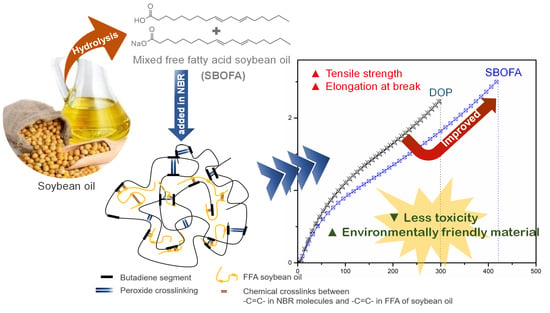
Effect of a Natural Processing Aid on the Properties of Acrylonitrile-Butadiene Rubber: Study on Soybean Oil Fatty Acid from Seed Crop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
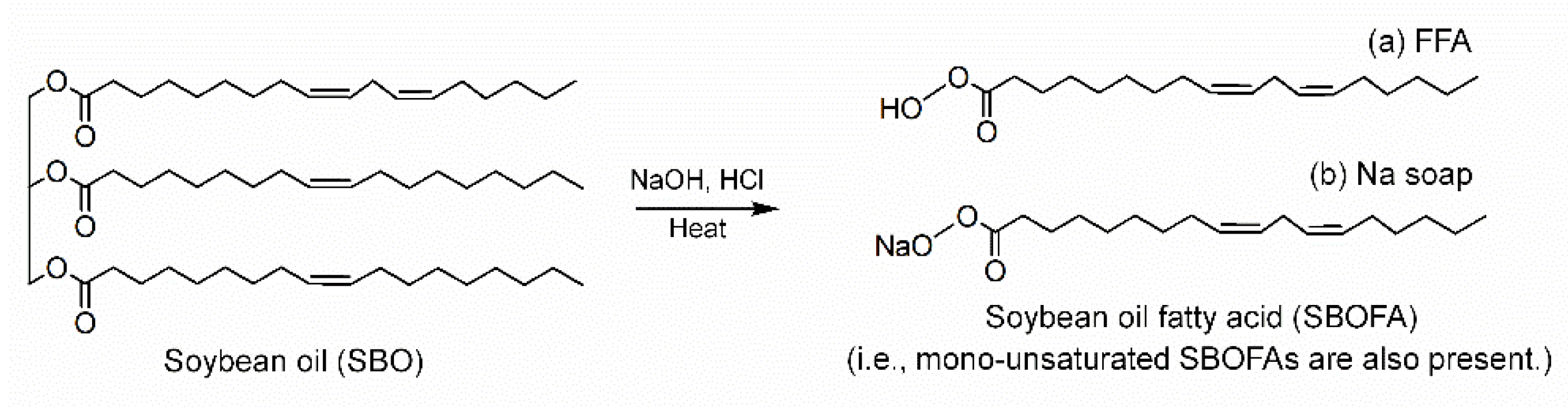
2.2. Preparation of SBOFA
2.3. Characterization of SBO and SBOFA
2.4. Preparation of Rubber Compounds
2.5. Cure Characteristics of Rubber Compounds
2.6. Preparation of Rubber Vulcanizates
2.7. Characterization
2.7.1. Mooney Viscosity of Rubber Compounds
2.7.2. ATR-FTIR Spectrum of Unvulcanized and Peroxide-Vulcanized Rubbers
2.7.3. Crosslink Density of Rubber Vulcanizates
2.7.4. Tensile Property of Rubber Vulcanizates
2.7.5. Thermal Aging Property of Rubber Vulcanizates
2.7.6. Hardness of Rubber Vulcanizates
2.7.7. Temperature Scanning Stress Relaxation (TSSR) of Rubber Vulcanizates
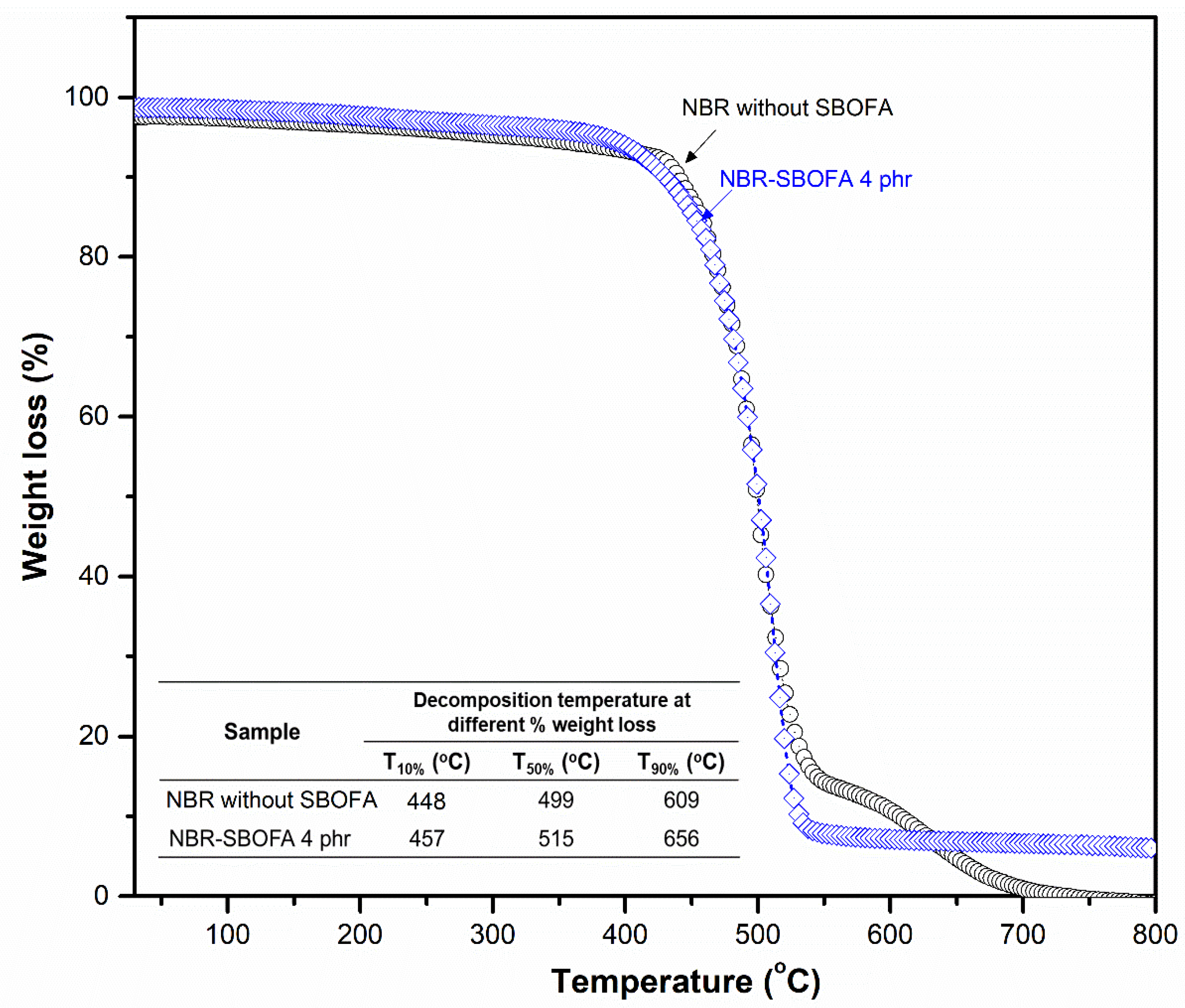
2.7.8. Thermogravimetric Analysis (TGA) of Rubber Vulcanizates
3. Results and Discussion
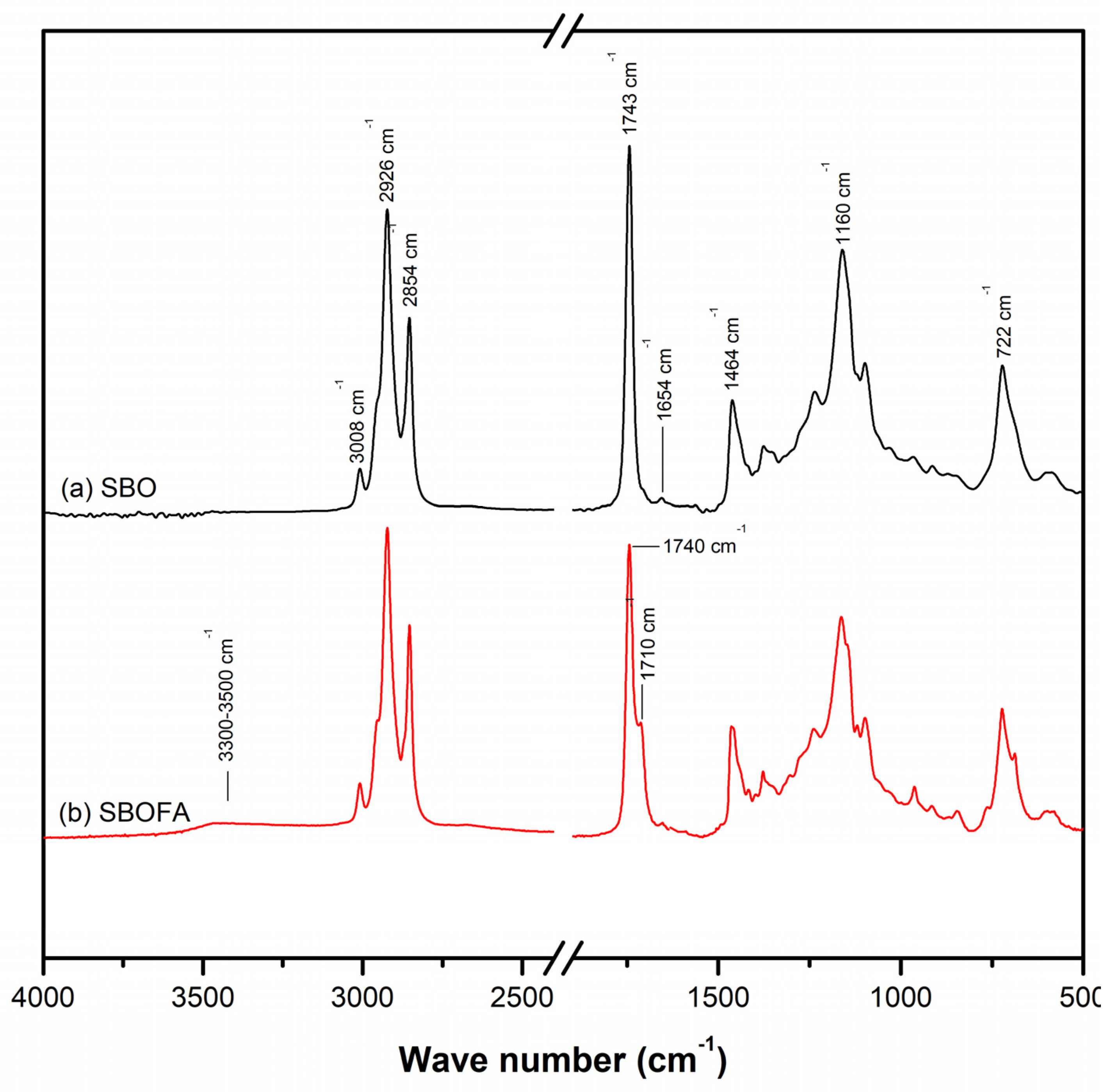
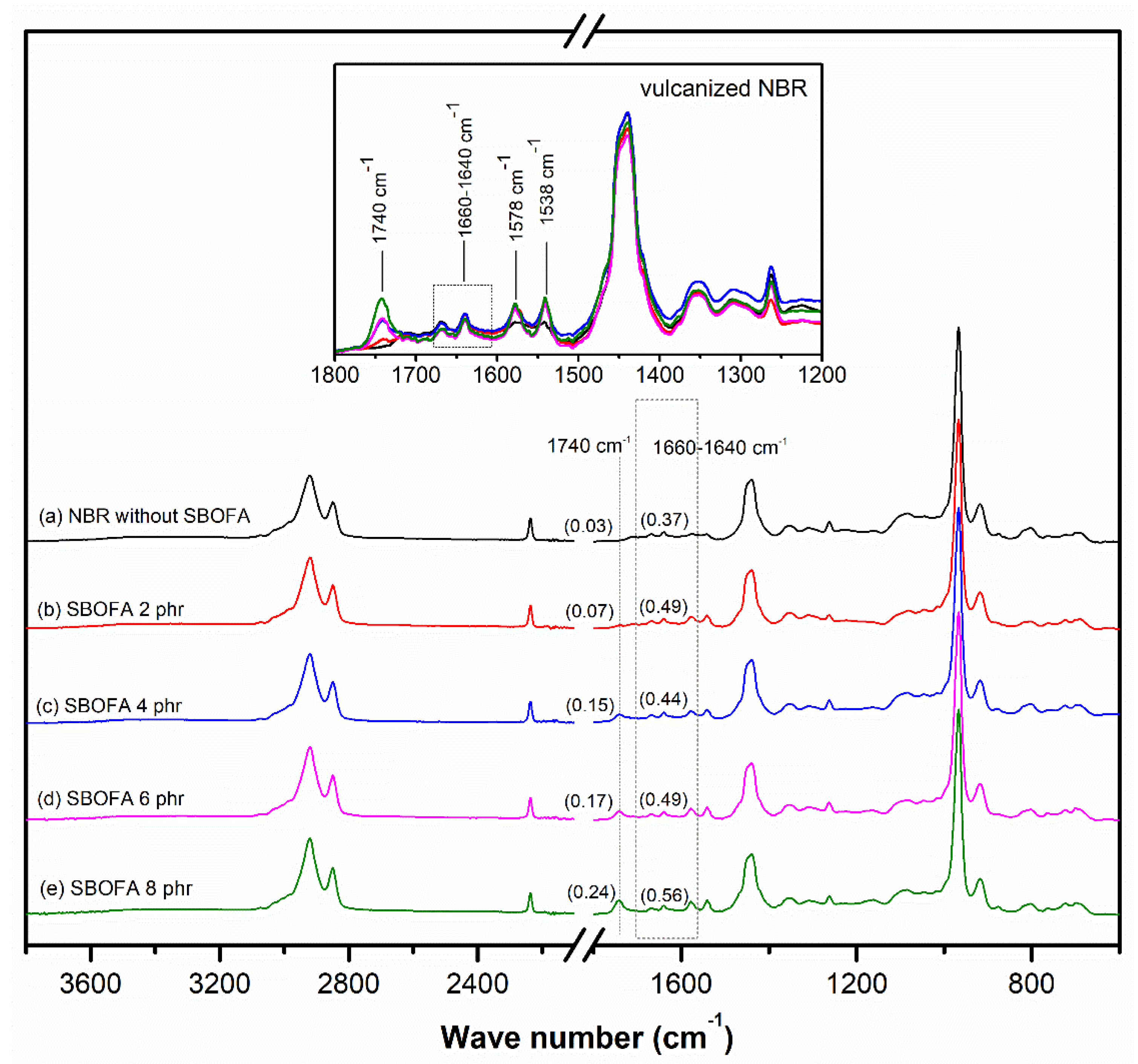
3.1. Chemical Structure of the Obtained SBOFA
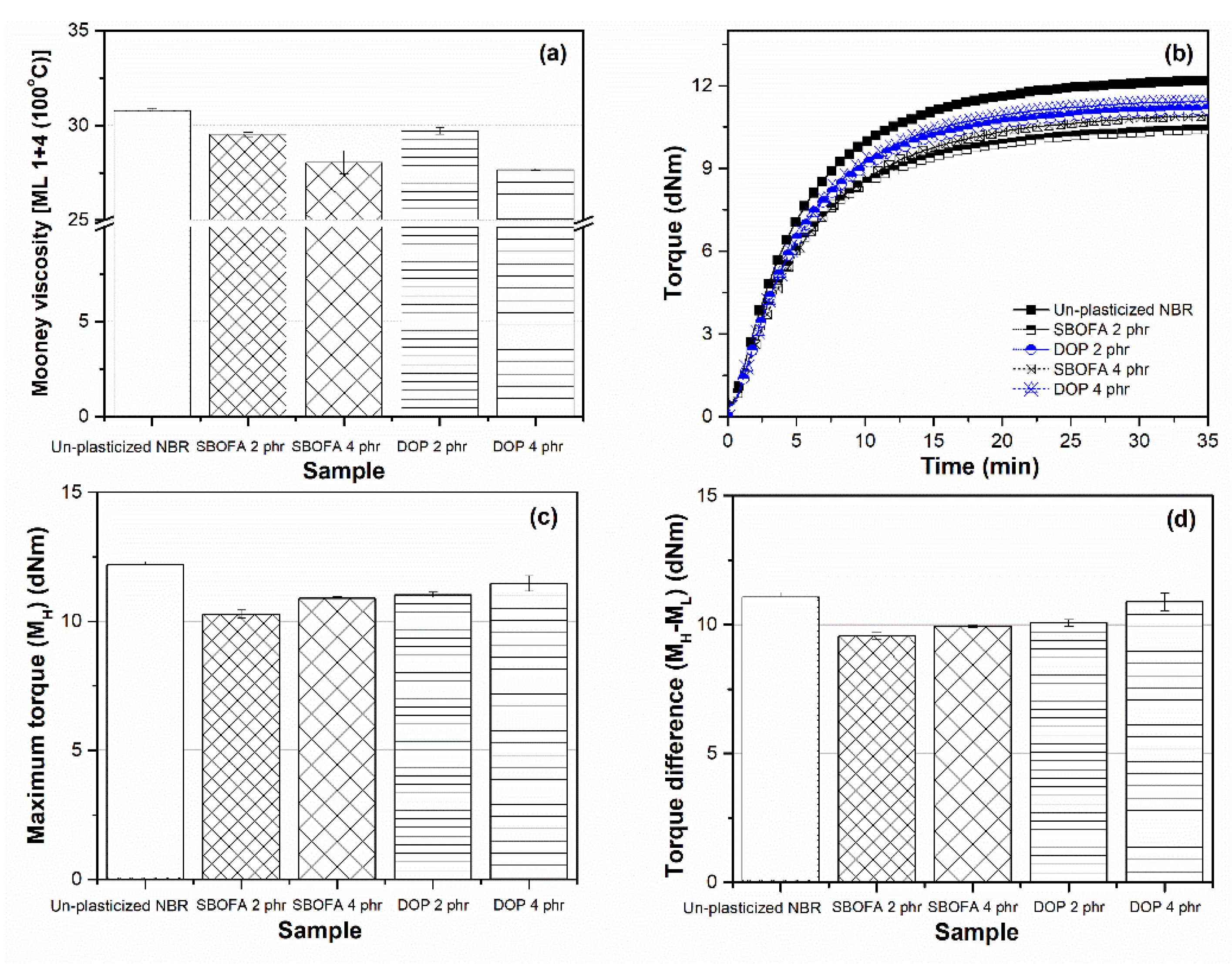
3.2. Effect of the SBOFA Loading on the Mooney Viscosity of NBR Compounds
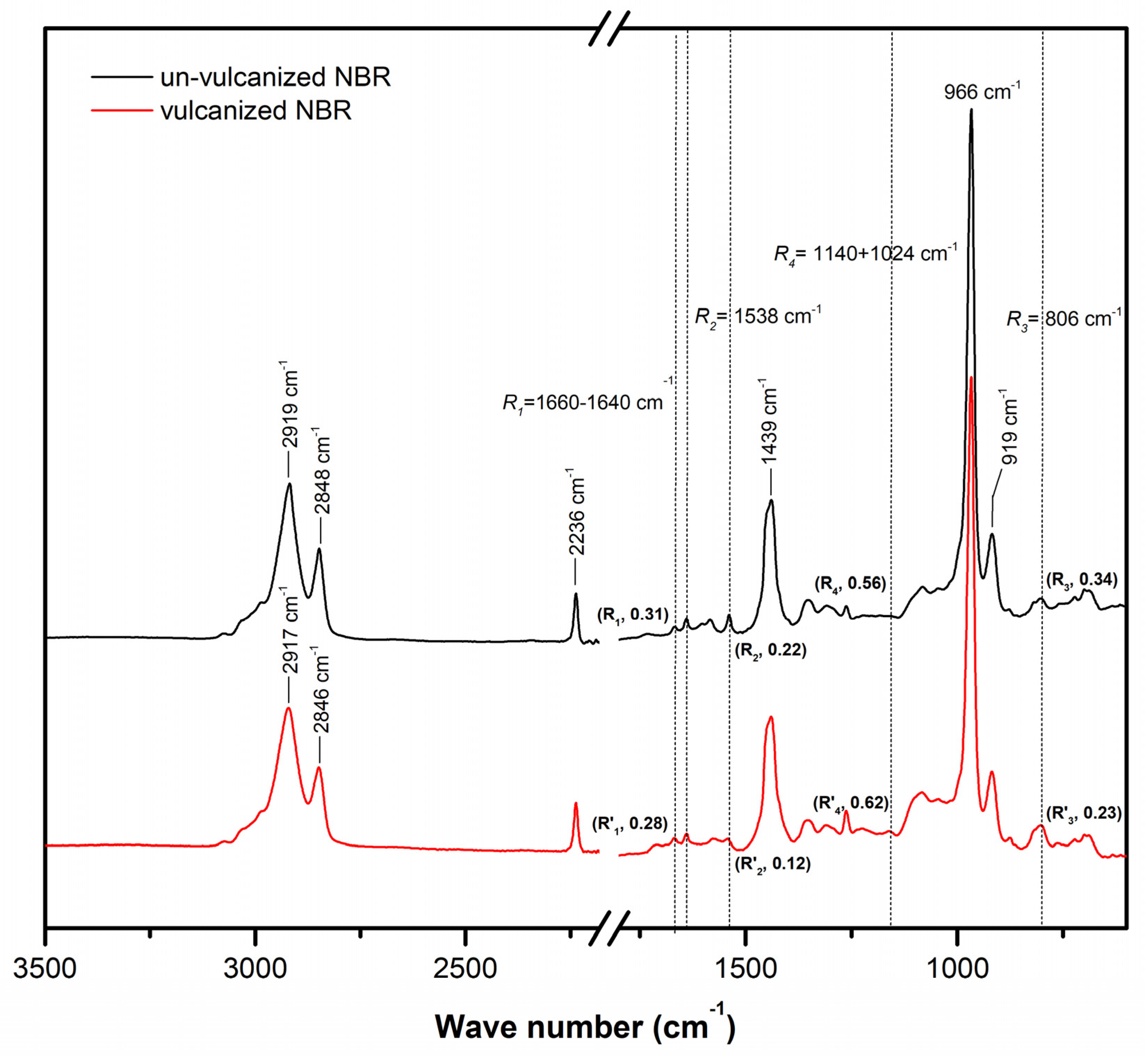
3.3. ATR-FTIR Spectra of the Unvulcanized and Peroxide-Vulcanized NBR without SBOFA
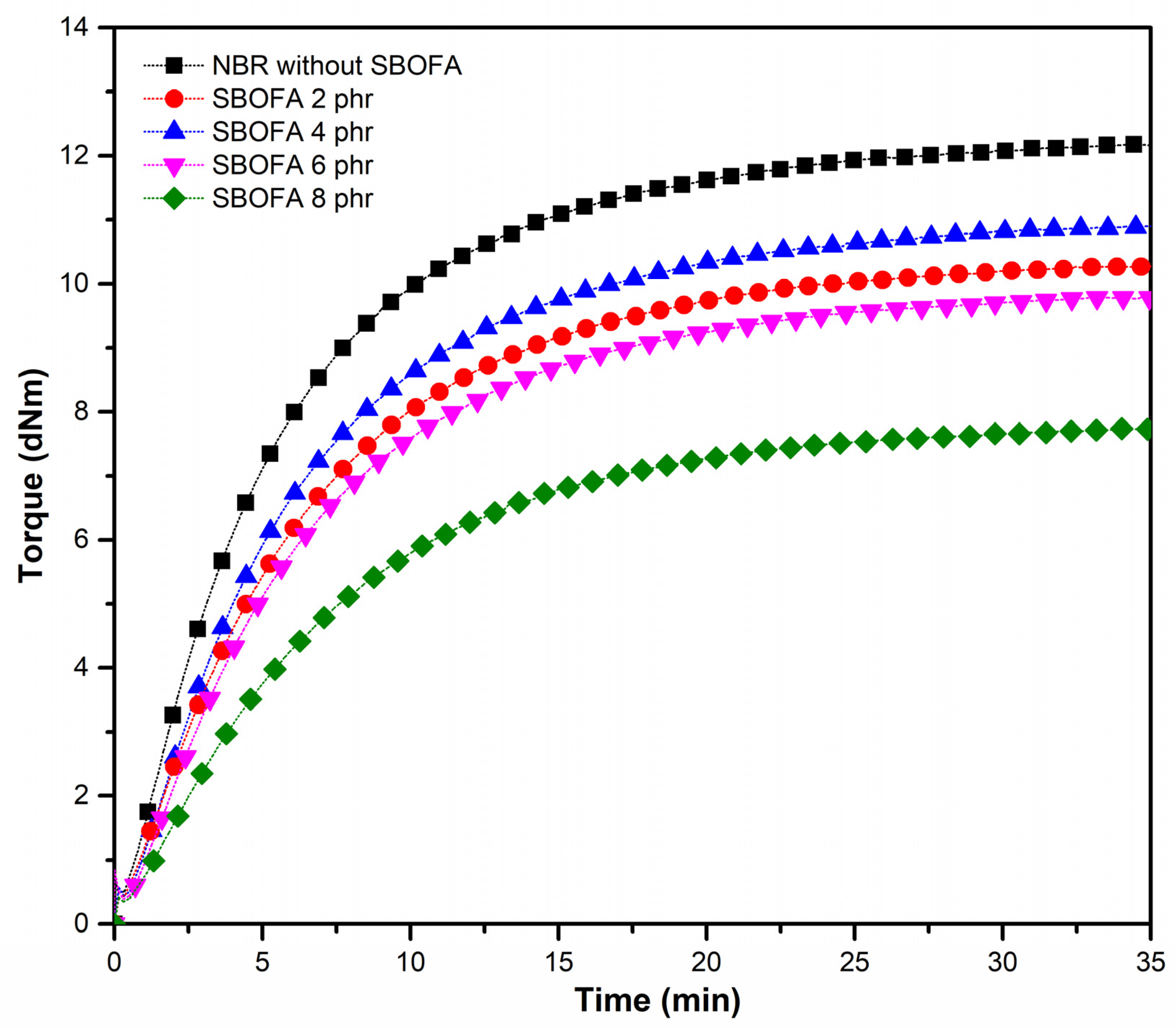
3.4. Effect of the SBOFA Loading on the Cure Characteristics of NBR Compounds
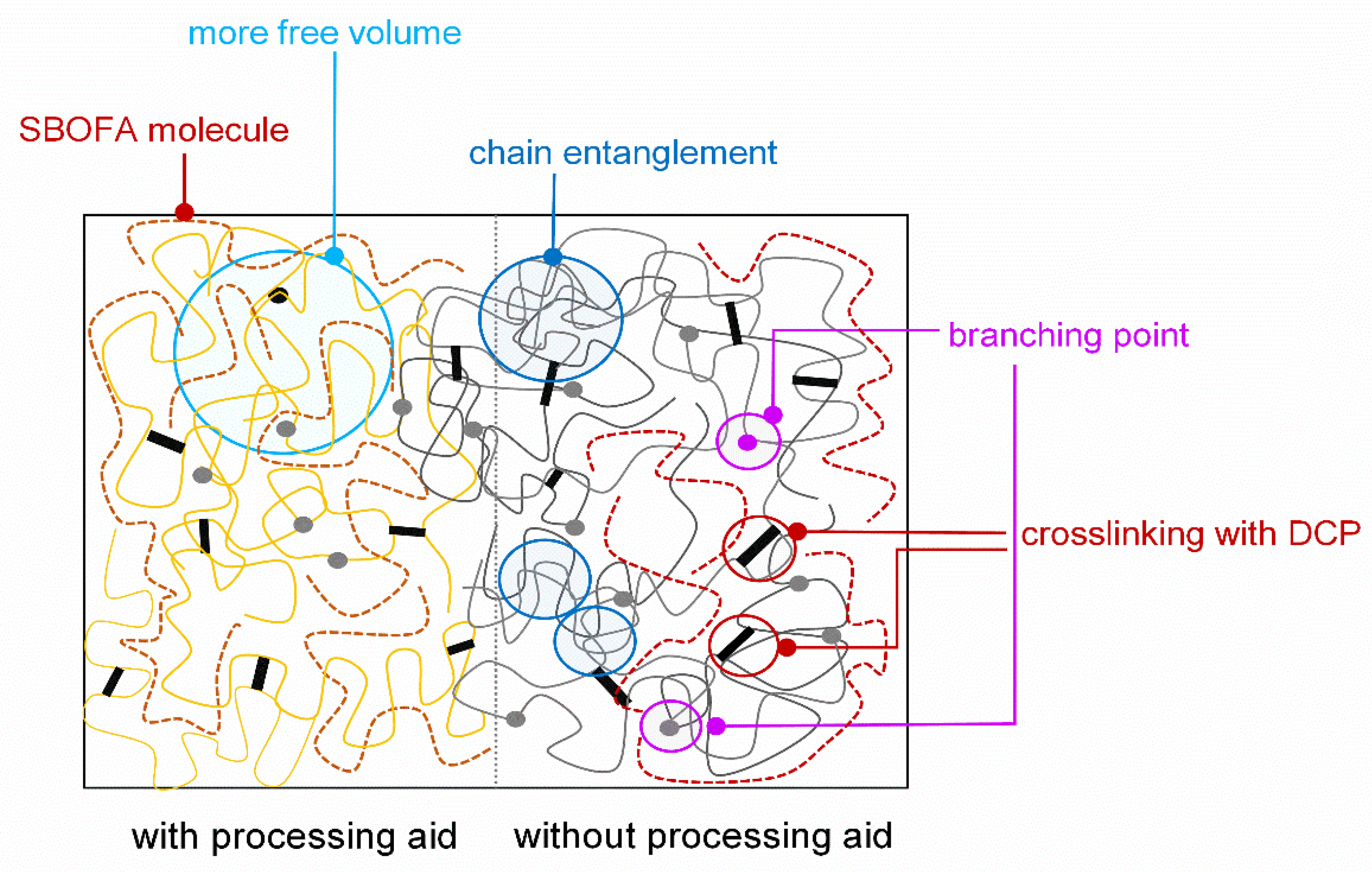
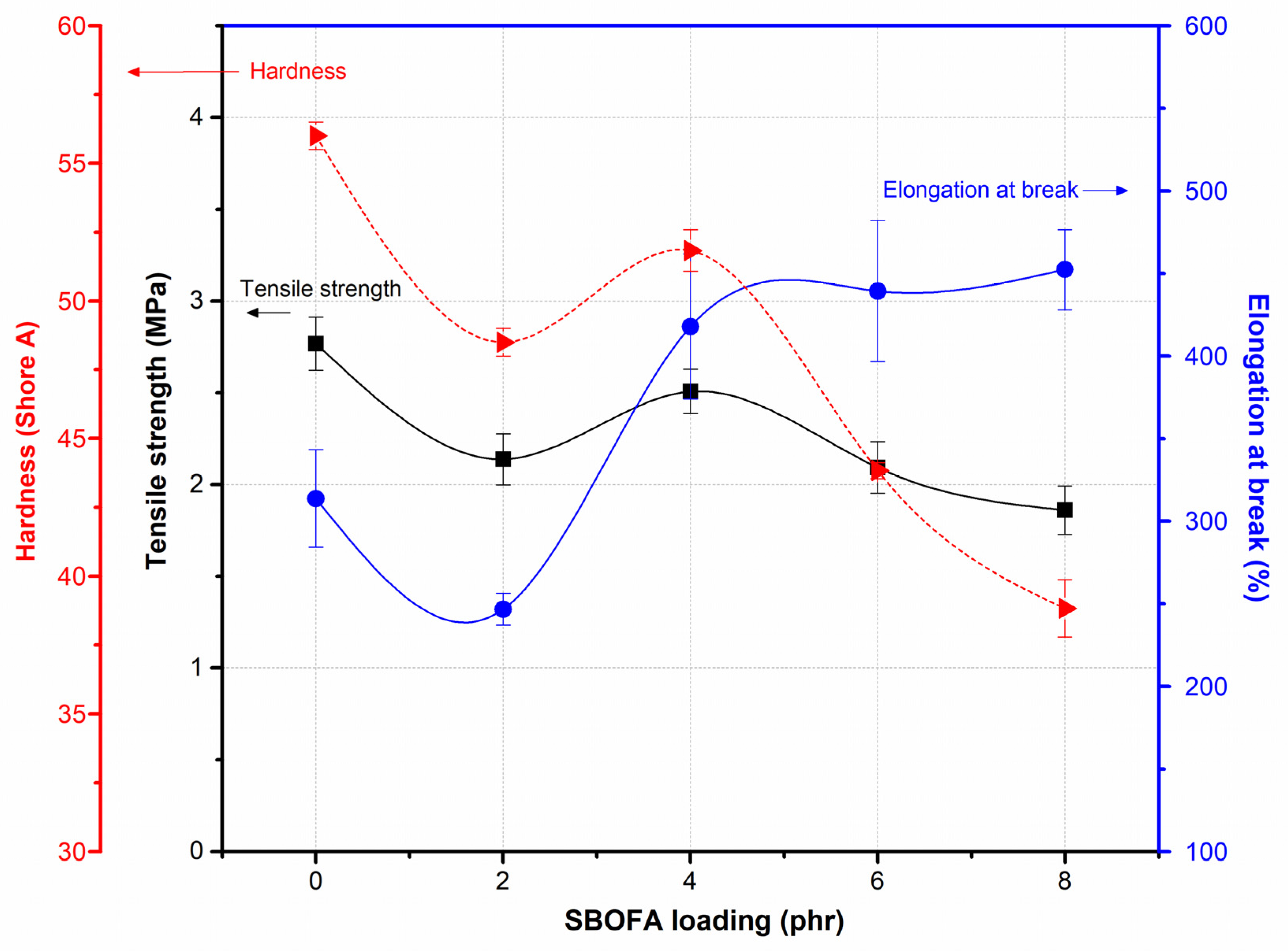
3.5. Influence of the SBOFA Loading on the Mechanical Properties of NBR Vulcanizates
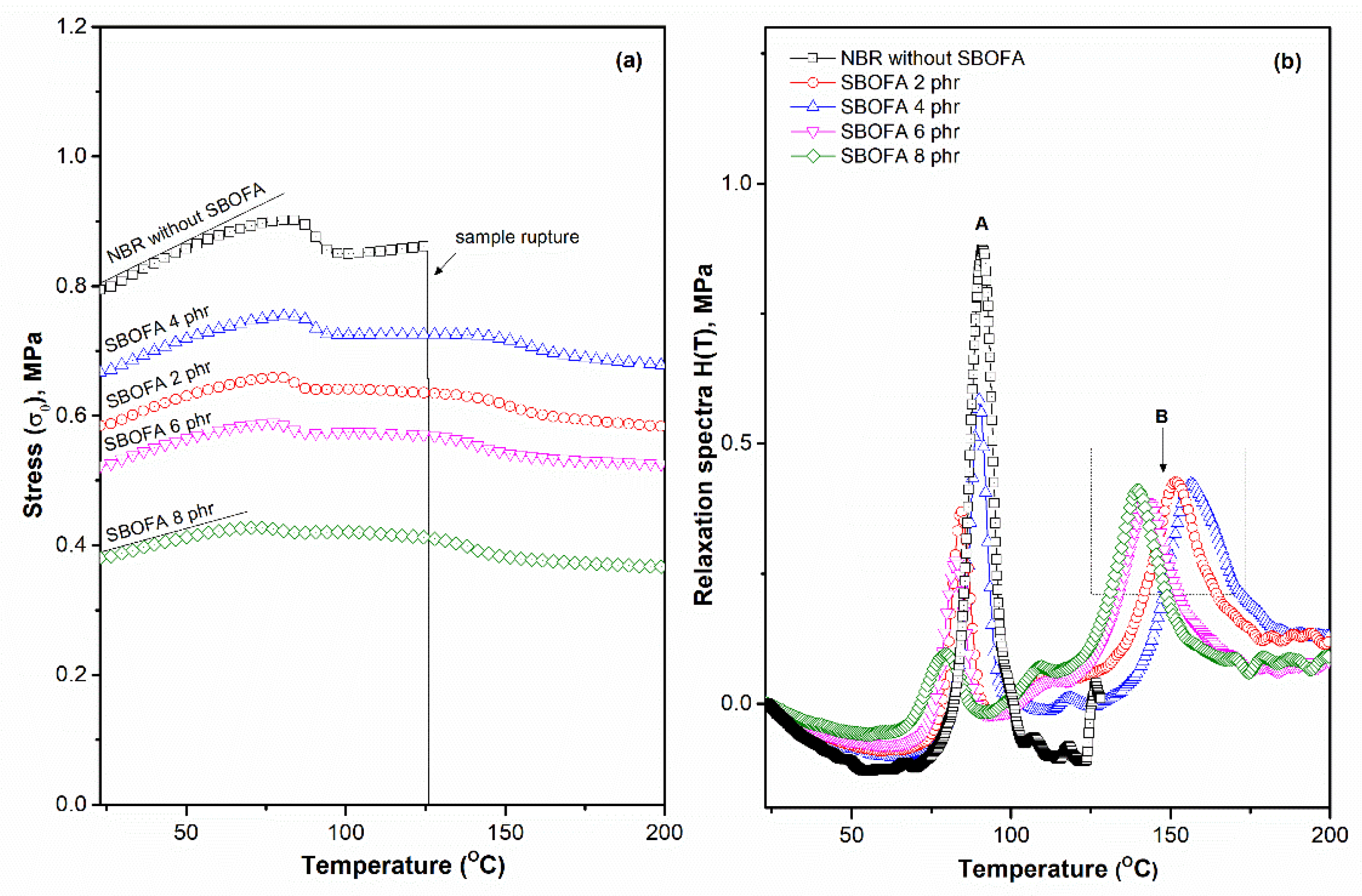
3.6. Influence of the SBOFA Loading Level on the Stress-Relaxation Behavior of NBR Vulcanizates
3.7. Comparison of the Effect of SBOFA and DOP on the Properties of NBR Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bodaghi, A. An overview on the recent developments in reactive plasticizers in polymers. Polym. Adv. Technol. 2020, 31, 355–367. [Google Scholar] [CrossRef]
