In Vitro and In Vivo Studies of Biodegradability and Biocompatibility of Poly(εCL)-b-Poly(EtOEP)-Based Films
Abstract
:1. Introduction
2. Materials and Methods
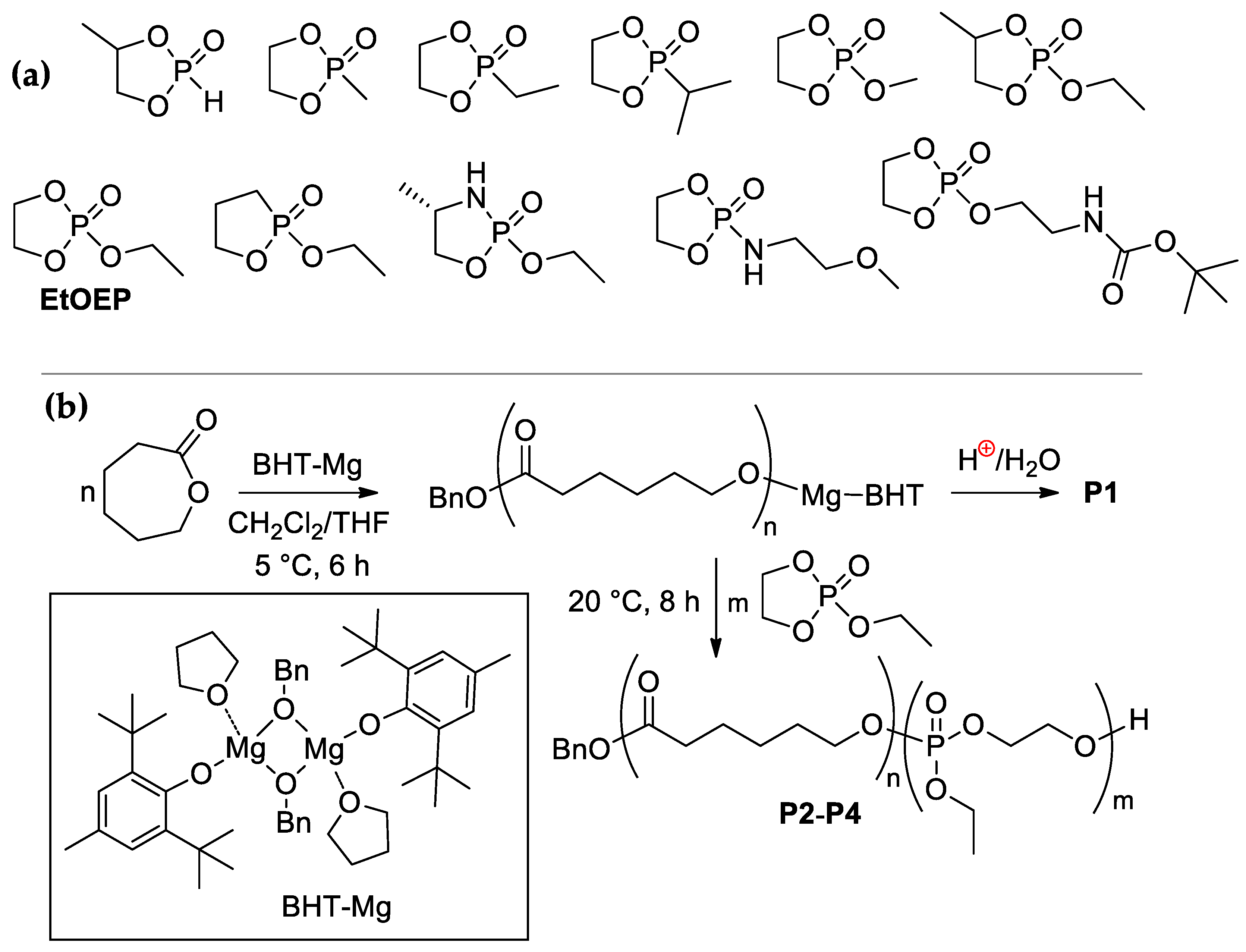
2.1. Synthesis of (co)Polymers
2.1.1. General Experimental Remarks
2.1.2. Synthesis of Poly(εCL) P1
2.1.3. Synthesis of Poly(εCL)-b-Poly(EtOEP) P2–P4
2.2. Preparation and Mechanical Testing of Polymer Films
2.3. Preparation of Polymer Films for Hydrolytic and Biomedical Testing
2.3.1. Preparation of Polymer Films
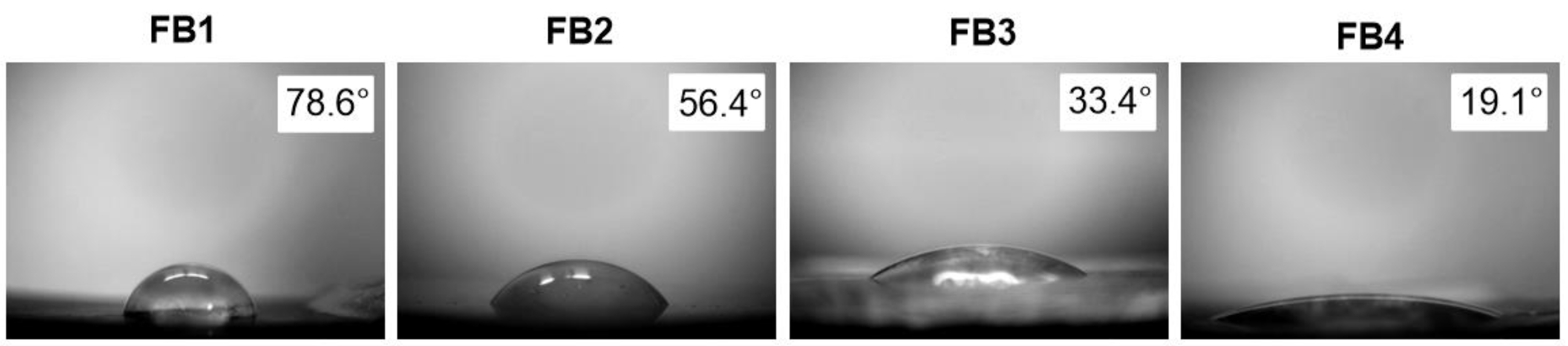
2.3.2. Contact Angle Measurements
2.3.3. Hydrolytic Degradation in Vitro
2.3.4. Preparation of the Samples for Biomedical Testing
2.4. Protein Adhesion
2.5. Cultivation of UC MSCs
2.6. Cell Visualization and Count
2.7. Cytotoxicity Studies
2.8. Immunocytochemical Studies
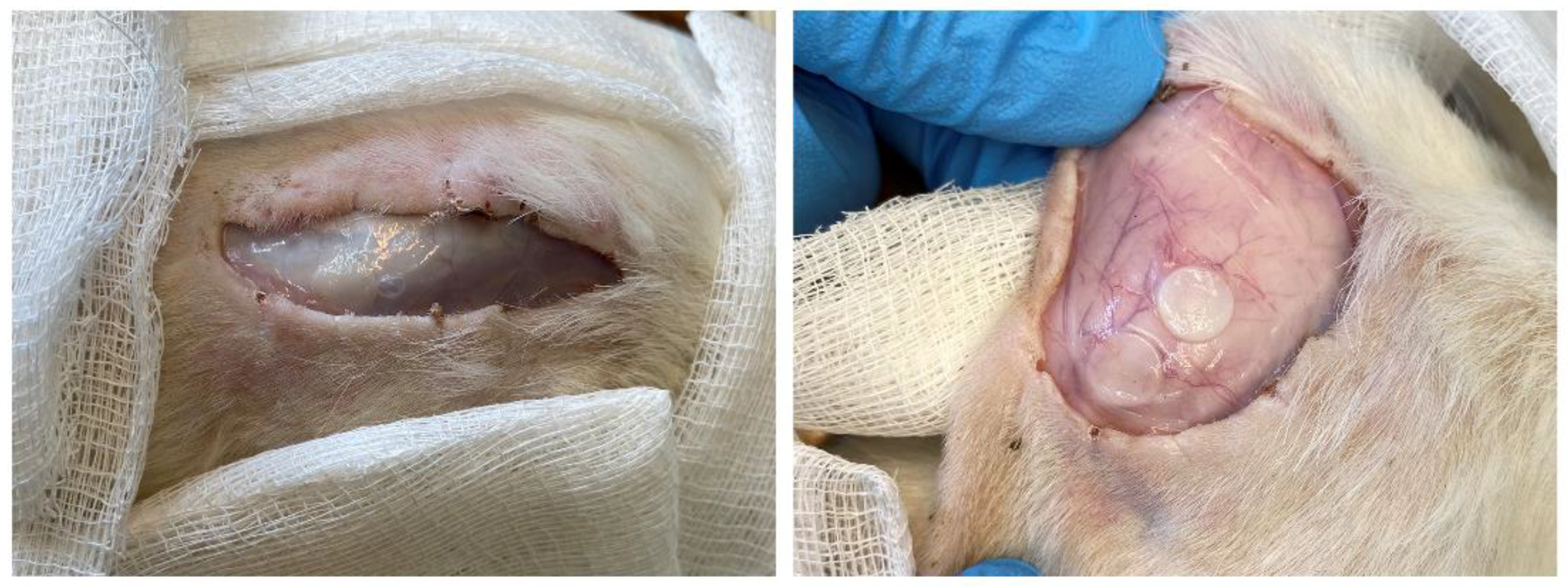
2.9. Subcutaneous Implantation Experiments
2.9.1. Animals
2.9.2. Subcutaneous Administration of the Polymer Films
2.9.3. Morphometric Studies
2.9.4. Immunohistochemical Studies
2.10. Statistical Analysis
3. Results and Discussion
3.1. Polymerization and (co)Polymer Characteristics
3.2. Mechanical Properties of Polymers
3.3. Hyrdophilicity and Hydrolysis In Vitro
3.3.1. The Results of the Contact Angle Measurements
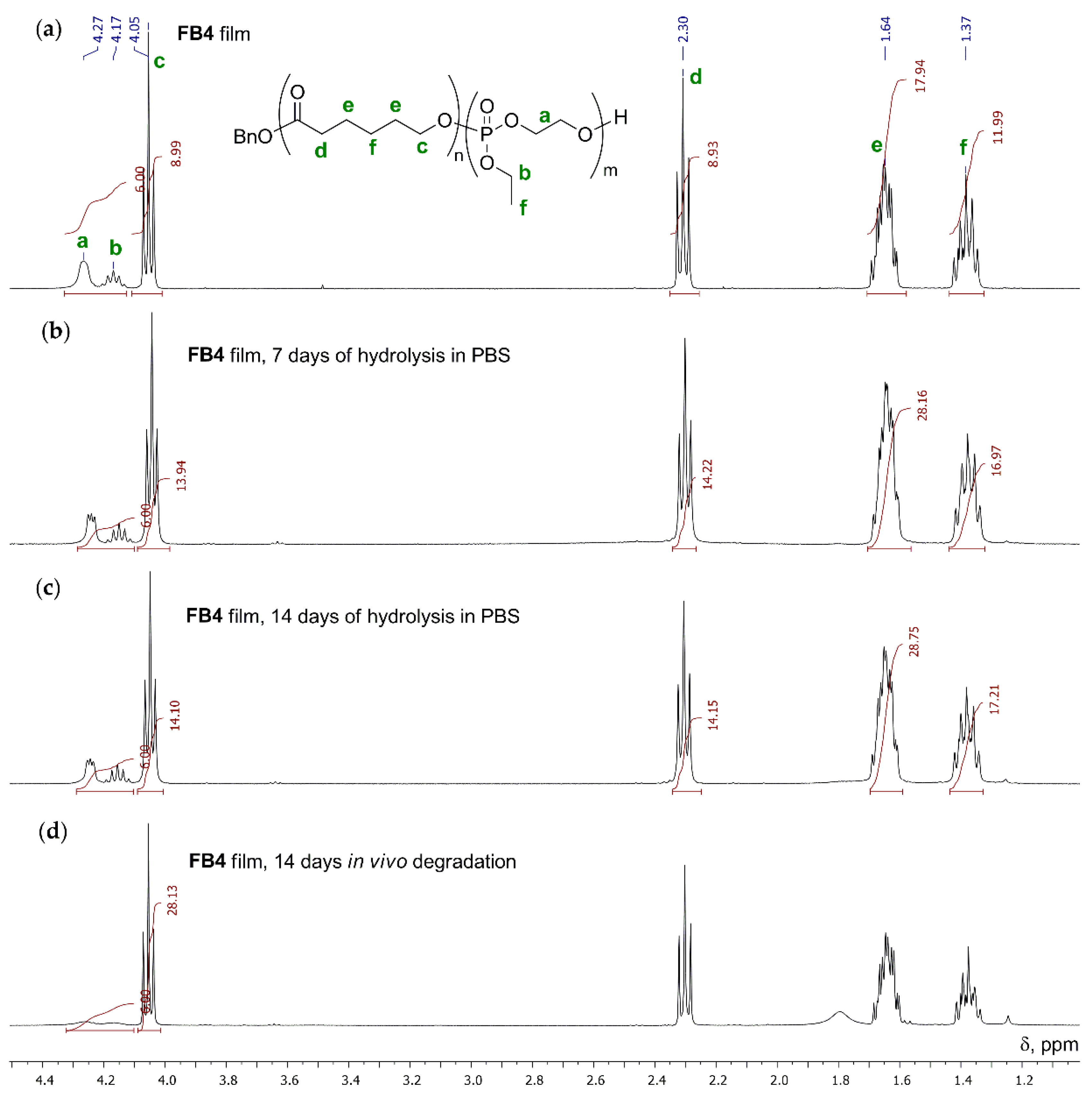
3.3.2. Hydrolytic Degradation in Buffer Solution
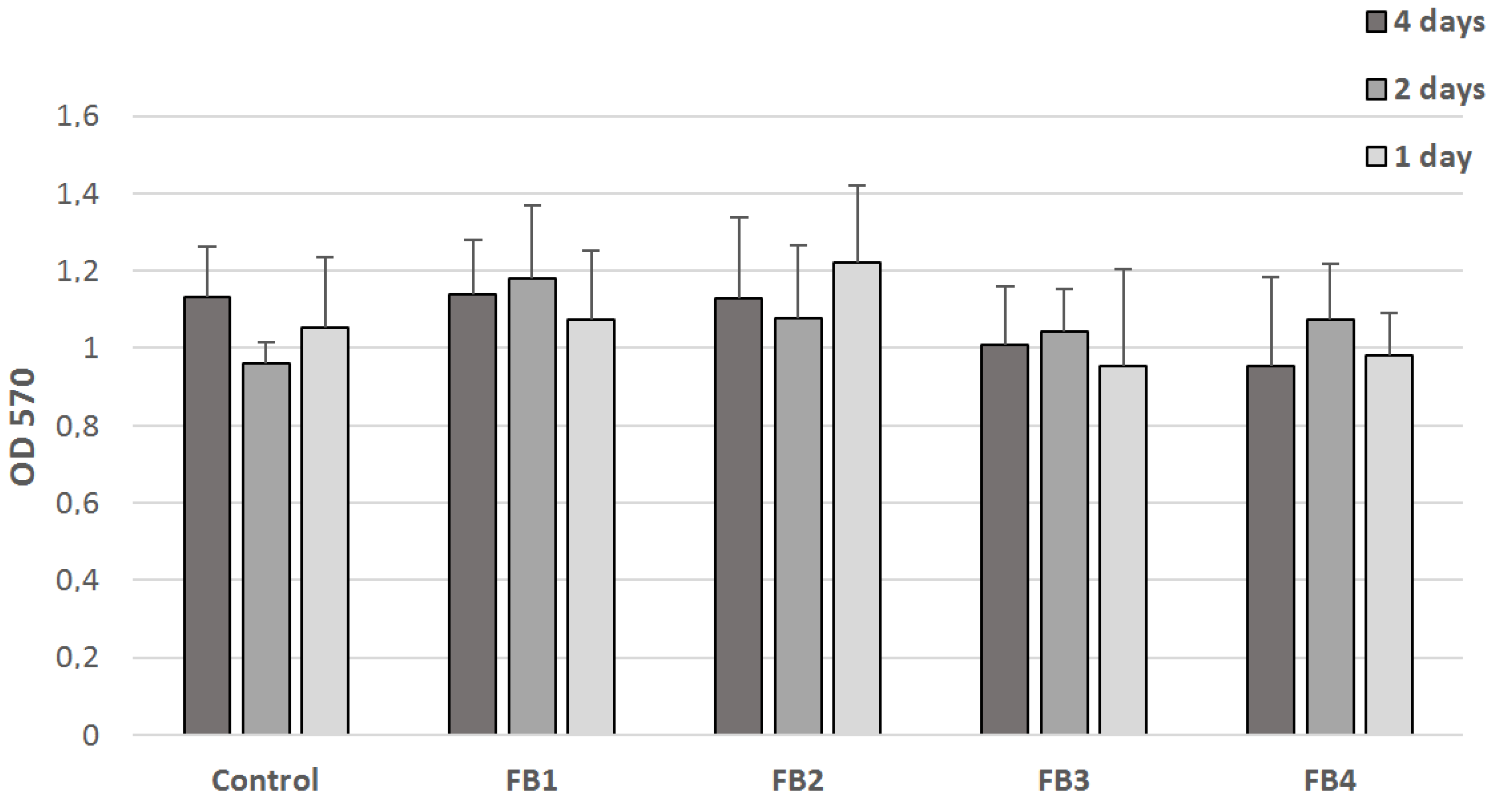
3.4. Cytotoxicity
3.5. Protein Adhesion
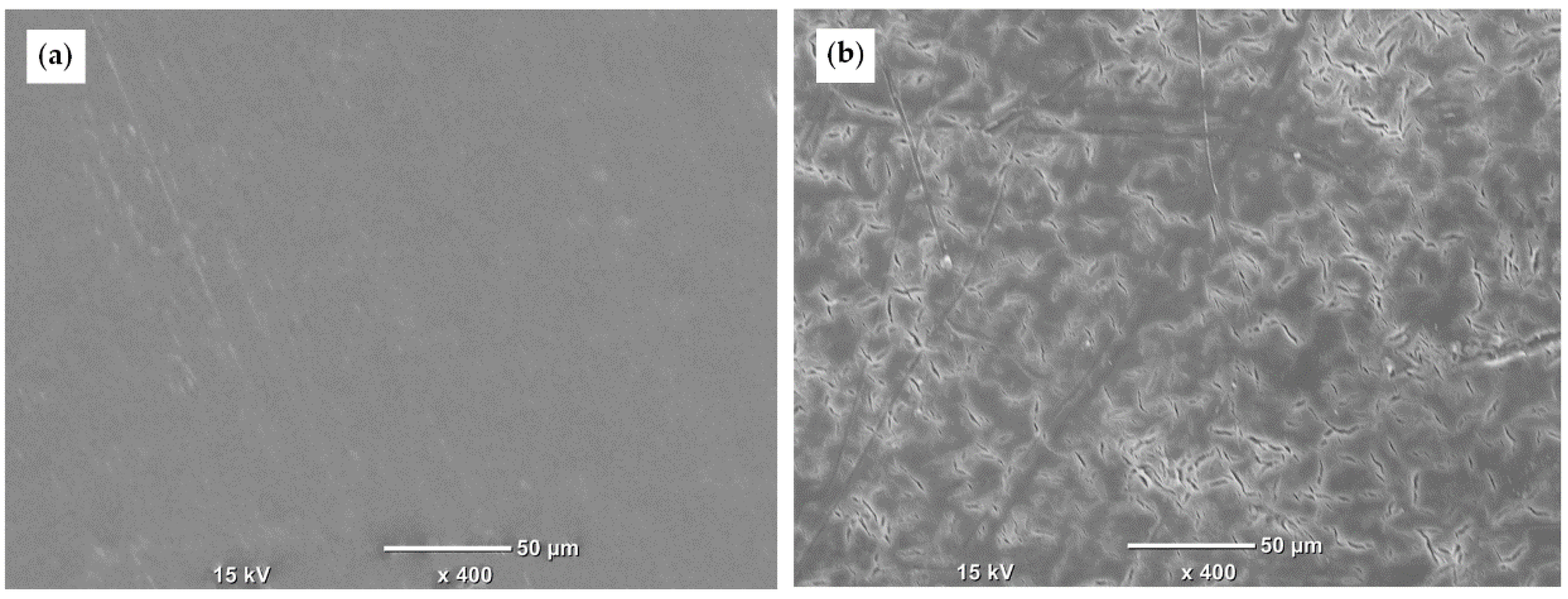
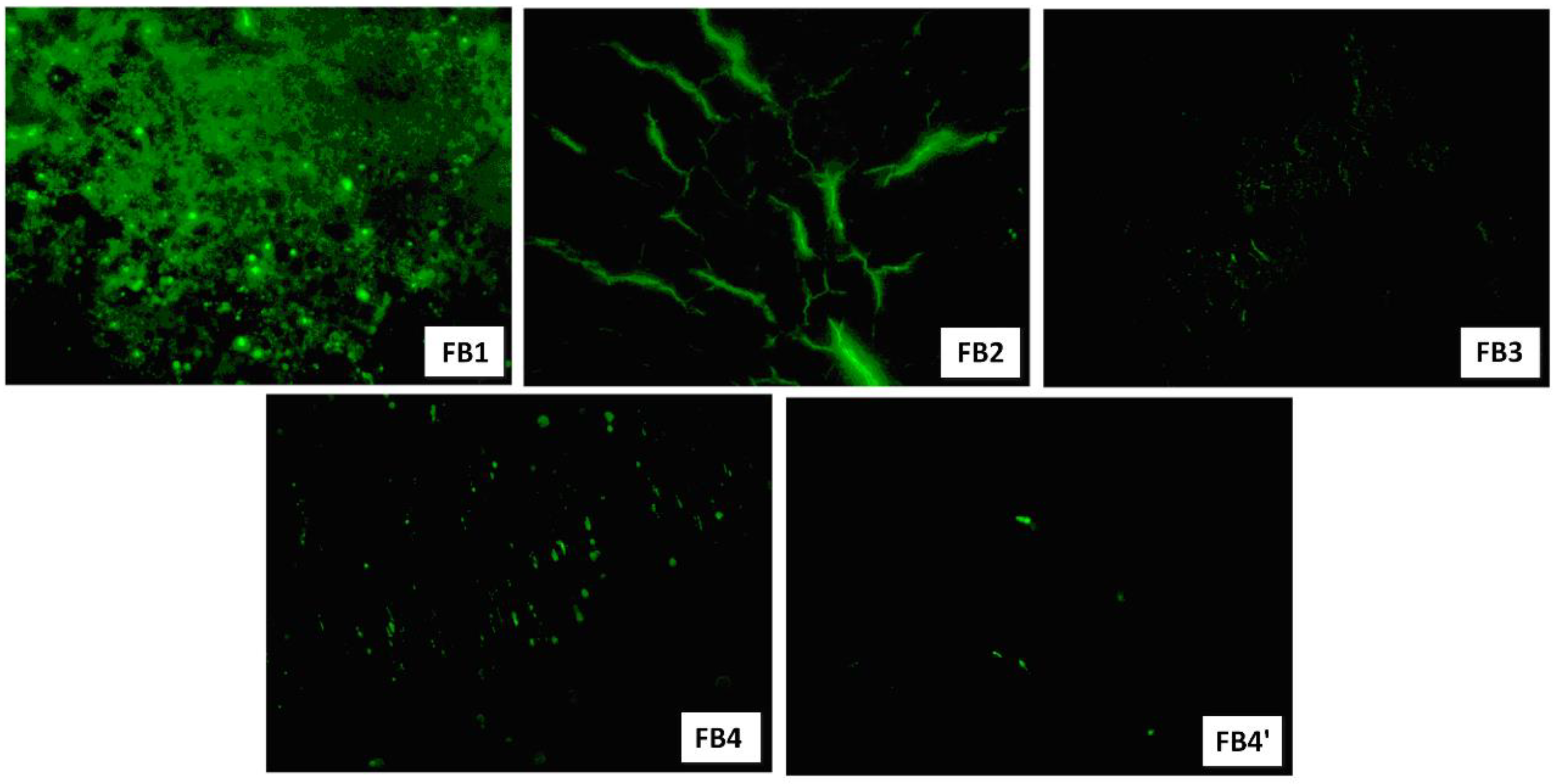
