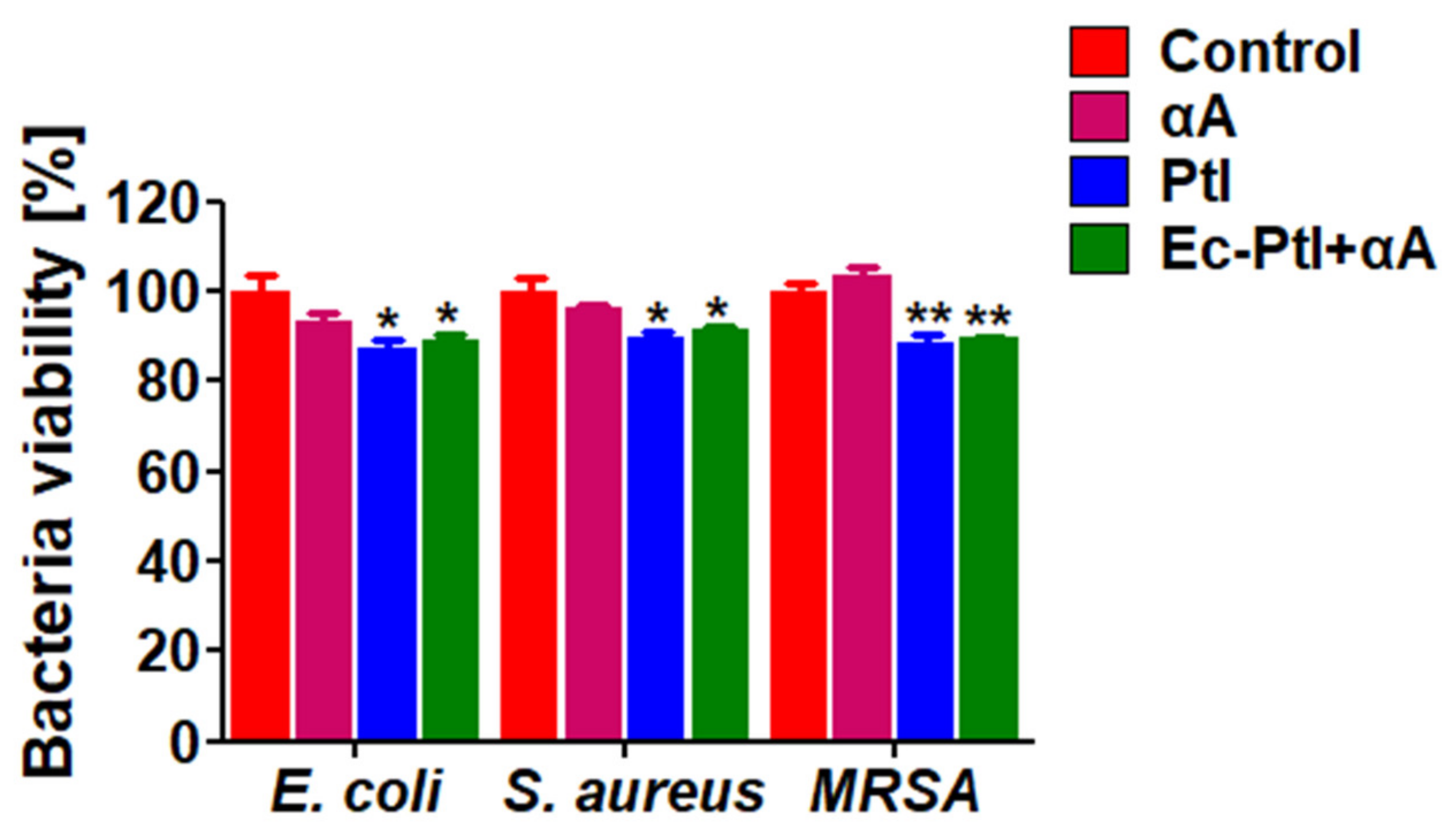
3.4. Anti-Bacterial Assessment of the Enzymes αA, PtI, and Ec-PtI+αA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Knetsch, M.L.W.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Sung, D.; Moon, E. Enhanced Antibiofilm Effects of N2 Plasma-Treated Buffer Combined with Antimicrobial Hexapeptides Against Plant Pathogens. Polymers 2020, 12, 1992. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Eradication of marine biofilms by atmospheric pressure non-thermal plasma: A potential approach to control biofouling? Int. Biodeterior. Biodegrad. 2014, 86, 14–18. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. New Perspectives in Biofilm Eradication. ACS Infect. Dis. 2018, 4, 93–106. [Google Scholar] [CrossRef]
- Faria, S.I.; Teixeira-Santos, R.; Romeu, M.J.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J. The relative importance of shear forces and surface hydrophobicity on biofilm formation by coccoid cyanobacteria. Polymers 2020, 12, 653. [Google Scholar] [CrossRef] [Green Version]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef] [Green Version]
- Buhmann, M.T.; Stiefel, P.; Maniura-Weber, K.; Ren, Q. In Vitro Biofilm Models for Device-Related Infections. Trends Biotechnol. 2016, 34, 945–948. [Google Scholar] [CrossRef] [Green Version]
- Segura González, E.A.; Olmos, D.; Lorente, M.Á.; Vélaz, I.; González-Benito, J. Preparation and characterization of polymer composite materials based on PLA/TiO2 for antibacterial packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef] [Green Version]
- Lotlikar, S.R.; Gallaway, E.; Grant, T.; Popis, S.; Whited, M.; Guragain, M.; Rogers, R.; Hamilton, S.; Gerasimchuk, N.G.; Patrauchan, M.A. Polymeric composites with silver (I) cyanoximates inhibit biofilm formation of gram-positive and gram-negative bacteria. Polymers 2019, 11, 1018. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Borges, A.; Teodósio, J.; Araújo, P.; Mergulhão, F.; Melo, L.; Simões, M. The effects of ferulic and salicylic acids on Bacillus cereus and Pseudomonas fluorescens single- and dual-species biofilms. Int. Biodeterior. Biodegrad. 2014, 86, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Morton, L.H.G.; Surman, S.B. Biofilms in biodeterioration—A review. Int. Biodeterior. Biodegrad. 1994, 34, 203–221. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef]
- Crouzet, M.; Le Senechal, C.; Brözel, V.S.; Costaglioli, P.; Barthe, C.; Bonneu, M.; Garbay, B.; Vilain, S. Exploring early steps in biofilm formation: Set-up of an experimental system for molecular studies. BMC Microbiol. 2014, 14, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watters, C.M.; Burton, T. Enzymatic degradation of in vitro Staphylococcus aureus biofilms supplemented with human plasma. Infect. Drug Resist. 2016, 2016, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.S.; Koo, O.K.; Kim, M.; Kim, J. Bio-enzymes for inhibition and elimination of Escherichia coli O157: H7 biofilm and their synergistic effect with sodium hypochlorite. Sci. Rep. 2019, 9, 9920. [Google Scholar] [CrossRef]
