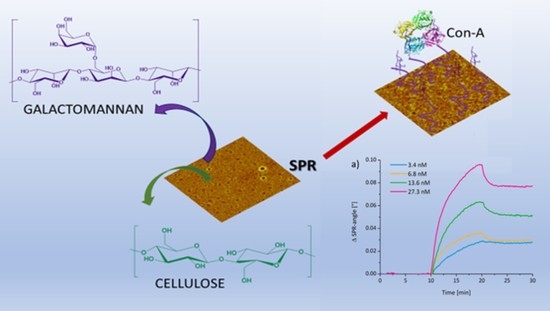
Interactions and Dissociation Constants of Galactomannan Rendered Cellulose Films with Concavalin A by SPR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions
2.3. Substrate Cleaning and Film Preparation
2.4. Infrared Spectroscopy
2.5. Profilometry
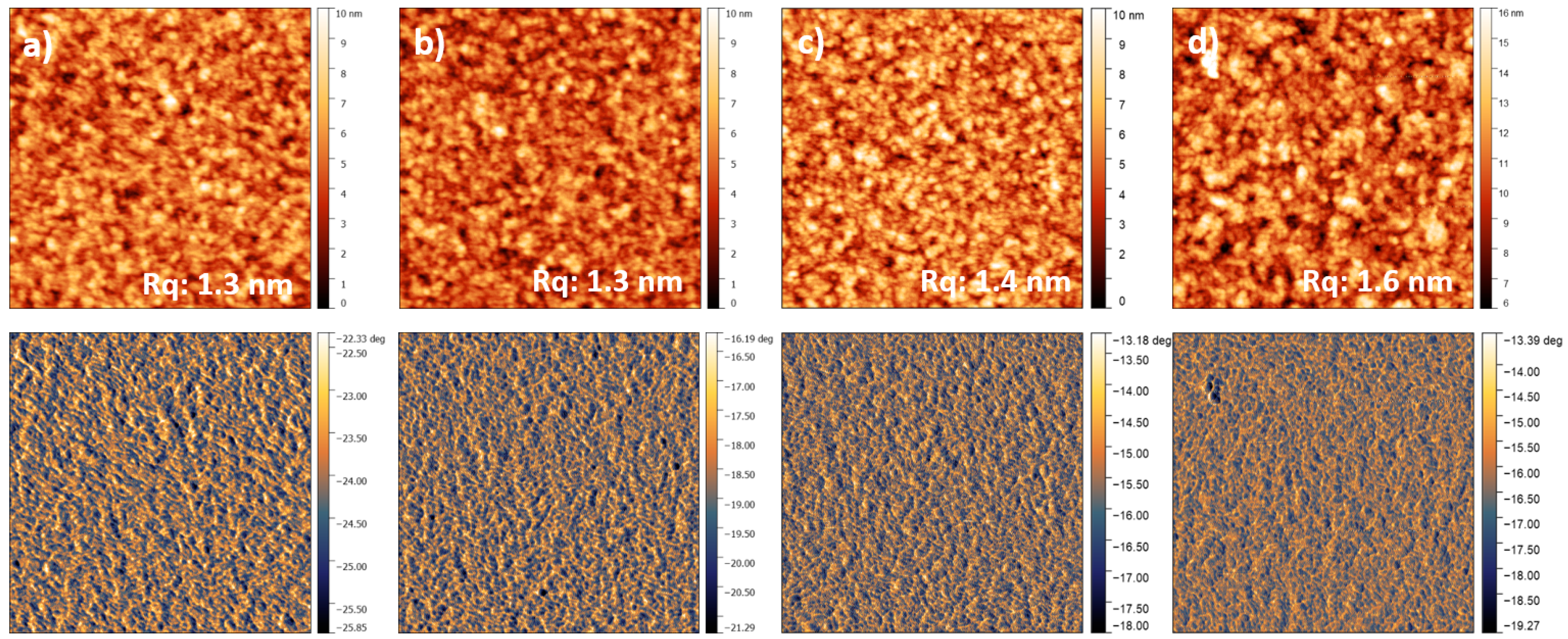
2.6. Atomic Force Microscopy—AFM
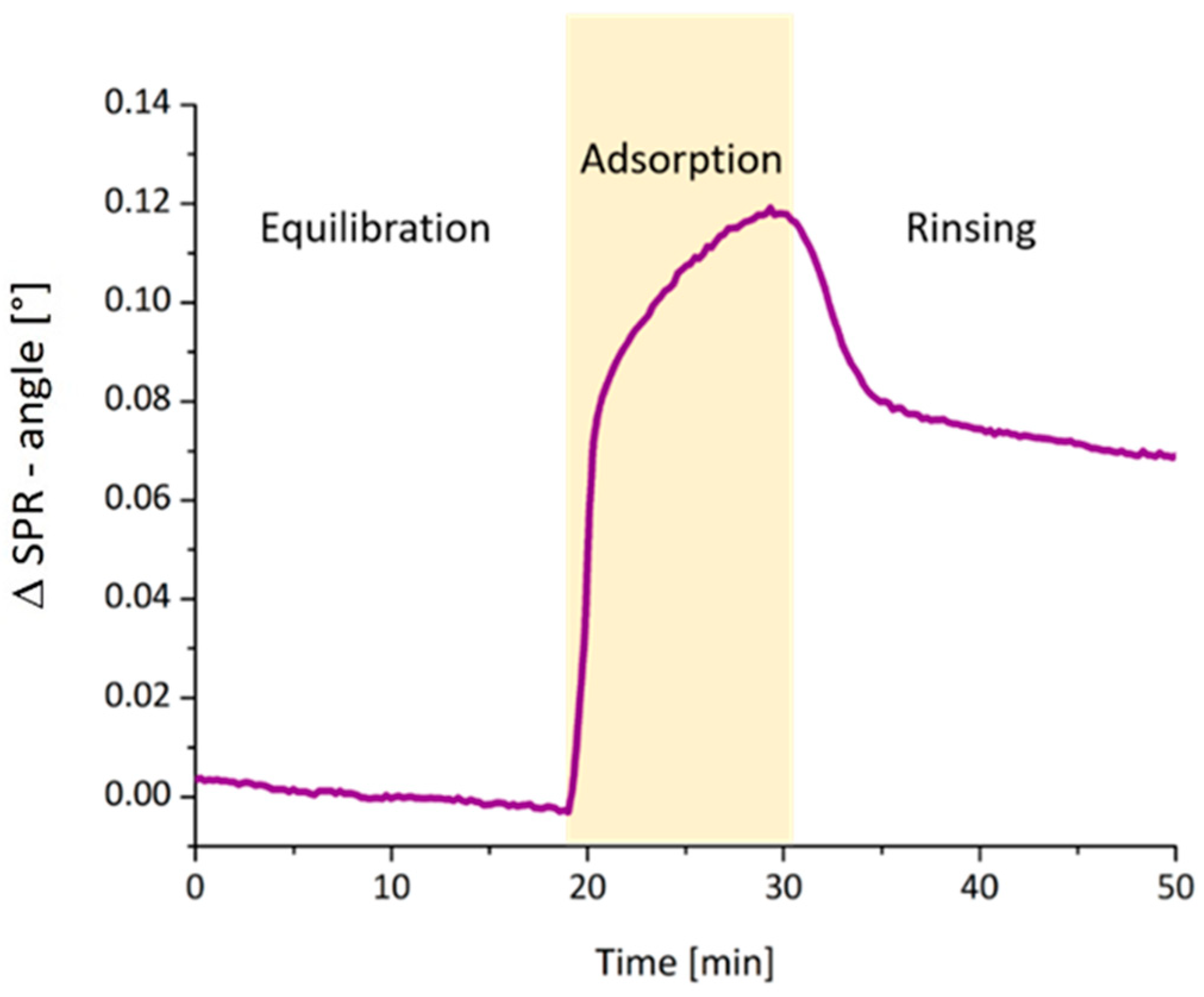
2.7. Multi-Parameter Sruface Plasmon Resonance Spectroscopy: MP-SPR
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hassan, A.; Balakrishnan, H.; Akbari, A. Polylactic Acid Based Blends, Composites and Nanocomposites. In Advances in Natural Polymers. Advanced Structured Materials; Thomas, S., Visakh, P., Mathew, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Ebringerova, A.; Heinze, T. Naturally occurring xylans structures, isolation procedures and properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-based products from xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, K.S.; Tenkanen, M. Sustainable food-packaging materials based on future biorefinery products: Xylans and mannans. Trends Food Sci. Technol. 2012, 28, 90–102. [Google Scholar] [CrossRef]
- McCleary, B.V.; Matheson, N.K.; Small, D.M. Galactomannans and a galactoglucomannan in legume seed endosperms: Structural requirements for b-mannanase hydrolysis. Phytochemistry 1976, 15, 1111–1117. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Clark, A.H.; McCleary, B.V. Effect of the molecular fine structure of galactomannans on their interaction properties—The role of unsubstituted sides. Food Hydrocoll. 1986, 1, 129–140. [Google Scholar] [CrossRef]
- Fox, J.E. Seed gums. In Thickening and Gelling Agents for Food; Imeson, A., Ed.; Chapman Hall: Glasgow, UK, 1997; pp. 262–283. [Google Scholar]
- Mikkonen, K.S.; Rita, H.; Helén, H.; Talja, R.A.; Hyvönen, L.; Tenkanen, M. Effect of polysaccharide structure on mechanical and thermal properties of galactomannan-based films. Biomacromolecules 2007, 8, 3198–3205. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Morrison, A. Chemistry and interactions of seed galactomannans. In Advances in Carbohydrate Chemistry and BioChemistry; Academic Press: Cambridge, MA, USA, 1975; Volume 31, pp. 241–312. [Google Scholar]
- Morris, V.J. Gelation of polysaccharides. In Functional Properties of Food Macromolecules, 2nd ed.; Hill, S.E., Ledward, D.A., Mitchell, J.R., Eds.; Mitchell, Aspen Publishers Inc.: Gaithersburg, MD, USA, 1998; pp. 143–226. [Google Scholar]