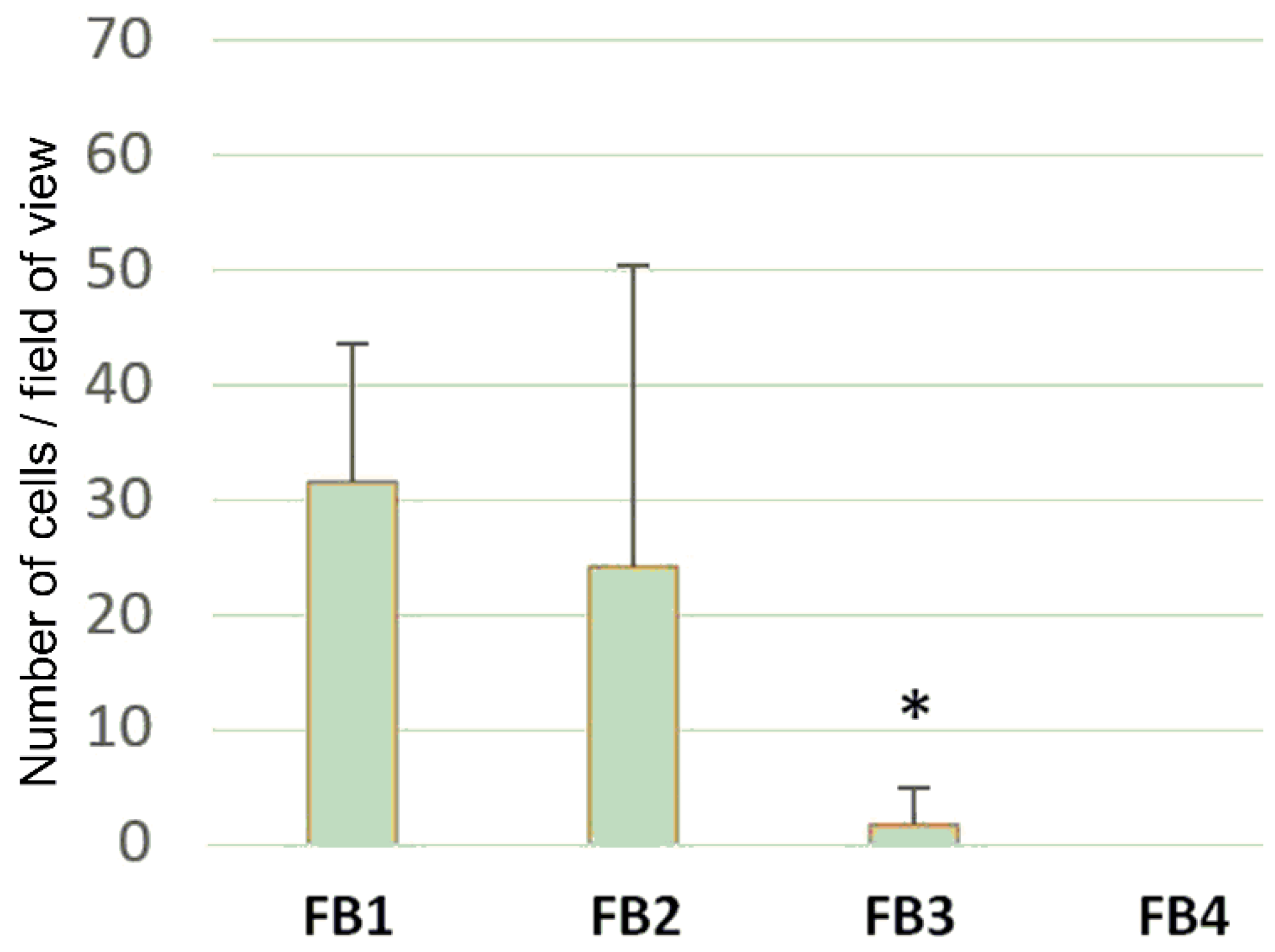
3.6. Cell Adhesion and Cell Proliferation
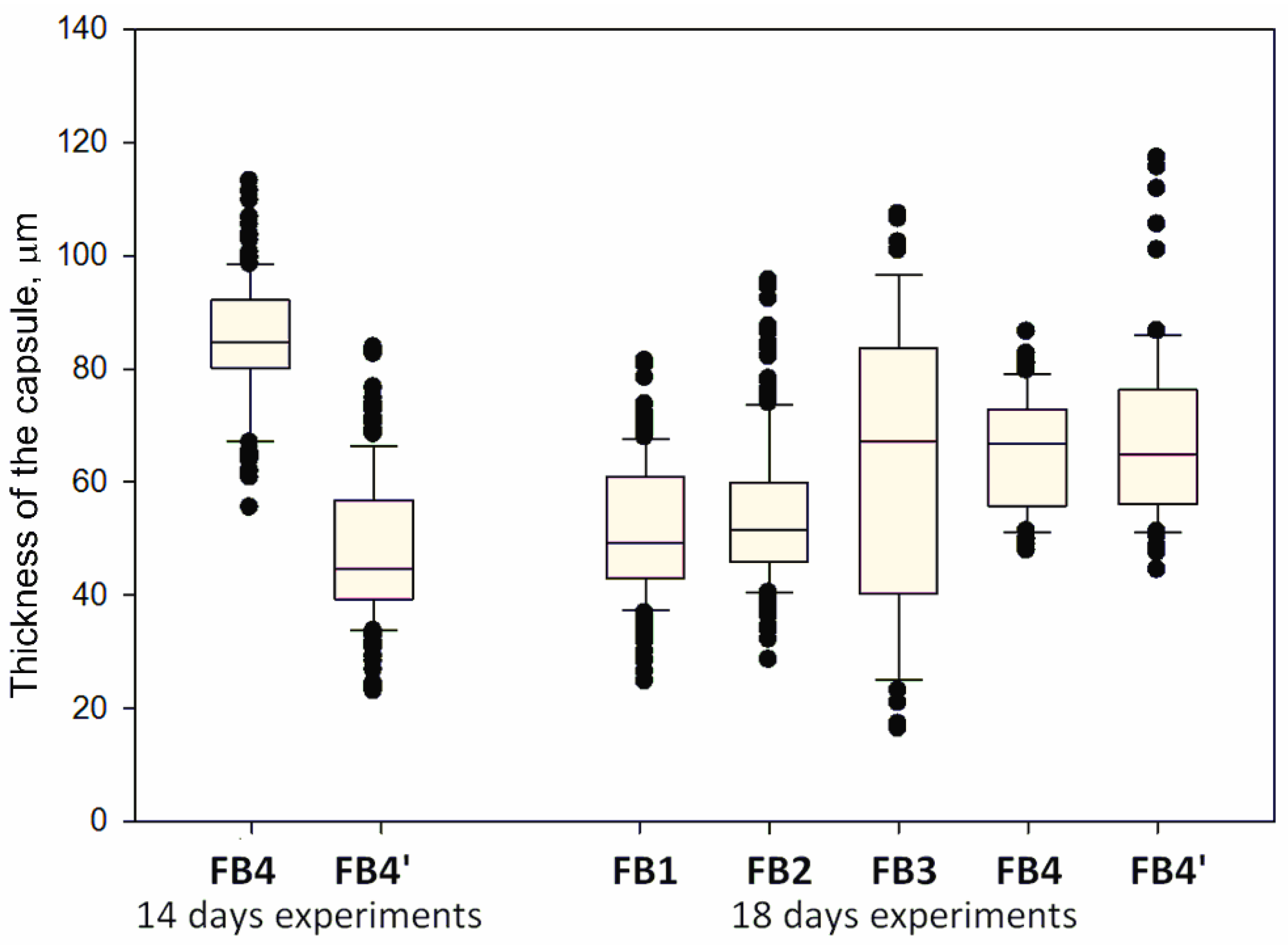
3.7. Subcutaneous Implantation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schulte, V.A.; Díez, M.; Möller, M.; Lensen, M.C. Surface Topography Induces Fibroblast Adhesion on Intrinsically Nonadhesive Poly(ethylene glycol) Substrates. Biomacromolecules 2009, 10, 2795–2801. [Google Scholar] [CrossRef]
- Nair, A.; Zou, L.; Bhattacharyya, D.; Timmons, R.B.; Tang, L. Species and Density of Implant Surface Chemistry Affect the Extent of Foreign Body Reactions. Langmuir 2008, 24, 2015–2024. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yuan, L.; Song, W.; Wu, Z.; Li, D. Biocompatible polymer materials: Role of protein–surface interactions. Progr. Polym. Sci. 2008, 33, 1059–1087. [Google Scholar] [CrossRef]
- Ngo, B.K.D.; Grunlan, M.A. Protein Resistant Polymeric Biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, C.; Tinè, M.R.; Wurm, F.R. Main-chain water-soluble polyphosphoesters: Multi-functional polymers as degradable PEG-alternatives for biomedical applications. Eur. Polym. J. 2020, 141, 110079. [Google Scholar] [CrossRef]
- Abraham, A.A.; Means, A.K.; Clubb, F.J., Jr.; Fei, R.; Locke, A.K.; Gacasan, E.G.; Coté, G.L.; Grunlan, M.A. Foreign Body Reaction to a Subcutaneously Implanted Self-Cleaning, Thermoresponsive Hydrogel Membrane for Glucose Biosensors. ACS Biomater. Sci. Eng. 2018, 4, 4104–4111. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yuan, Y.