- Xu, H.; Fan, T.; Ye, N.; Wu, W.; Huang, D.; Wang, D.; Wang, Z.; Zhang, L. Plasticization effect of bio-based plasticizers from soybean oil for tire tread rubber. Polymers 2020, 12, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzoddin, S.; Abbasian, A.; Aman-Alikhani, M.; Ganjali, S.T. The influence of non-carcinogenic petroleum-based process oils on tire compounds’ performance. Iran. Polym. J. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Kuta, A.; Hrdlicka, Z.; Voldanova, J.; Brejcha, J.; Pokorny, J. Dynamic Mechanical Properties of Rubbers with Standard Oils and Oils with Low Content of Polycyclic Aromatic Hydrocarbons. Kautsch. Gummi Kunstst. 2010, 63, 120–122. [Google Scholar]
- Joona, M. Non-carcinogenic tire extender oils providing good dynamic performance. In Proceedings of the Rubber Expo 05: 168th Technical Meeting of the American Chemical Society, Rubber Division, Pittsburgh, PA, USA, 1–3 November 2005. [Google Scholar]
- Regulation, E.C. No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration. Eval. Auth. Restrict. Chem. REACH Establ. Eur. Chem. Agency Amend. Dir. 1999, 45, 1–849. [Google Scholar]
- Dasgupta, S.; Agrawal, S.L.; Bandyopadhyay, S.; Chakraborty, S.; Mukhopadhyay, R.; Malkani, R.K.; Ameta, S.C. Characterization of eco-friendly processing aids for rubber compound. Polym. Test. 2007, 26, 489–500. [Google Scholar] [CrossRef]
- Null, V. Safe process oils for tires with low environmental impact. Kautsch. Gummi Kunstst. 1999, 52, 6. [Google Scholar]
- Wilson, R.F. Soybean: Market driven research needs. In Genetics and Genomics of Soybean; Springer: New York, NY, USA, 2008; pp. 3–15. [Google Scholar]
- Pechurai, W.; Chiangta, W.; Tharuen, P. Effect of vegetable oils as processing aids in SBR compounds. Macromol. Symp. 2015, 354, 191–196. [Google Scholar] [CrossRef]
- Jayewardhana, W.G.D.; Perera, G.M.; Edirisinghe, D.G.; Karunanayake, L. Study on natural oils as alternative processing aids and activators in carbon black filled natural rubber. J. Natl. Sci. Found. Sri Lanka 2009, 37, 187–193. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Ionescu, M.; Milić, J.; Halladay, J.R. Soybean oil plasticizers as replacement of petroleum oil in rubber. Rubber Chem. Technol. 2013, 86, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Siwarote, B.; Sae-Oui, P.; Wirasate, S.; Suchiva, K. Effects of bio-based oils on processing properties and cure characteristics of silica-filled natural rubber compounds. J. Rubber Res. 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Pylee, K.A.; Varghese, M. The compounding of nitrile and polychloroprene rubbers with rice bran oil. Iran. Polym. J. 1999, 8, 247–255. [Google Scholar]