- Suresh, M.K.; Biswas, R.; Biswas, L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019, 309, 1–12. [Google Scholar] [CrossRef]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J.; Jennings, L.K.; Tam, J.; Melnyk, R.A.; Parsek, M.R.; et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef] [Green Version]
- Fleming, G.; Aveyard, J.; Fothergill, J.L.; McBride, F.; Raval, R.; D’Sa, R.A. Effect of polymer demixed nanotopographies on bacterial adhesion and biofilm formation. Polymers 2019, 11, 1921. [Google Scholar] [CrossRef] [Green Version]
- Lequette, Y.; Boels, G.; Clarisse, M.; Faille, C. Using enzymes to remove biofilms of bacterial isolates sampled in the food-industry. Biofouling 2010, 26, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Thallinger, B.; Prasetyo, E.N.; Nyanhongo, G.S.; Guebitz, G.M. Antimicrobial enzymes: An emerging strategy to fight microbes and microbial biofilms. Biotechnol. J. 2013, 8, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Saggu, S.K.; Jha, G.; Mishra, P.C. Enzymatic degradation of biofilm by metalloprotease from microbacterium sp. SKS10. Front. Bioeng. Biotechnol. 2019, 7, 192. [Google Scholar] [CrossRef] [Green Version]
- Banar, M.; Emaneini, M.; Beigverdi, R.; Fanaei Pirlar, R.; Node Farahani, N.; van Leeuwen, W.B.; Jabalameli, F. The efficacy of lyticase and β-glucosidase enzymes on biofilm degradation of Pseudomonas aeruginosa strains with different gene profiles. BMC Microbiol. 2019, 19, 291. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, G.; Bassegoda, A.; Hoyo, J.; Torrent-Burgués, J.; Tzanov, T. Metal-Enzyme Nanoaggregates Eradicate Both Gram-Positive and Gram-Negative Bacteria and Their Biofilms. ACS Appl. Mater. Interfaces 2018, 10, 40434–40442. [Google Scholar] [CrossRef]
- Molobela, I.; Cloete, T.; Beukes, M. Protease and amylase enzymes for biofilm removal and degradation of extracellular polymeric substances (EPS) produced by Pseudomonas fluorescens bacteria. Afr. J. Microbiol. Res. 2010, 4, 1515–1524. [Google Scholar]
- Barrett, A.J.; McDonald, J.K. Nomenclature: Protease, proteinase and peptidase. Biochem. J. 1986, 237, 935. [Google Scholar] [CrossRef] [Green Version]
- Forsmark, C.E.; Tang, G.; Xu, H.; Tuft, M.; Hughes, S.J.; Yadav, D. The use of pancreatic enzyme replacement therapy in patients with a diagnosis of chronic pancreatitis and pancreatic cancer in the US is infrequent and inconsistent. Aliment. Pharmacol. Ther. 2020, 51, 958–967. [Google Scholar] [CrossRef]
- Ogretmen, B. Pancreatic Enzyme Replacement Therapy: A Concise Review. Physiol. Behav. 2019, 176, 139–148. [Google Scholar]