-Y.; Du, J.-Z.; Yang, X.-Z.; Wang, J. Recent progress in polyphosphoesters: From controlled synthesis to biomedical applications. Macromol. Biosci. 2009, 9, 1154–1164. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.B.; Kaluzynski, K.; Lapienis, G. Polymers with Esters of Phosphoric Acid Units: From Synthesis, Models of Biopolymers to Polymer—Inorganic Hybrids. Isr. J. Chem. 2012, 52, 306–319. [Google Scholar] [CrossRef]
- Steinbach, T.; Wurm, F.R. Poly(phosphoester)s: A new platform for degradable polymers. Angew. Chem. Int. Ed. 2015, 54, 6098–6108. [Google Scholar] [CrossRef]
- Yilmaz, Z.E.; Jérôme, C. Polyphosphoesters: New trends in synthesis and drug delivery applications. Macromol. Biosci. 2016, 16, 1745–1761. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Controlled ring-opening polymerisation of cyclic phosphates, phosphonates and phosphoramidates catalysed by hereroleptic BHT-alkoxy magnesium complexes. Polym. Chem. 2017, 8, 6806–6816. [Google Scholar] [CrossRef]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar] [CrossRef] [Green Version]
- Bauer, K.N.; Liu, L.; Wagner, M.; Andrienko, D.; Wurm, F.R. Mechanistic study on the hydrolytic degradation of polyphosphates. Eur. Polym. J. 2018, 108, 286–294. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Liu, X.-Q.; Sun, T.-M.; Xiong, M.-H.; Wang, J. Functionalized micelles from block copolymer of polyphosphoester and poly(ɛ-caprolactone) for receptor-mediated drug delivery. J. Contr. Release 2008, 128, 32–40. [Google Scholar] [CrossRef]