- Goycoolea, F.M.; Morris, E.R.; Gidley, M.J. Viscosity of galactomannans at alkaline and neutral pH: Evidence of ’hyperentanglement’ in solution. Carbohydr. Polym. 1995, 27, 69–71. [Google Scholar] [CrossRef]
- Sébastien, G.; Christophe, B.; Mario, A.; Pascal, L.; Michel, P.; Aurore, R. Impact of purification and fractionation process on the chemical structure and physical properties of locust bean gum. Carbohydr. Polym. 2014, 108, 159–168. [Google Scholar] [CrossRef]
- Morris, E. Shear-Thinning of ‘Random Coil’ Polysaccharides: Characterisation by Two Parameters from a Simple Linear Plot. Carbohydr. Polym. 1990, 13, 85–96. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Reicher, F.; Mazeau, K. Conformational analysis of galactomannans: From oligomeric segments to polymeric chains. Carbohydr. Polym. 1998, 37, 25–39. [Google Scholar] [CrossRef]
- Tenkanen, M. Enzymatic Tailoring of Hemicelluloses. In Hemicelluloses: Science and Technology; American Chemical Society: Washington, DC, USA, 2003; pp. 292–311. [Google Scholar]
- Burkart, A. The genus Prosopis and annoted key to the species of the world. In Mesquite: Its Biology in Two Desert Ecosystems; Dowden, Hutchinson & Ross Inc.: Stroudsburg, PA, USA, 1977; pp. 201–215. [Google Scholar]
- Pasiecznik, N.M.; Harris, P.J.; Smith, J.S. Identifying Tropical Prosopis Species. A Field Guide; Hydra Publishing: Coventry, UK, 2004. [Google Scholar]
- Echeverría, R.D. Monitoreo de los Recursos Forestales–Inventario Forestal Nacional–Resumen de Resultados–Etapa I; Ministerio de Ganadería, Agricultura y Pesca: Montevideo, Uruguay, 2010. [Google Scholar]
- Valenga, F.; Petri, D.F.S.; Lucyszyn, N.; Jó, T.A.; Sierakowski, M.R. Galactomannan thin films as supports for the immobilization of Concanavalin A and/or dengue viruses. Int. J. Biol. Macromol. 2012, 50, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.S.; Youan, B.-B.C. Concanavalin A-polysaccharides binding affinity analysis using a quartz crystal microbalance. Biosens. Bioelectron. 2014, 59, 404–411. [Google Scholar] [PubMed] [Green Version]