- Jayaveni, S.; Nithyanandham, K.; Rose, C. In vitro secretion of zymogens by bovine pancreatic acini and ultra-structural analysis of exocytosis. Biochem. Biophys. Rep. 2016, 5, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Ianiro, G.; Pecere, S.; Giorgio, V.; Gasbarrini, A.; Cammarota, G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr. Drug Metab. 2016, 17, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, U.T.; Burrows, L.L. DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int. J. Food Microbiol. 2014, 187, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Scoarughi, G.L.; Poggiali, F.; Cellini, A.; Carpentieri, A.; Seganti, L.; Pucci, P.; Amoresano, A.; Cocconcelli, P.S.; Artini, M.; et al. Protease treatment affects both invasion ability and biofilm formation in Listeria monocytogenes. Microb. Pathog. 2008, 45, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Mizan, M.F.R.; Ha, A.J.W.; Ha, S.D. Do Advances and Future Prospects of Enzyme-Based Biofilm Prevention Approaches in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1484–1502. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.Y.; Kim, B.R.; Bae, Y.M.; Lee, S.Y. Biofilm formation, attachment, and cell hydrophobicity of foodborne pathogens under varied environmental conditions. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 207–220. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6, 1–42. [Google Scholar]
- Park, J.H.; Lee, J.H.; Cho, M.H.; Herzberg, M.; Lee, J. Acceleration of protease effect on Staphylococcus aureus biofilm dispersal. FEMS Microbiol. Lett. 2012, 335, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loughran, A.J.; Atwood, D.N.; Anthony, A.C.; Harik, N.S.; Spencer, H.J.; Beenken, K.E.; Smeltzer, M.S. Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. Microbiologyopen 2014, 3, 897–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elchinger, P.H.; Delattre, C.; Faure, S.; Roy, O.; Badel, S.; Bernardi, T.; Taillefumier, C.; Michaud, P. Effect of proteases against biofilms of Staphylococcus aureus and Staphylococcus epidermidis. Lett. Appl. Microbiol. 2014, 59, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari-Ayezloy, E.; Hosseini-Jazani, N.; Yousefi, S.; Habibi, N. Eradication of methicillin resistant S. Aureus biofilm by the combined use of fosfomycin and β-chloro-L-alanine. Iran. J. Microbiol. 2017, 9, 1–10. [Google Scholar] [PubMed]
- Chakraborti, S.; Mandal, A.K.; Sarwar, S.; Singh, P.; Chakraborty, R.; Chakrabarti, P. Bactericidal effect of polyethyleneimine capped ZnO nanoparticles on multiple antibiotic resistant bacteria harboring genes of high-pathogenicity island. Colloids Surf. B Biointerfaces 2014, 121, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloete, T.E.; Brözel, V.S.; Von Holy, A. Practical aspects of biofouling control in industrial water systems. Int. Biodeterior. Biodegrad. 1992, 29, 299–341. [Google Scholar] [CrossRef]