- Steinbach, T.; Becker, G.; Spiegel, A.; Figueiredo, T.; Russo, D.; Wurm, F.R. Reversible bioconjugation: Biodegradable poly(phosphate)-protein conjugates. Macromol. Biosci. 2017, 17, 1600377. [Google Scholar] [CrossRef]
- Pelosi, C.; Duce, C.; Russo, D.; Tiné, M.R.; Wurm, F.R. PPEylation of proteins: Synthesis, activity, and stability of myoglobin-polyphosphoester conjugates. Eur. Polym. J. 2018, 108, 357–363. [Google Scholar] [CrossRef]
- Zhai, X.; Huang, W.; Liu, J.; Pang, Y.; Zhu, X.; Zhou, Y.; Yan, D. Micelles from amphiphilic block copolyphosphates for drug delivery. Macromol. Biosci. 2011, 11, 1603–1610. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Waymouth, R.M.; Wender, P.A. Cell-Penetrating, Guanidinium-Rich Oligophosphoesters: Effective and Versatile Molecular Transporters for Drug and Probe Delivery. J. Am. Chem. Soc. 2016, 138, 3510–3517. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, S.; Pollack, S.F.; Li, R.; Gonzalez, A.M.; Fan, J.; Zou, J.; Leininger, S.E.; Pavía-Sanders, A.; Johnson, R.; et al. Improving paclitaxel delivery: In vitro and in vivo characterization of PEGylated polyphosphoester-based nanocarriers. J. Am. Chem. Soc. 2015, 137, 2056–2066. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotech. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Pranantyo, D.; Xu, L.Q.; Kang, E.-T.; Mya, M.K.; Chan-Park, M.B. Conjugation of polyphosphoester and antimicrobial peptide for enhanced bactericidal activity and biocompatibility. Biomacromolecules 2016, 17, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein–Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.