- Mohan, T.; Niegelhell, K.; Nagaraj, C.; Reishofer, D.; Spirk, S.; Olschewski, A.; Stana Kleinschek, K.; Kargl, R. Interaction of Tissue Engineering Substrates with Serum Proteins and Its Influence on Human Primary Endothelial Cells. Biomacromolecules 2017, 18, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Strasser, S.; Niegelhell, K.; Kaschowitz, M.; Markus, S.; Kargl, R.; Stana-Kleinschek, K.; Slugovc, C.; Mohan, T.; Spirk, S. Exploring Nonspecific Protein Adsorption on Lignocellulosic Amphiphilic Bicomponent Films. Biomacromolecules 2016, 17, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Vilaró, P.; Bennadji, Z.; Budelli, E.; Moyna, G.; Panizzolo, L.; Ferreira, F. Isolation and characterization of galactomannans from Prosopis affinis as potential gum substitutes. Food Hydrocoll. 2018, 77, 711–719. [Google Scholar] [CrossRef]
- Niegelhell, K.; Chemelli, A.; Hobisch, J.; Griesser, T.; Reiter, H.; Hirn, U.; Spirk, S. Interaction of industrially relevant cationic starches with cellulose. Carbohydr. Polym. 2018, 179, 290–296. [Google Scholar] [CrossRef]
- Mohan, T.; Spirk, S.; Kargl, R.; Doliška, A.; Ehmann, H.M.A.; Köstler, S.; Ribitsch, V.; Stana-Kleinschek, K. Watching cellulose grow—Kinetic investigations on cellulose thin film formation at the gas–solid interface using a quartz crystal microbalance with dissipation (QCM-D). Colloids Surf. A Physicochem. Eng. Asp. 2012, 400, 67–72. [Google Scholar] [CrossRef]
- Mohan, T.; Niegelhell, K.; Zarth, C.S.; Kargl, R.; Kostler, S.; Ribitsch, V.; Heinze, T.; Spirk, S.; Stana-Kleinschek, K. Triggering protein adsorption on tailored cationic cellulose surfaces. Biomacromolecules 2014, 15, 3931–3941. [Google Scholar] [CrossRef]
- Mohan, T.; Kargl, R.; Doliska, A.; Ehmann, H.M.; Ribitsch, V.; Stana-Kleinschek, K. Enzymatic digestion of partially and fully regenerated cellulose model films from trimethylsilyl cellulose. Carbohydr. Polym. 2013, 93, 191–198. [Google Scholar] [CrossRef]
- Theisen, A.; Johann, C.; Deacon, M.P.; Harding, S.E. Refractive Increment Data-Book; Nottingham University Press: Nottingham, UK, 2000. [Google Scholar]
- Niegelhell, K.; Süßenbacher, M.; Jammernegg, K.; Ganner, T.; Schwendenwein, D.; Schwab, H.; Stelzer, F.; Plank, H.; Spirk, S. Enzymes as Biodevelopers for Nano- and Micropatterned Bicomponent Biopolymer Thin Films. Biomacromolecules 2016, 17, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, E.; Thüne, P.C.; Niemantsverdriet, J.W. Cellulose Model Surfaces Simplified Preparation by Spin Coating and Characterization by X-ray Photoelectron Spectroscopy, Infrared Spectroscopy, and Atomic Force Microscopy. Langmuir 2003, 19, 5735–5741. [Google Scholar] [CrossRef]
