Reactive Sintering of Ground Tire Rubber (GTR) Modified by a Trans-Polyoctenamer Rubber and Curing Additives
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
3. Results and Discussion
3.1. Curing Characteristics
3.2. FTIR Analysis
3.3. Physico-Mechanical Properties
3.4. Thermogravimetric Analysis
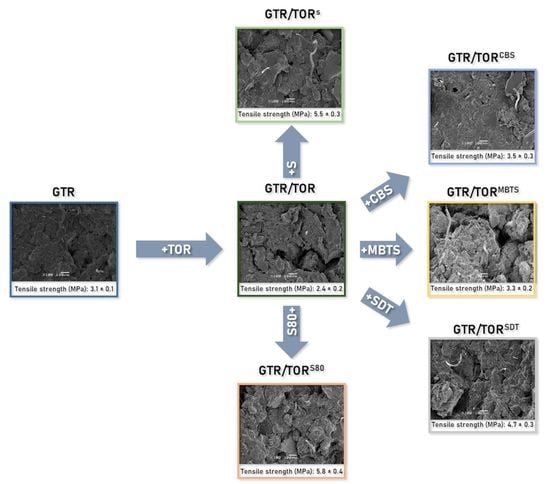
3.5. Scanning Electron Microscopy
3.6. Acoustic Properties
3.7. Differential Scanning Calorimetry
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Former, C.; Osen, E. State and prospects of rubber recycling. Kautsch. Gummi Kunstst. 2003, 56, 81–89. [Google Scholar]
- European Tyre Rubber Manufactures Association (ETRMA). European Tyre and Rubber Industry—Statistics. 2017. Available online: http://www.etrma.org/statistics-2 (accessed on 15 September 2020).
- Sienkiewicz, M.; Kucińska-Lipka, J.; Janik, H.; Balas, A. Progress in used tyres management in the European Union: A review. Waste Manag. 2012, 32, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Torretta, V.; Rada, E.C.; Ragazzi, M.; Trulli, E.; Istrate, I.A.; Cioca, L.I. Treatment and disposal of tyres: Two EU approaches. A review. Waste Manag. 2015, 45, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gieré, R.; Smith, K.; Blackford, M. Chemical composition of fuels and emissions from a coal + tire combustion experiment in a power station. Fuel 2006, 85, 2278–2285. [Google Scholar] [CrossRef]
- Tao, G.; He, Q.; Xia, Y.; Jia, G.; Yang, H.; Ma, W. The effect of devulcanization level on mechanical properties of reclaimed rubber by thermal-mechanical shearing devulcanization. J. Appl. Polym. Sci. 2013, 129, 2598–2605. [Google Scholar] [CrossRef]
- Saiwari, S.; Dierkes, W.K.; Noordermeer, J.W.M. Comparative investigation of the devulcanization parameters of tire rubbers. Rubber Chem. Technol. 2014, 87, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.M.; George, B.; Madhusoodanan, K.N.; Rosamma, A. Cure characteristics of devulcanized rubber: The issue of low scorch. Rubber Chem. Technol. 2017, 90, 536–549. [Google Scholar] [CrossRef]
- Movahed, S.O.; Ansarifar, A.; Estagy, S. Review of the reclaiming of rubber waste and recent work on the recycling of ethylene-propylene-diene rubber waste. Rubber Chem. Technol. 2016, 89, 54–78. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, C.; Liang, M. Properties of natural rubber vulcanizates containing mechanochemically devulcanized ground tire rubber. J. Polym. Res. 2009, 16, 411–419. [Google Scholar] [CrossRef]
- Sripornsawat, B.; Saiwari, S.; Pichaiyut, S.; Nakason, C. Influence of ground tire rubber devulcanization conditions on properties of its thermoplastic vulcanizate blends with copolyester. Eur. Polym. J. 2016, 85, 279–297. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Seghar, S.; Aït Hocine, N. Recycling of rubber wastes by devulcanization. Resour. Conserv. Recycl. 2018, 133, 250–262. [Google Scholar] [CrossRef]