-Z.; Sun, T.-M.; Dou, S.; Wu, J.; Wang, Y.-C.; Wang, J. Block Copolymer of Polyphosphoester and Poly(l-Lactic Acid) Modified Surface for Enhancing Osteoblast Adhesion, Proliferation, and Function. Biomacromolecules 2009, 10, 2213–2220. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Minyaev, M.E.; Churakov, A.V.; Karchevsky, S.G.; Birin, K.P.; Ivchenko, P.V. Mono-BHT heteroleptic magnesium complexes: Synthesis, molecular structure and catalytic behavior in the ring-opening polymerization of cyclic esters. Dalton Trans. 2017, 46, 12132–12146. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, T.; Schröder, R.; Ritz, S.; Wurm, F.R. Microstructure analysis of biocompatible phosphoester copolymers. Polym. Chem. 2013, 4, 4469–4479. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Stayshich, R.M.; Meyer, T.Y. Exploiting Sequence to Control the Hydrolysis Behavior of Biodegradable PLGA Copolymers. J. Am. Chem. Soc. 2011, 133, 6910–6913. [Google Scholar] [CrossRef]
- Pogorielov, M.; Hapchenko, A.; Deineka, V.; Rogulska, L.; Oleshko, O.; Vodseďálková, K.; Berezkinová, L.; Vysloužilová, L.; Klápšťová, A.; Erben, J. In vitro degradation and in vivo toxicity of NanoMatrix3D® polycaprolactone and poly(lactic acid) nanofibrous scaffolds. J. Biomed. Mater. Res. 2018, 106, 2200–2212. