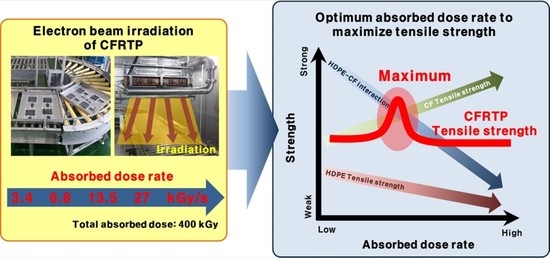
Influences of Absorbed Dose Rate on the Mechanical Properties and Fiber–Matrix Interaction of High-Density Polyethylene-Based Carbon Fiber Reinforced Thermoplastic Irradiated by Electron-Beam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Electron-Beam Irradiation
2.4. Tensile Testing of CFRTP and HDPE Dog-Bone Specimens
2.5. Tensile Testing of CF Filaments
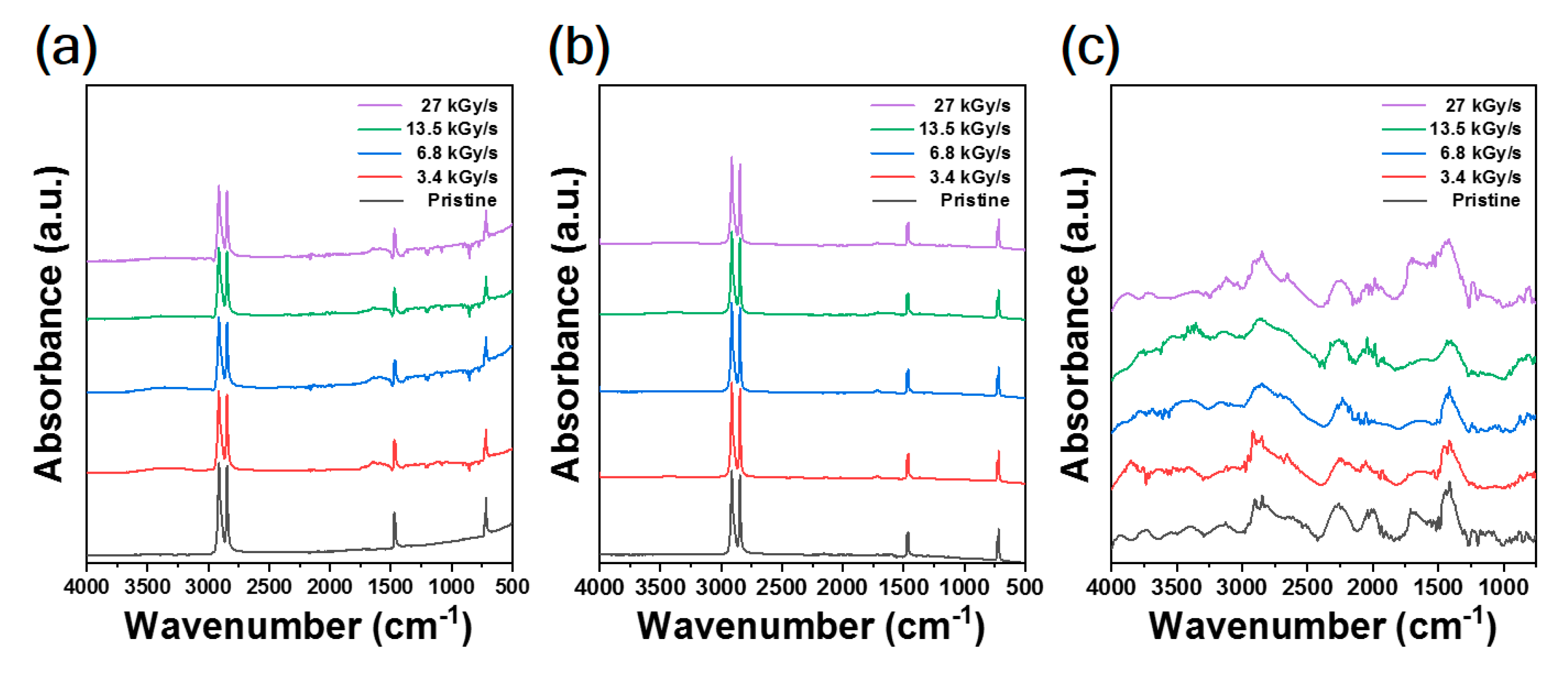
2.6. Fourier Transform-Infrared (FT-IR) Analysis
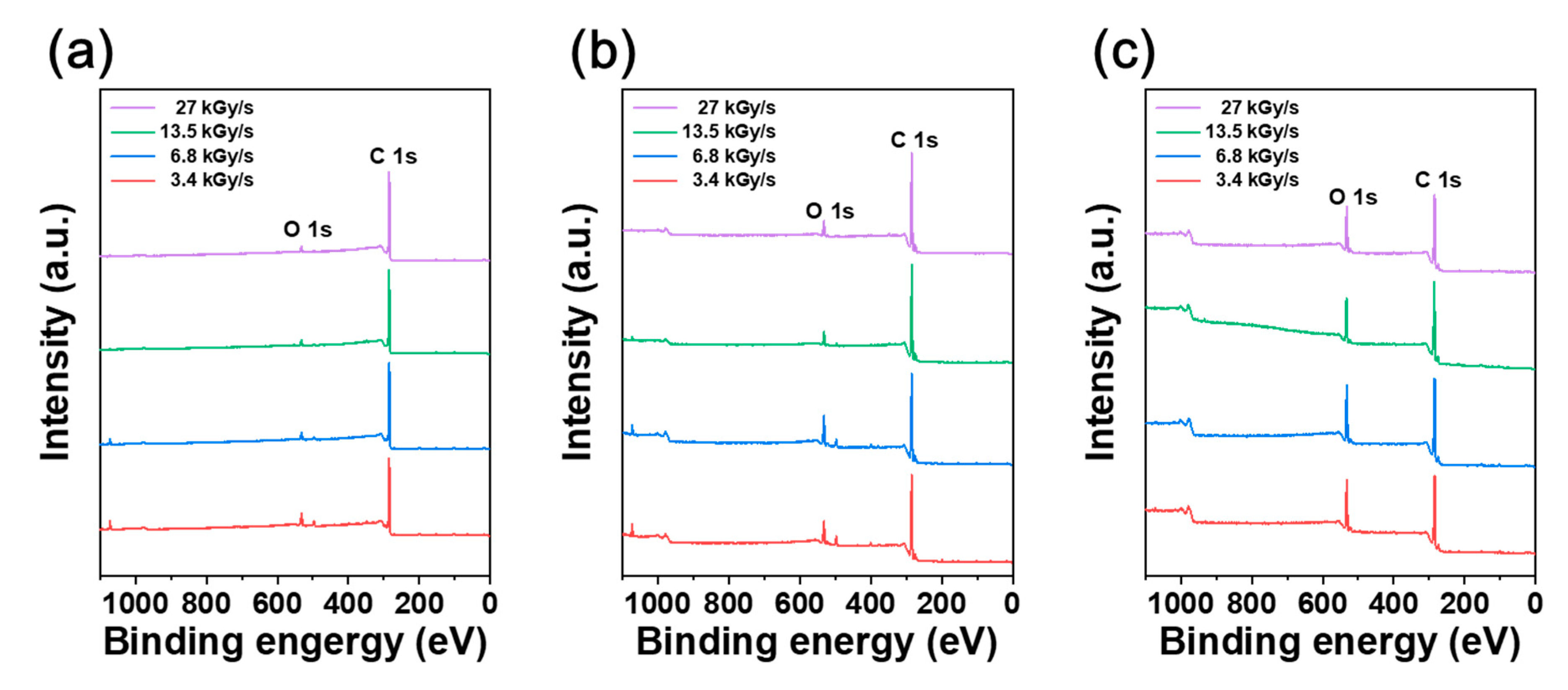
2.7. X-ray Photoelectron Spectroscopy (XPS) Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martin, A.M. Introduction of Fibre-Reinforced Polymers and Composites: Concepts, Properties and Processes, Fiber Reinforced Polymers. In The Technology Applied for Concrete Repair; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Guermazi, N.; Tarjem, A.B.; Ksouri, I.; Ayedi, H.F. On the durability of FRP composites for aircraft structures in hygrothermal conditioning. Compos. Part. B 2016, 85, 294–304. [Google Scholar] [CrossRef]
- Karatas, M.A.; Gokkaya, H. A review on machinability of carbon fiber reinforced polymer (CFRP) and glass fiber reinforced polymer (GFRP) composite materials. Def. Technol. 2018, 14, 318–326. [Google Scholar] [CrossRef]
- Abbood, I.S.; Odaa, S.A.; Hasan, K.F.; Jasim, M.A. Properties evaluation of fiber reinforced polymers and their constituent materials used in structures—A review. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Langston, T. The Tensile Behavior of High-Strength Carbon Fibers. Microsc. Microanal. 2016, 22, 841–844. [Google Scholar] [CrossRef]