- Kontturi, E.; Lankinen, A. Following the kinetics of a chemical reaction in ultrathin supported polymer films by reliable mass density determination with x-ray reflectivity. J. Am. Chem. Soc. 2010, 132, 3678–3679. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Spirk, S.; Kargl, R.; Doliška, A.; Vesel, A.; Salzmann, I.; Resel, R.; Ribitsch, V.; Stana-Kleinschek, K. Exploring the rearrangement of amorphous cellulose model thin films upon heat treatment. Soft Matter 2012, 8, 9807–9815. [Google Scholar] [CrossRef]
- Lenz, R.W. Cellulose, structure, accessibility and reactivity, by H. A. Krässig, Gordon and Breach Publishers, 5301 Tacony Street, Philadelphia, PA, 1993; xvi + 376 pp. Price: $260.00. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 2401. [Google Scholar] [CrossRef]
- Ehmann, H.M.A.; Werzer, O.; Pachmajer, S.; Mohan, T.; Amenitsch, H.; Resel, R.; Kornherr, A.; Stana-Kleinschek, K.; Kontturi, E.; Spirk, S. Surface-Sensitive Approach to Interpreting Supramolecular Rearrangements in Cellulose by Synchrotron Grazing Incidence Small-Angle X-ray Scattering. ACS Macro Lett. 2015, 4, 713–716. [Google Scholar] [CrossRef]
- Jones, A.O.F.; Resel, R.; Schrode, B.; Machado-Charry, E.; Röthel, C.; Kunert, B.; Salzmann, I.; Kontturi, E.; Reishofer, D.; Spirk, S. Structural Order in Cellulose Thin Films Prepared from a Trimethylsilyl Precursor. Biomacromolecules 2020, 21, 653–659. [Google Scholar] [CrossRef]
- Kontturi, E.; Spirk, S. Ultrathin Films of Cellulose: A Materials Perspective. Front. Chem. 2019, 7, 488. [Google Scholar] [CrossRef] [Green Version]
- Raghuwanshi, V.S.; Garnier, G. Cellulose Nano-Films as Bio-Interfaces. Front. Chem. 2019, 7, 2296–2646. [Google Scholar] [CrossRef] [Green Version]
- Dam, T.K.; Roy, R.; Das, S.K.; Oscarson, S.; Brewer, C.F. Binding of Multivalent Carbohydrates to Concanavalin A and Dioclea grandiflora Lectin. Thermodynamic Analysis of the ‘Multivalency effect’. J. Biol. Chem. 2000, 275, 14223–14230. [Google Scholar] [CrossRef] [Green Version]
- Sekharudu, Y.C.; Biswas, M.; Rao, V.S.R. Complex carbohydrates: 2. The modes of binding of complex carbohydrates to Concanavalin A—A computer modelling approach. Int. J. Biol. Macromol. 1986, 8, 9–19. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Hollerman, C.E.; Smith, E.E. Protein-Carbohydrate Interaction. II. Inhibition Studies on the Interaction of Concanavalin A with Polysaccharides. Biochemistry 1965, 4, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Sekharudu, Y.C.; Rao, V.S.R. Theoretical studies on the modes of binding of some of the derivatives of d-mannose to Concanavalin A. Int. J. Biol. Macromol. 1984, 6, 337–347. [Google Scholar] [CrossRef]