- Indrajati, I.N.; Dewi, I.R. Performance of maleated castor oil based plasticizer on rubber: Rheology and curing characteristic studies. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012001. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, H.; Roser, M. Meat and Seafood Production & Consumption. Our World Data 2017. Available online: https://ourworldindata.org/grapher/meat-production-tonnes (accessed on 12 July 2021).
- Boontawee, H.; Nakason, C.; Kaesaman, A.; Thitithammawong, A.; Chewchanwuttiwong, S. Benzyl esters of vegetable oils as processing oil in carbon black-filled SBR compounding: Chemical modification, characterization, and performance. Adv. Polym. Technol. 2017, 36, 320–330. [Google Scholar] [CrossRef]
- Vennemann, N.; Bökamp, K.; Bröker, D. Crosslink density of peroxide cured TPV. Macromol. Symp. 2006, 245, 641–650. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular size distribution in three dimensional polymers. II. Trifunctional branching units. J. Am. Chem. Soc. 1941, 63, 3091–3096. [Google Scholar] [CrossRef]
- Mathew, S.; Varghese, S.; Joseph, R. Degradation behaviour of natural rubber layered silicate nanocomposites. Prog. Rubber Plast. Recycl. Technol. 2013, 29, 1–20. [Google Scholar] [CrossRef]
- Dziemidkiewicz, A.; Maciejewska, M. Manganese and Nickel Acetylacetonates as Curatives for Chloroprene Rubber Based on Heck’s Reaction. Materials 2021, 14, 807. [Google Scholar] [CrossRef]
- Barbe, A.; Bökamp, K.; Kummerlöwe, C.; Sollmann, H.; Vennemann, N.; Vinzelberg, S. Investigation of modified SEBS-based thermoplastic elastomers by temperature scanning stress relaxation measurements. Polym. Eng. Sci. 2005, 45, 1498–1507. [Google Scholar] [CrossRef]
- Vennemann, N. Characterization of thermoplastic elastomers by means of temperature scanning stress relaxation measurements. In Thermoplastic Elastomers; InTech: Rijeka, Croatia, 2012; pp. 347–370. [Google Scholar]
- Mahdavi, H.; Kamyabi, A.; Shahalizade, T.; Taheri, H.A. Preparation of highly flexible cellulose acetate membranes modified by hyperbranched poly (amine ester)-epoxidized soybean oil and evaluation of its filtration properties. Cellulose 2017, 24, 5389–5402. [Google Scholar] [CrossRef]