- Pettit, R.K.; Weber, C.A.; Kean, M.J.; Hoffmann, H.; Pettit, G.R.; Tan, R.; Franks, K.S.; Horton, M.L. Microplate alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 2005, 49, 2612–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adetunji, V.O.; Odetokun, I.O. Assesment of Biofilms in E. coli O157:H7 and Salmonella Strains: Influence of culture conditions. Am. J. Food Technol. 2012, 7, 582–595. [Google Scholar] [CrossRef] [Green Version]
- Kalpana, B.J.; Aarthy, S.; Pandian, S.K. Antibiofilm Activity of α-Amylase from Bacillus subtilis S8-18 Against Biofilm Forming Human Bacterial Pathogens. Appl. Biochem. Biotechnol. 2012, 167, 1778–1794. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Mukherji, R.; Patil, A.; Prabhune, A. Role of Extracellular Proteases in Biofilm Disruption of Gram Positive Bacteria with Special Emphasis on Staphylococcus aureus Biofilms. Enzym. Eng. 2014, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Landini, P.; Antoniani, D.; Burgess, J.G.; Nijland, R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol. 2010, 86, 813–823. [Google Scholar] [CrossRef]
- Beloin, C.; Roux, A.; Ghigo, J. Escherichia coli biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 249–289. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.F.; Li, Q.; Tan, L.R.; Sun, X.F.; Song, C.; Wang, S.G. Extracellular polymeric substances protect Escherichia coli from organic solvents. RSC Adv. 2016, 6, 59438–59444. [Google Scholar] [CrossRef]
- Bales, P.M.; Renke, E.M.; May, S.L.; Shen, Y.; Nelson, D.C. Purification and Characterization of Biofilm-Associated EPS Exopolysaccharides from ESKAPE Organisms and Other Pathogens. PLoS ONE 2013, 8, e67950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Hiltunen, A.K.; Savijoki, K.; Nyman, T.A.; Miettinen, I.; Ihalainen, P.; Peltonen, J.; Fallarero, A. Structural and functional dynamics of Staphylococcus aureus biofilms and biofilm matrix proteins on different clinical materials. Microorganisms 2019, 7, 584. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Veeregowda, D.H.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C. Extracellular polymeric matrix production and relaxation under fluid shear and mechanical pressure in Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2018, 84, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Reffuveille, F.; Josse, J.; Vallé, Q.; Mongaret, C.; Gangloff, S.C. Staphylococcus aureus Biofilms and their impact on the medical field. In Rise Virulence Antibiotic Resistance in Staphylococcus aureus; IntechOpen: London, UK, 2017. [Google Scholar]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hedin, P.A.; Davis, F.M.; Callahan, F.E.; Dollar, D.A. Wheat germ hemicellulose is an absolute requirement for growth and development of the southwestern corn borer. J. Nutr. 1994, 124, 2458–2465. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Bhatia, S. Introduction to enzymes and their applications. In Introduction to Pharmaceutical Biotechnology; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 2. [Google Scholar]