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. J. Quant. Cell Sci. 2018, 93, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Lamanna, R.; Corti, A.; Iorio, M.; Nocchi, F.; Urciuoli, P.; Lapi, S.; Scatena, F.; Franzini, M.; Vanacore, R.; Lorenzini, E.; et al. Are standard cell culture conditions adequate for human umbilical cord blood mesenchymal stem cells? Blood Transfus. 2014, 12, s375–s377. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Ivchenko, P.V.; Borisov, R.S.; Churakov, A.V. Monomeric and dimeric magnesium mono-BHT complexes as effective ROP catalysts. Catal. Commun. 2016, 87, 106–111. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Kosarev, M.A.; Tavtorkin, A.N.; Minyaev, M.E.; Roznyatovsky, V.A.; Ivchenko, P.V. Synthesis and ring-opening polymerization of glycidyl ethylene phosphate with a formation of linear and branched polyphosphates. Mendeleev Commun. 2018, 28, 155–157. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Komarov, P.D.; Tavtorkin, A.N.; Minyaev, M.E.; Kosarev, M.A.; Ivchenko, P.V. Synthesis in aqueous media of poly(ethylene phosphoric acids) by mild thermolysis of homopolymers and block copolymers based on tert-butyl ethylene phosphate. Eur. Polym. J. 2018, 106, 249–256. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Karchevsky, S.; Ivchenko, P. Mechanistic Insights of BHT-Mg-Catalyzed Ethylene Phosphate’s Coordination Ring-Opening Polymerization: DFT Modeling and Experimental Data. Polymers 2018, 10, 1105. [Google Scholar] [CrossRef] [Green Version]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Gavrilov, D.; Karchevsky, S.; Ivchenko, P. DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers 2019, 11, 1641. [Google Scholar] [CrossRef] [Green Version]