- Dong, T.; Yu, L.; Gao, D.; Yu, X.; Miao, C.; Zheng, Y.; Lian, J.; Li, T.; Chen, S. Direct quantification of fatty acids in wet microalgal and yeast biomass via a rapid in situ fatty acid methyl ester derivatization approach. Appl. Microbiol. Biotechnol. 2015, 99, 10237–10247. [Google Scholar] [CrossRef] [PubMed]
- Poulenat, G.; Sentenac, S.; Mouloungui, Z. Fourier-transform infrared spectra of fatty acid salts-Kinetics of high-oleic sunflower oil saponification. J. Surfactants Deterg. 2003, 6, 305–310. [Google Scholar] [CrossRef]
- Zielinski, W.; Rajca, A. Spectroscopic Methods and their Application to Identification Organic Compounds; WNT: Warszaw, Poland, 2000. [Google Scholar]
- Krzemińska, S.M.; Smejda-Krzewicka, A.A.; Leniart, A.; Lipińska, L.; Woluntarski, M. Effects of curing agents and modified graphene oxide on the properties of XNBR composites. Polym. Test. 2020, 83, 106368. [Google Scholar] [CrossRef]
- Rajan, R.; Varghese, S.; George, K. Role of coagents in peroxide vulcanization of natural rubber. Rubber Chem. Technol. 2013, 86, 488–502. [Google Scholar] [CrossRef]
- Dogadkin, B.; Dobromyslova, A.; Belyatskaya, O. The Scorching of Rubber Mixes. II. Effect of Retarders on the Kinetics of Sulfur Combination. Rubber Chem. Technol. 1962, 35, 501–508. [Google Scholar] [CrossRef]
- Nandanan, V.; Joseph, F.D.; Rani, K. Studies on the Use of Drying Oils as Ingredient in the Vulcanization of Elastomers. Ph.D. Thesis, Cochin University of Science and Technology, Kochi, India, June 2000. [Google Scholar]
- Lee, H.K.; Kim, D.S.; Won, J.S.; Jin, D.Y.; Lee, H.J.; Lee, S.G. Effects of thermal and humidity aging on the interfacial adhesion of polyketone fiber reinforced natural rubber composites. Adv. Mater. Sci. Eng. 2016, 2016, 4159072. [Google Scholar] [CrossRef] [Green Version]
- Cunneen, J. Oxidative aging of natural rubber. Rubber Chem. Technol. 1968, 41, 182–208. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, R.; Iervolino, R.; Barbera, S. Changes of chemical structure and mechanical property levels during thermo-oxidative aging of NBR. Rubber Chem. Technol. 2013, 86, 591–603. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, R.; Iervolino, R.; Van der vorst, B.; Barbera, S. The effect of thermo-oxidation on the continuous stress relaxation behavior of nitrile rubber. Polym. Degrad. Stab. 2015, 115, 32–37. [Google Scholar] [CrossRef]