- Craik, C.S.; Page, M.J.; Madison, E.L. Proteases as therapeutics. Biochem. J. 2011, 435, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.E.; Rocha, G.F.; Kise, F.; Rosso, A.M.; Guevara, M.G.; Parisi, M.G. Antimicrobial activity of an aspartic protease from Salpichroa origanifolia fruits. Lett. Appl. Microbiol. 2018, 67, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachanamol, R.S.; Lipton, A.P.; Thankamani, V.; Sarika, A.R.; Selvin, J. Production of Protease Showing Antibacterial Activity by Bacillus subtilis VCDA Associated with Tropical Marine Sponge Callyspongia diffusa. J. Microb. Biochem. Technol. 2017, 9, 270–276. [Google Scholar]

| Enzyme | Sources | Biofilms Inhibition (%) | Target Bacteria | Reference |
|---|---|---|---|---|
| PtI | Bovine pancreas | 87 | E. coli | This work |
| 89 | S. aureus | |||
| 67 | MRSA | |||
| Flavourzyme | Aspergillus oryzae | 50 | S. epidermidis | [39] |
| Neutrase | Bacillus amyloliquefaciens | 72 | S. aureus | [39] |
| 35 | S. epidermidis | |||
| Alcalase | B. licheniformis | 25 | S. epidermidis | [39] |
| α-amylase | B. subtilis | 50 | Pseudomonas aeruginosa | [46] |
| 65 | Vibrio cholerae | |||
| 70 | MRSA | |||
| Aureolysin | S. aureus | 50 | S. aureus | [38] |
| 33 | S. epidermidis | |||
| Dispersin B | Aggregatibacter actinomycetemcomitans | 50 | S. epidermidis | [38] |
| Proteinase K | Tritirachium album | 5 | Pseudomonas aeruginosa | [47] |
| 10 | Vibrio cholerae | |||
| 5 | MRSA | |||
| 75 | S. aureus | |||
| 90 | L. monocytogenes | |||
| Papain | Papaya | 80 | L. monocytogenes | [32] |
| Trypsin | PA clan superfamily | 20 | Pseudomonas aeruginosa | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, S.-C.; Kim, M.; Sung, J.-S.; Kadam, A.A. Efficient Biofilms Eradication by Enzymatic-Cocktail of Pancreatic Protease Type-I and Bacterial α-Amylase. Polymers 2020, 12, 3032. https://doi.org/10.3390/polym12123032
Jee S-C, Kim M, Sung J-S, Kadam AA. Efficient Biofilms Eradication by Enzymatic-Cocktail of Pancreatic Protease Type-I and Bacterial α-Amylase. Polymers. 2020; 12(12):3032. https://doi.org/10.3390/polym12123032
Chicago/Turabian StyleJee, Seung-Cheol, Min Kim, Jung-Suk Sung, and Avinash A. Kadam. 2020. "Efficient Biofilms Eradication by Enzymatic-Cocktail of Pancreatic Protease Type-I and Bacterial α-Amylase" Polymers 12, no. 12: 3032. https://doi.org/10.3390/polym12123032
APA StyleJee, S.-C., Kim, M., Sung, J.-S., & Kadam, A. A. (2020). Efficient Biofilms Eradication by Enzymatic-Cocktail of Pancreatic Protease Type-I and Bacterial α-Amylase. Polymers, 12(12), 3032. https://doi.org/10.3390/polym12123032