- Kosarev, M.A.; Gavrilov, D.E.; Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Dyadchenko, V.P.; Roznyatovsky, V.A.; Ivchenko, P.V. Ultrafast hydrolytic degradation of 2,3-dihydroxypropyl functionalized poly(ethylene phosphates). Mendeleev Commun. 2019, 29, 509–511. [Google Scholar] [CrossRef]
- Pannecouque, C.; Daelemans, D.; De Clercq, E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: Revisited 20 years later. Nat. Protoc. 2008, 3, 427–434. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Höglund, A.; Odelius, K.; Albertsson, A.-C. Tuning the Release Rate of Acidic Degradation Products through Macromolecular Design of Caprolactone-Based Copolymers. J. Am. Chem. Soc. 2007, 129, 6308–6312. [Google Scholar] [CrossRef]



| Run | εCL/EtOEP/BHT-Mg Initial Ratio | εCL Conv., % 1 | EtOEP Conv., % 1 | Polymer Composition DPn (εCL)/ DPn (EtOEP) 2 | Mntheo × 103 3 | MnNMR × 103 2 | MnSEC × 103 4 | ÐM4 |
|---|---|---|---|---|---|---|---|---|
| P1628 | 200/–/1 | >99 | — | — | 22.9 | 30.0 | 36.0 | 1.38 |
| P2 | 200/20/1 | >99 | 83 | 31.6 | 25.2 | 34.9 | 32.7 | 1.81 |
| P3 | 200/40/1 | >99 | 92 | 8.3 | 27.9 | 31.3 | 28.8 | 2.00 |
| P4 | 200/60/1 | >99 | 87 | 2.8 | 32.4 | 32.6 | 46.5 | 2.04 |
| Polymer | Average Cross-Sectional Area, mm2 | Tensile Strength, MPa | Yield Stress σ, MPa | Young’s Modulus E, MPa | Elongation at Break εp, % |
|---|---|---|---|---|---|
| FM1 | 0.81 | 20.2 ± 9.6 | 14.5 ± 0.6 | 207 ± 19 | 72 ± 78 |