- Kukreja, T.R.; Chauhan, R.C.; Choe, S.; Kundu, P.P. Effect of the doses and nature of vegetable oil on carbon black/rubber interactions: Studies on castor oil and other vegetable oils. J. Appl. Polym. Sci. 2003, 87, 1574–1578. [Google Scholar] [CrossRef]
- Vennemann, N.; Wu, M.; Heinz, M. Thermoelastic properties and relaxation behavior of S-SBR/silica vulcanizates. Rubber World 2012, 246, 18–23. [Google Scholar]
- Pongdong, W.; Nakason, C.; Kummerlöwe, C.; Vennemann, N. Influence of filler from a renewable resource and silane coupling agent on the properties of epoxidized natural rubber vulcanizates. J. Chem. 2015, 2015, 796459. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Tan, J.; Wang, F.; Zhu, X. Study on the Synthesis of Castor Oil-Based Plasticizer and the Properties of Plasticized Nitrile Rubber. Polymers 2020, 12, 2584. [Google Scholar] [CrossRef] [PubMed]
- Tee, Y.B.; Talib, R.A.; Abdan, K.; Chin, N.L.; Basha, R.K.; Yunos, K.F.M. Comparative study of chemical, mechanical, thermal, and barrier properties of poly (lactic acid) plasticized with epoxidized soybean oil and epoxidized palm oil. BioResources 2016, 11, 1518–1540. [Google Scholar] [CrossRef] [Green Version]
- Avalos, F.; Tellez-Rosas, M.M.; Martínez-Casado, F.J.; Rodríguez-Cheda, J.A.; Arroyo, M.; López-Manchado, M.A. Effect of mesogenic organic salts on vulcanization and physical properties of rubber compounds. Polym. Int. 2014, 63, 136–144. [Google Scholar] [CrossRef]
- Kuriakose, A.; Varghese, M. Use of rice bran oil and epoxidized rice bran oil in carbon black–filled natural rubber–polychloroprene blends. J. Appl. Polym. Sci. 2003, 90, 4084–4092. [Google Scholar] [CrossRef]





| Chemicals | Sources |
|---|---|
| Soybean oil (SBO) | Lotus’s, Ek-Chai Distribution System Co., Ltd., Khet Buengkoom, Bangkok, Thailand |
| Sodium hydroxide (NaOH) | Fisher Scientific, Loughborough, UK |
| Sodium chloride (NaCl) | Merck KGaA, Darmstadt, Germany |
| Hydrochloric acid (HCl) | Merck KGaA, Darmstadt, Germany |
| Acrylonitrile-butadiene rubber (NBR) (1052, acrylonitrile content 33%) | Nantex Industry Co., Ltd., Zhenjiang, Jiangsu, China |