- Miller, M.C.; Ribeiro, J.P.; Roldós, V.; Martín-Santamaría, S.; Cañada, F.J.; Nesmelova, I.A.; André, S.; Pang, M.; Klyosov, A.A.; Baum, L.G.; et al. Structural aspects of binding of-linked digalactosides to human galectin-1. Glycobiology 2011, 21, 1627–1641. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2016, 26, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.; Pérez, S. Thermodynamics and chemical characterization of protein-carbohydrate interactions: The multivalency issue. Comptes Rendus Chimie 2011, 14, 74–95. [Google Scholar] [CrossRef]
- Perez, S.; Tvaroska, I. Carbohydrate-protein interactions: Molecular modeling insights. Adv. Carbohydr. Chem. Biochem. 2014, 71, 9–136. [Google Scholar]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef]
- Lombardo, S.; Eyley, S.; Schütz, C.; van Gorp, H.; Rosenfeldt, S.; van den Mooter, G.; Thielemans, W. Thermodynamic Study of the Interaction of Bovine Serum Albumin and Amino Acids with Cellulose Nanocrystals. Langmuir 2017, 33, 5473–5481. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilaró, P.; Sampl, C.; Teichert, G.; Schlemmer, W.; Hobisch, M.; Weissl, M.; Panizzolo, L.; Ferreira, F.; Spirk, S. Interactions and Dissociation Constants of Galactomannan Rendered Cellulose Films with Concavalin A by SPR Spectroscopy. Polymers 2020, 12, 3040. https://doi.org/10.3390/polym12123040
Vilaró P, Sampl C, Teichert G, Schlemmer W, Hobisch M, Weissl M, Panizzolo L, Ferreira F, Spirk S. Interactions and Dissociation Constants of Galactomannan Rendered Cellulose Films with Concavalin A by SPR Spectroscopy. Polymers. 2020; 12(12):3040. https://doi.org/10.3390/polym12123040
Chicago/Turabian StyleVilaró, Pilar, Carina Sampl, Gundula Teichert, Werner Schlemmer, Mathias Hobisch, Michael Weissl, Luis Panizzolo, Fernando Ferreira, and Stefan Spirk. 2020. "Interactions and Dissociation Constants of Galactomannan Rendered Cellulose Films with Concavalin A by SPR Spectroscopy" Polymers 12, no. 12: 3040. https://doi.org/10.3390/polym12123040
APA StyleVilaró, P., Sampl, C., Teichert, G., Schlemmer, W., Hobisch, M., Weissl, M., Panizzolo, L., Ferreira, F., & Spirk, S. (2020). Interactions and Dissociation Constants of Galactomannan Rendered Cellulose Films with Concavalin A by SPR Spectroscopy. Polymers, 12(12), 3040. https://doi.org/10.3390/polym12123040