| FM2 | 0.84 | 11.9 ± 5.0 | 16.6 ± 0.7 | 251 ± 20 | 455 ± 147 |
| FM3 | 0.82 | 9.5 ± 6.2 | 14.8 ± 0.5 | 200 ± 39 | 515 ± 100 |
| FM4 | 0.82 | 4.0 ± 1.4 | 6.3 ± 2.0 | 163 ± 68 | 4.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Shlyakhtin, A.; Komarov, P.; Tavtorkin, A.; Kananykhina, E.; Elchaninov, A.; Vishnyakova, P.; Fatkhudinov, T.; Ivchenko, P. In Vitro and In Vivo Studies of Biodegradability and Biocompatibility of Poly(εCL)-b-Poly(EtOEP)-Based Films. Polymers 2020, 12, 3039. https://doi.org/10.3390/polym12123039
Nifant’ev I, Shlyakhtin A, Komarov P, Tavtorkin A, Kananykhina E, Elchaninov A, Vishnyakova P, Fatkhudinov T, Ivchenko P. In Vitro and In Vivo Studies of Biodegradability and Biocompatibility of Poly(εCL)-b-Poly(EtOEP)-Based Films. Polymers. 2020; 12(12):3039. https://doi.org/10.3390/polym12123039
Chicago/Turabian StyleNifant’ev, Ilya, Andrey Shlyakhtin, Pavel Komarov, Alexander Tavtorkin, Evgeniya Kananykhina, Andrey Elchaninov, Polina Vishnyakova, Timur Fatkhudinov, and Pavel Ivchenko. 2020. "In Vitro and In Vivo Studies of Biodegradability and Biocompatibility of Poly(εCL)-b-Poly(EtOEP)-Based Films" Polymers 12, no. 12: 3039. https://doi.org/10.3390/polym12123039
APA StyleNifant’ev, I., Shlyakhtin, A., Komarov, P., Tavtorkin, A., Kananykhina, E., Elchaninov, A., Vishnyakova, P., Fatkhudinov, T., & Ivchenko, P. (2020). In Vitro and In Vivo Studies of Biodegradability and Biocompatibility of Poly(εCL)-b-Poly(EtOEP)-Based Films. Polymers, 12(12), 3039. https://doi.org/10.3390/polym12123039