| Dioctyl phthalate (DOP) | Hansen & Rosenthal Chemical Co., Ltd., Hamburg, Germany |
| Dicumyl peroxide (DCP) | A.F. Supercell Co., Ltd., Khlong Toei, Bangkok, Thailand |
| Ingredients | Processing Aid Loading (Parts per Hundred Rubber; phr) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 2 | 4 | |
| NBR (ACN 33%) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| DCP | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| SBOFA | - | 2.0 | 4.0 | 6.0 | 8.0 | - | - |
| DOP | - | - | - | - | - | 2.0 | 4.0 |
| Wave Number cm−1 | Assignment |
|---|---|
| 3500–3300 | O–H stretching vibration of carboxyl and hydroxyl groups |
| 3008 | –C=CH stretching |
| 2926 | C–H of CH3 stretching |
| 2919 | CH2 stretching vibration |
| 2854 | C–H of CH2 stretching |
| 2848 | CH2 stretching vibration |
| 2236 | –C≡N stretching vibrations in the acrylonitrile segments |
| 1743–1740 | C=O stretching vibration of ester |
| 1710 | C=O stretching vibration of carboxylic acid |
| 1660–1640 | C=C stretching vibration |
| 1578 | Carboxylate ion (COO–) stretching vibration |
| 1538 | C=C symmetric stretching vibration of double bond |
| 1464 | C–H of CH2 stretching |
| 1443 | C–H bending vibration in –CH2 |
| 1262 | Stretching vibrations of C–O in C–OH groups |
| 1160 | –CH in plane |
| 1024, 1140 | C–C stretching vibration |
| 966 | C–H out-of-plane bending vibration of trans-1,4 –HC=CH– |
| 919 | Out of plane –CH=CH2 deformations |
| 806 | Vibrations of residual –C=C– bonds |
| 722 | –HC=CH– bending (out of plane) |
| Sample | Level of –C=C– Bonds | Level of C–C Linkages | ||
|---|---|---|---|---|
| Peak Ratio R1 = A1660−1640/A1443 | Peak Ratio R2 = A1538/A1443 | Peak Ratio R3 =A806/A1443 | Peak Ratio R4 =A1140+1024/A1443 | |
| NBR without SBOFA | 0.37 | 0.13 | 0.13 | 0.79 |
| NBR-SBOFA 2 phr | 0.49 | 0.21 | 0.18 | 0.71 |
| NBR-SBOFA 4 phr | 0.44 | 0.19 | 0.17 | 0.76 |
| NBR-SBOFA 6 phr | 0.49 | 0.22 | 0.25 | 0.69 |
| NBR-SBOFA 8 phr | 0.56 | 0.22 | 0.34 | 0.48 |
| Cure Parameters | SBOFA Content (phr) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |
| Scorch time (ts2) (min) | 2.06 ± 0.05 | 2.45 ± 0.05 | 2.52 ± 0.04 | 2.63 ± 0.06 | 3.87 ± 0.09 |
| Cure time (t90) (min) | 15.01 ± 0.06 | 16.05 ± 0.08 | 16.08 ± 0.13 | 16.48 ± 0.18 | 17.21 ± 0.2 |