- Formela, K.; Klein, M.; Colom, X.; Saeb, M.R. Investigating the combined impact of plasticizer and shear force on the efficiency of low temperature reclaiming of ground tire rubber (GTR). Polym. Degrad. Stab. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Tolstov, A.; Grigoryeva, O.; Fainleib, A.; Danilenko, I.; Spanoudaki, A.; Pissis, P.; Grenet, J. Reactive compatibilization of polyethylene/ground tire rubber inhomogeneous blends via interactions of pre-functionalized polymers in interface. Macromol. Symp. 2007, 254, 226–232. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Carrillo, F.; Velasco, J.I.; Pagès, P.; Mujal, R.; Nogués, F. Structural and mechanical studies on modified reused tyres composites. Eur. Polym. J. 2006, 42, 2369–2378. [Google Scholar] [CrossRef]
- Rajalingam, P.; Baker, W.E. The role of functional polymers in ground rubber tire-polyethylene composite. Rubber Chem. Technol. 1992, 65, 908–916. [Google Scholar] [CrossRef]
- Paasche, B.A.; Hannen, P. Surface modification of ground rubber with trans-polyoctenamer. Rubber Plast. News 2020, 14–19. [Google Scholar]
- Yadollahi, G.; Sabbagh Mollahosseini, H. Improving the performance of crumb rubber bitumen by means of polyphosphoric acid (PPA) and vestenamer additives. Constr. Build. Mater. 2011, 25, 3108–3116. [Google Scholar] [CrossRef]
- Burns, B.J. Rubber-Modified Asphalt Paving Binder. U.S. Patent 5,936,015, 10 August 1999. [Google Scholar]
- Ko, M.B.; Hong, Y.K. Improvement of deformation resistancy of asphalt by modification with tire rubber. Elastomers 2008, 43, 72–81. [Google Scholar]
- Arti, D.K.; Hidayat, A.S.; Ulfah, I.M.; Susanto, H.; Wisojodharmo, L.A.; Dandi, A. Mastication process of natural rubber/butadiene rubber blending: Validation method on influence of peptizer. Macromol. Symp. 2020, 391, 1900175. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, Y.K.; Rodrigue, D. Production of thermoplastic elastomers based on recycled pe and ground tire rubber: Morphology, mechanical properties and effect of compatibilizer addition. Int. Polym. Process. 2018, 33, 525–534. [Google Scholar] [CrossRef]
- Herrmann, V.; Heinrich, A. Influence of the modification of ground truck tyres as an additive on the properties of a truck tread compound. Int. Polym. Sci. Technol. 2017, 44, T1–T10. [Google Scholar] [CrossRef]
- Nik Yahya, N.Z.; Zulkepli, N.N.; Kamarudin, H.; Ismail, H.; Ting, S.S.; Jamalul Nasir, M.R.; Abdullah, M.M.A.B.; Hamzah, R. Effect of recycled nitrile glove (rNBRg) particle sizes on curing characteristics and physical properties of natural rubber/styrene butadiene rubber/recycled nitrile glove (NR/SBR/rNBRg) blends. Appl. Mech. Mater. 2015, 815, 54–58. [Google Scholar] [CrossRef]