| Cure rate index (CRI) (min−1) | 7.72 ± 0.04 | 7.35 ± 0.02 | 7.37 ± 0.05 | 7.22 ± 0.06 | 7.5 ± 0.06 |
| Crosslink density (mol/m3) | 226.87 ± 3.10 | 156.45 ± 1.79 | 198.21 ± 3.43 | 147.32 ± 2.83 | 138.08 ± 2.42 |
| Mechanical Properties | SBOFA Loading (phr) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |
| 100% modulus (MPa) | 1.19 ± 0.02 | 1.17 ± 0.02 | 0.91 ± 0.02 | 0.77 ± 0.05 | 0.70 ± 0.02 |
| 200% Modulus (MPa) | 1.78 ± 0.03 | 1.70 ± 0.06 | 1.37 ± 0.04 | 1.10 ± 0.09 | 1.02 ± 0.02 |
| 300% Modulus (MPa) | - | - | 1.86 ± 0.06 | 1.44 ± 0.14 | 1.33 ± 0.02 |
| Sample | Retention in Tensile Strength (%) | Retention in Elongation at Break (%) | Aging Coefficient (Af) |
|---|---|---|---|
| NBR without SBOFA | 82.86 | 75.01 | 0.62 |
| SBOFA 2 phr | 94.07 | 97.06 | 0.91 |
| SBOFA 4 phr | 94.37 | 57.59 | 0.54 |
| SBOFA 6 phr | 75.17 | 40.04 | 0.30 |
| SBOFA 8 phr | 67.80 | 33.47 | 0.23 |
| Mechanical Properties (after Aging at 100 °C) | Soybean Oil Fatty Acid Content (phr) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |
| 100% modulus (MPa) | 1.27 ± 0.09 | 1.19 ± 0.06 | 1.22 ± 0.12 | 1.06 ± 0.09 | 0.93 ± 0.14 |
| 200% Modulus (MPa) | 1.99 ± 0.13 | 1.73 ± 0.08 | 1.89 ± 0.16 | - | - |
| Hardness (Shore A) | 58.0 ± 0.5 | 49.7 ± 1.0 | 54.5 ± 0.4 | 46.9 ± 0.4 | 41.1 ± 0.8 |
| Sample | Concentration of the Correlative Functional Groups (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| (1660–1640 cm−1)/1443 cm−1 | (1140 + 1024) cm−1/1443 cm−1 | (1740 + 1710) cm−1/1443 cm−1 | (3310 + 3450) cm−1/1443 cm−1 | |||||
| Unaged | Aged | Unaged | Aged | Unaged | Aged | Unaged | Aged | |
| NBR without SBOFA | 0.37 | 0.28 | 0.79 | 0.42 | 0.11 | 0.33 | 0.11 | 0.23 |
| SBOFA 2 phr | 0.49 | 0.28 | 0.71 | 0.69 | 0.15 | 0.35 | 0.17 | 0.31 |
| SBOFA 4 phr | 0.44 | 0.30 | 0.76 | 0.57 | 0.24 | 0.62 | 0.18 | 0.33 |
| SBOFA 6 phr | 0.49 | 0.25 | 0.69 | 0.48 | 0.25 | 0.68 | 0.19 | 0.35 |
| SBOFA 8 phr | 0.56 | 0.25 | 0.48 | 0.56 | 0.34 | 0.69 | 0.22 | 0.38 |
| Rubber Sample | Initial Stress (σ0) (MPa) | Peak Area (MPa*K) (Temperature Range 50–80 °C) | Crosslink Density by TSSR Measurement (mol/m3) |
|---|---|---|---|
| NBR without SBOFA | 0.79 | 4.27 | 237 |
| SBOFA 2 phr | 0.58 | 1.77 | 169 |
| SBOFA 4 phr | 0.67 | 2.34 | 211 |
| SBOFA 6 phr | 0.52 | 1.31 | 157 |