- Jacob, C.; De, P.P.; Bhowmick, A.K.; De, K.S. Recycling of EPDM waste. I. Effect of ground EPDM vulcanizate on properties of EPDM rubber. J. Appl. Polym. Sci. 2001, 82, 3293–3303. [Google Scholar] [CrossRef]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Vulcanization of natural rubber modified with cashew nut shell liquid and its phosphorylated derivative—A comparative study. Polymer (Guildf.) 1998, 39, 4033–4036. [Google Scholar] [CrossRef]
- Khang, T.H.; Ariff, Z.M. Vulcanization kinetics study of natural rubber compounds having different formulation variables. J. Therm. Anal. Calorim. 2012, 109, 1545–1553. [Google Scholar] [CrossRef]
- Song, P.; Wan, C.; Xie, Y.; Formela, K.; Wang, S. Vegetable derived-oil facilitating carbon black migration from waste tire rubbers and its reinforcement effect. Waste Manag. 2018, 78, 238–248. [Google Scholar] [CrossRef]
- Formela, K.; Haponiuk, J.T. Curing characteristics, mechanical properties and morphology of butyl rubber filled with ground tire rubber (GTR). Iran. Polym. J. 2014, 23, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Gradwell, M.H.S.; Mcgill, W.J. The thermal decomposition of sulfenamide accelerators. J. Appl. Polym. Sci. 1994, 51, 169–176. [Google Scholar] [CrossRef]
- Akiba, M.; Hashim, A.S. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Hejna, A.; Zedler, Ł.; Przybysz-Romatowska, M.; Cañavate, J.; Colom, X.; Formela, K. Reclaimed rubber/poly(ε-caprolactone) blends: Structure, mechanical, and thermal properties. Polymers 2020, 12, 1204. [Google Scholar] [CrossRef]
- Zedler, Ł.; Burger, P.; Wang, S.; Formela, K. Ground tire rubber modified by ethylene-vinyl acetate copolymer: Processing, physico-mechanical properties, volatile organic compounds emission and recycling possibility. Materials 2020, 13, 4669. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, L.; Georgescu, M.; Sönmez, M.; Nițuică, M. Biodegradable polymeric composite based on recycled polyurethane and rubber wastes: Material for green shoe manufacturing. Leather Footwear J. 2020, 20, 323–331. [Google Scholar] [CrossRef]
- Mészáros, L.; Fejős, M.; Bárány, T. Mechanical properties of recycled LDPE/EVA/ground tyre rubber blends: Effects of EVA content and postirradiation. J. Appl. Polym. Sci. 2012, 125, 512–519. [Google Scholar] [CrossRef]
- Awang, M.; Ismail, H.; Hazizan, M.A. Polypropylene-based blends containing waste tire dust: Effects of trans-polyoctylene rubber (TOR) and dynamic vulcanization. Polym. Test. 2007, 26, 779–787. [Google Scholar] [CrossRef]
- Garcia, P.S.; de Sousa, F.D.B.; de Lima, J.A.; Cruz, S.A.; Scuracchio, C.H. Devulcanization of ground tire rubber: Physical and chemical changes after different microwave exposure times. Express Polym. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Ubaidillah; Harjana; Yahya, I.; Kristiani, R.; Muqowi, E.; Mazlan, S.A. Perfect sound insulation property of reclaimed waste tire rubber. AIP Conf. Proc. 2016, 1717. [Google Scholar] [CrossRef]
- Pfretzschner, J.; Rodriguez, R.M. Acoustic properties of rubber crumbs. Polym. Test. 1999, 18, 81–92. [Google Scholar] [CrossRef]
- Zhang, H. Building materials in civil engineering. Woodhead publishing in materials. Heat-insulating materials and sound-absorbing materials. Build. Mater. Civ. Eng. 2011, 304–423. [Google Scholar]
- Sobral, M.; Samagaio, A.J.B.; Ferreira, J.M.F.; Labrincha, J.A. Mechanical and acoustical characteristics of bound rubber granulate. J. Mater. Process. Technol. 2003, 142, 427–433. [Google Scholar] [CrossRef]
- Chough, S.H.; Chang, D.H. Kinetics of sulfur vulcanization of NR, BR, SBR, and their blends using arheometer and DSC. J. Appl. Polym. Sci. 1996, 61, 449–454. [Google Scholar] [CrossRef]








| Property | Mooney Viscosity ML(1 + 4) at 100 °C (MU) | Glass Transition Temperature (°C) | Melting Point (°C) | Crystallinity (%) | Thermal Degradation (°C) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|
| Method | DIN 53 523 | ISO 11357-1/-2 | ISO 11357-1/-3 | DSC (2nd heating) | TGA | ISO 527 | ISO 527 |
| Value | <10 | −65 | 54 | ~30 | 275 | 8.5 | 400 |
| Components (phr) | Sample Code | ||||||
|---|---|---|---|---|---|---|---|
| GTR | GTR/TOR | GTR/TORS | GTR/TORCBS | GTR/TORMBTS | GTR/TORSDT | GTR/TORS80 | |
| GTR | 100 | 90 | 90 | 90 | 90 | 90 | 90 |
| TOR | - | 10 | 10 | 10 | 10 | 10 | 10 |
| Sulfur | - | - | 3 | - | - | - | - |
| CBS | - | - | - | 3 | - | - | - |
| MBTS-80 | - | - | - | - | 3.75 | - | - |
| SDT-50 | - | - | - | - | - | 6 | - |
| S-80 | - | - | - | - | - | - | 3.75 |
| Properties | GTR | GTR/TOR | GTR/TORS | GTR/TORCBS | GTR/TORMBTS | GTR/TORSDT | GTR/TORS80 |
|---|---|---|---|---|---|---|---|
| Minimal torque (dNm) | - | - | 19.2 | 15.8 | 15.5 | 18.9 | 18.7 |
| Maximal torque (dNm) | - | - | 44.5 | 29.7 | 27.9 | 34.8 | 41.6 |
| ΔM (dNm) | - | - | 25.3 | 13.9 | 12.4 | 15.9 | 22.9 |
| Scorch time (min) | - | - | 1.5 | 2.3 | 2.2 | 1.1 | 1.5 |
| Optimum cure time (min) | 5.0 | 5.0 | 4.6 | 4.5 | 6.3 | 3.4 | 4.2 |
| Cure rate index (min−1) | - | - | 31.9 | 44.6 | 24 | 42.7 | 38 |
| Thermal aging resistance (%) | - | - | 0.7 | 2.1 | 0.5 | 0.9 | −3.5 |
| Properties | GTR | GTR/TOR | GTR/TORS | GTR/TORCBS | GTR/TORMBTS | GTR/TORSDT | GTR/TORS80 |
|---|---|---|---|---|---|---|---|
| Tensile strength (MPa) | 3.1 ± 0.1 | 2.4 ± 0.2 | 5.5 ± 0.3 | 3.5 ± 0.3 | 3.3 ± 0.2 | 4.7 ± 0.3 | 5.8 ± 0.4 |
| Elongation at break (%) | 198 ± 3 | 111 ± 13 | 167 ± 8 | 128 ± 14 | 132 ± 11 | 164 ± 8 | 186 ± 5 |
| M100 (MPa) | 1.6 ± 0.1 | 2.2 ± 0.1 | 3.3 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 3.0 ± 0.1 | 3.2 ± 0.1 |
| Hardness (Shore A) | 56 ± 1 | 68 ± 1 | 72 ± 1 | 72 ± 1 | 70 ± 1 | 71 ± 1 | 72 ± 1 |