| SBOFA 8 phr | 0.38 | 1.29 | 143 |
| Cure Parameters | Unplasticized NBR | SBOFA 2 phr | SBOFA 4 phr | DOP 2 phr | DOP 4 phr |
|---|---|---|---|---|---|
| Scorch times, ts2 (min) | 2.06 ± 0.05 | 1.99 ± 0.08 | 2.46 ± 0.01 | 2.05 ± 0.06 | 2.07 ± 0.08 |
| Cure time, t90 (min) | 15.01 ± 0.06 | 14.85 ± 0.10 | 16.05 ± 0.05 | 15.09 ± 0.08 | 14.94 ± 0.70 |
| Cure rate index, CRI (min−1) | 7.72 ± 0.04 | 7.77 ± 0.01 | 7.36 ± 0.02 | 7.67 ± 0.01 | 7.78 ± 0.36 |
| Crosslink density (mol/m3) | 226.87 ± 3.10 | 159.84 ± 1.54 | 203.42 ± 2.51 | 144.75 ± 0.51 | 198.65 ± 1.72 |
| Properties/Sample | Unplasticized NBR | SBOFA 2 phr | SBOFA 4 phr | DOP 2 phr | DOP 4 phr |
|---|---|---|---|---|---|
| 100% modulus (MPa) | 1.19 ± 0.02 | 1.17 ± 0.02 | 0.91 ± 0.02 | 1.23 ± 0.02 | 1.09 ± 0.04 |
| 200% modulus (MPa) | 1.78 ± 0.03 | 1.70 ± 0.06 | 1.37 ± 0.04 | 2.06 ± 0.04 | 1.64 ± 0.03 |
| 300% modulus (MPa) | - | - | 1.86 ± 0.06 | - | - |
| Tensile strength (MPa) | 2.77 ± 0.20 | 2.14 ± 0.14 | 2.51 ± 0.29 | 2.08 ± 0.14 | 2.24 ± 0.43 |
| Elongation at break (%) | 314 ± 29 | 247 ± 9 | 418 ± 43 | 202 ± 8 | 299 ± 11 |
| Hardness (Shore A) | 56.0 ± 0.5 | 48.5 ± 0.5 | 51.8 ± 0.8 | 42.1 ± 0.6 | 47.2 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nun-Anan, P.; Hayichelaeh, C.; Boonkerd, K. Effect of a Natural Processing Aid on the Properties of Acrylonitrile-Butadiene Rubber: Study on Soybean Oil Fatty Acid from Seed Crop. Polymers 2021, 13, 3459. https://doi.org/10.3390/polym13203459
Nun-Anan P, Hayichelaeh C, Boonkerd K. Effect of a Natural Processing Aid on the Properties of Acrylonitrile-Butadiene Rubber: Study on Soybean Oil Fatty Acid from Seed Crop. Polymers. 2021; 13(20):3459. https://doi.org/10.3390/polym13203459
Chicago/Turabian StyleNun-Anan, Phattarawadee, Chesidi Hayichelaeh, and Kanoktip Boonkerd. 2021. "Effect of a Natural Processing Aid on the Properties of Acrylonitrile-Butadiene Rubber: Study on Soybean Oil Fatty Acid from Seed Crop" Polymers 13, no. 20: 3459. https://doi.org/10.3390/polym13203459
APA StyleNun-Anan, P., Hayichelaeh, C., & Boonkerd, K. (2021). Effect of a Natural Processing Aid on the Properties of Acrylonitrile-Butadiene Rubber: Study on Soybean Oil Fatty Acid from Seed Crop. Polymers, 13(20), 3459. https://doi.org/10.3390/polym13203459