| Density (g/cm3) | 1.178 ± 0.002 | 1.145 ± 0.008 | 1.164 ± 0.001 | 1.146 ± 0.003 | 1.150 ± 0.008 | 1.141 ± 0.002 | 1.153 ± 0.002 |
| Swelling degree (%) | 163.5 ± 3.2 | 172.1 ± 4.2 | 138.9 ± 8.7 | 170.7 ± 1.6 | 176.4 ± 1.3 | 159.8 ± 0.4 | 149.3 ± 0.4 |
| Sol fraction (%) | 10.5 ± 0.1 | 20.5 ± 0.5 | 9.8 ± 0.4 | 15.9 ± 0.3 | 17.9 ± 0.1 | 12.5 ± 0.1 | 10.4 ± 0.1 |
| Sample Composition | Sample Preparation | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Shore A) | Ref. |
|---|---|---|---|---|---|
| GTR/TOR/active compound 90/10/3 | mixing at ambient temperature compression molding 180 °C | 3.1–5.8 | 128–198 | 56–72 | This study |
| GTR/recycled PE/TOR 90/10/9 | extrusion at 150–180 °C injection molding at 180–190 °C | ~2.1 * | ~70 * | ~78 * | [22] |
| GTR/bitumen/PCL 90/10/10 | mixing GTR with bitumen at ambient temperature, then mixing with PCL at 120 °C compression molding at 120 °C | 2.1–2.4 | 89–92 | 61–63 | [32] |
| GTR/EVA 100/10 | extrusion at 60 °C, compression molding at 140–180 °C | 2.7–3.4 | 125–164 | 63–65 | [33] |
| waste thermoplastic polyurethane/waste SBR/PE-g-MA 90/10/5 | mixing at 170–175 °C compression molding at 170 °C | 4.8 | 280 | 81 | [34] |
| recycled LDPE/GTR/EVA 30/40/30 | extrusion at 165–175 °C injection molding at 165–190 °C | ~7.8 * | ~180 * | unknown | [35] |
| GTR/PP/TOR/curing system 60/40/10/9.5 | mixing at 180 °C (dynamic vulcanization) | ~8.0 | ~25 | unknown | [36] |
| Sample | T−2% | T−5% | T−10% | T−50% | Char Residues at 750 °C |
|---|---|---|---|---|---|
| GTR | 258.0 | 315.5 | 355.5 | 450.5 | 35.7 |
| GTR/TOR | 261.2 | 318.7 | 356.2 | 453.7 | 29.7 |
| GTR/TORS | 245.9 | 313.4 | 358.4 | 448.4 | 31.6 |
| GTR/TORCBS | 225.9 | 290.9 | 345.9 | 453.4 | 32.3 |
| GTR/TORMBTS | 253.2 | 303.2 | 348.2 | 453.2 | 31.3 |
| GTR/TORSDT | 250.6 | 295.6 | 348.1 | 450.6 | 28.1 |
| GTR/TORS80 | 243.1 | 305.6 | 353.1 | 450.6 | 31.4 |
| Frequency (Hz) | Sample Code | ||||||
|---|---|---|---|---|---|---|---|
| GTR | GTR/TOR | GTR/TORS | GTR/TORCBS | GTR/TORMBTS | GTR/TORSDT | GTR/TORS80 | |
| Sound Absorption Coefficient (α) | |||||||
| 125 | −0.07661 | 0.10734 | 0.03289 | 0.0307 | −0.07653 | 0.02072 | −0.07766 |
| 250 | 0.01146 | 0.01599 | 0.01566 | 0.01529 | 0.01709 | 0.00817 | 0.01525 |
| 500 | 0.02254 | 0.01958 | 0.02348 | 0.01994 | 0.02095 | 0.02025 | 0.01888 |
| 1000 | 0.02352 | 0.02251 | 0.02832 | 0.02433 | 0.02644 | 0.02423 | 0.0233 |
| 2000 | 0.02689 | 0.0224 | 0.03775 | 0.02053 | 0.03622 | 0.02178 | 0.0209 |
| 4000 | 0.05472 | 0.0623 | 0.05146 | 0.03849 | 0.04037 | 0.03726 | 0.03814 |
| Average | 0.01042 | 0.041687 | 0.031593 | 0.02488 | 0.010757 | 0.022068 | 0.006468 |
| Frequency (Hz) | Sample Code | ||||||
|---|---|---|---|---|---|---|---|
| GTR | GTR/TOR | GTR/TORS | GTR/TORCBS | GTR/TORMBTS | GTR/TORSDT | GTR/TORS80 | |
| Sound Absorption Coefficient (α) | |||||||
| 100–315 | 0.06323 | 0.103741 | 0.055114 | 0.056852 | 0.038187 | 0.087613 | 0.051914 |
| 400–1250 | 0.024173 | 0.02308 | 0.028704 | 0.023036 | 0.025176 | 0.024022 | 0.022998 |
| 1600–4000 | 0.038194 | 0.032915 | 0.058704 | 0.025429 | 0.036743 | 0.026061 | 0.025634 |
| Average | 0.041866 | 0.053245 | 0.047507 | 0.035106 | 0.033369 | 0.045899 | 0.033515 |
| Sample Code | Melting Enthalpy ΔHm (J/g) | Initial Melting Temperature (Tωm) (°C) | Peak Temperature (Tpm) (°C) | Melting Temperature Range D (°C) | Melting Time (tm) (min) | |||||
| 1st run | 2nd run | 1st run | 2nd run | 1st run | 2nd run | 1st run | 2nd run | 1st run | 2nd run | |
| GTR | - | - | - | - | - | - | - | - | - | - |
| GTR/TOR | 5.76 | 5.06 | 43.8 | 39.9 | 57.6 | 54.3 | 19.4 | 20.4 | 1.9 | 2.0 |
| GTR/TORS | 3.60; −20.83 | 2.85 | 36.5 | 1.5 | 45.9; 210.4 | 14.1 | 17.3; 72.4 | 24.4 | 1.7; 7.2 | 2.4 |
| GTR/TORCBS | 4.86 | 4.35 | 39.7 | 37.5 | 54.3 | 51.1 | 21.9 | 20.9 | 2.2 | 2.1 |
| GTR/TORMBTS | 3.32 | 4.13 | 47.3 | 35.4 | 55.4 | 51.3 | 15.3 | 24.2 | 1.5 | 2.4 |
| GTR/TORSDT | 4.88 | 3.97 | 36.0 | 35.2 | 53.2 | 49.4 | 25.4 | 21.4 | 2.5 | 2.1 |
| GTR/TORS80 | 4.30; −25.49 | 2.70 | 35.4 | −2.4 | 48.4; 205.7 | 13.4 | 20.8; 82.3 | 27.0 | 2.1; 8.2 | 2.7 |
| TOR | 85.88 | 70.79 | 40.2 | 25.4 | 60.1 | 56.6 | 36.9 | 37.6 | 2.9 | 3.7 |
| Sample Code | Crystallization Enthalpy ΔHc (J/g) | Initial Crystallization Temperature (Tωc) (°C) | Peak Temperature (Tpc) (°C) | Crystallization Temperature Range (D) (°C) | Crystalization Time (tc) (min) | |||||
| GTR | - | - | - | - | - | |||||
| GTR/TOR | −4.74 | 38.4 | 31.7 | 19.9 | 2.0 | |||||
| GTR/TORS | −2.47 | 5.3 | −10.2 | 34.0 | 3.4 | |||||
| GTR/TORCBS | −4.41 | 37.5 | 29.4 | 22.0 | 2.2 | |||||
| GTR/TORMBTS | −4.15 | 35.3 | 28.4 | 19.2 | 1.9 | |||||
| GTR/TORSDT | −3.86 | 35.3 | 26.7 | 20.8 | 2.1 | |||||
| GTR/TORS80 | −2.24 | 3.8 | −10.8 | 29.1 | 2.9 | |||||
| TOR | −79.07 | 39.9 | 32.4 | 33.9 | 3.4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedler, Ł.; Kowalkowska-Zedler, D.; Colom, X.; Cañavate, J.; Saeb, M.R.; Formela, K. Reactive Sintering of Ground Tire Rubber (GTR) Modified by a Trans-Polyoctenamer Rubber and Curing Additives. Polymers 2020, 12, 3018. https://doi.org/10.3390/polym12123018
Zedler Ł, Kowalkowska-Zedler D, Colom X, Cañavate J, Saeb MR, Formela K. Reactive Sintering of Ground Tire Rubber (GTR) Modified by a Trans-Polyoctenamer Rubber and Curing Additives. Polymers. 2020; 12(12):3018. https://doi.org/10.3390/polym12123018
Chicago/Turabian StyleZedler, Łukasz, Daria Kowalkowska-Zedler, Xavier Colom, Javier Cañavate, Mohammad Reza Saeb, and Krzysztof Formela. 2020. "Reactive Sintering of Ground Tire Rubber (GTR) Modified by a Trans-Polyoctenamer Rubber and Curing Additives" Polymers 12, no. 12: 3018. https://doi.org/10.3390/polym12123018