- Chand, S. Review Carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Wang, B.; Ma, S.; Yan, S.; Zhu, Z. Readily recyclable carbon fiber reinforced composites based on degradable thermosets: A review. Green Chem. 2019, 21, 5781. [Google Scholar] [CrossRef]
- Asmatulu, E.; Twomey, J.; Overcash, M. Recycling of fiber-reinforced composites and direct structural composite recycling concept. J. Compos. Mater. 2014, 48, 593–608. [Google Scholar] [CrossRef]
- Hu, C.; Liao, X.; Qin, Q.H.; Wang, G. The fabrication and characterization of high density polyethylene composites reinforced by carbon nanotube coated carbon fibers. Compos. Part A 2019, 121, 149–156. [Google Scholar] [CrossRef]
- Han, S.H.; Oh, H.J.; Kim, S.S. Evaluation of fiber surface treatment on the interfacial behavior of carbon fiber-reinforced polypropylene composites. Compos. Part B 2014, 60, 98–105. [Google Scholar] [CrossRef]
- Sang, L.; Wang, Y.; Chen, G.; Liang, J.; Wei, Z. A comparative study of the crystalline structure and mechanical properties of carbon fiber/polyamide 6 composites enhanced with/without silane treatment. RCS Adv. 2016, 6, 107739. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, S.; Yang, D.; Dodelet, J.P.; Sacher, E. The surface analytical characterization of carbon fibers functionalized by H2SO4/HNO3 treatment. Carbon 2008, 46, 196–205. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, D.H.; Yang, K.S.; Lee, B.C.; Kim, Y.A.; Endo, M. Electron Beam Irradiation-Enhanced Wettability of Carbon Fibers. Appl. Mater. Interfaces 2011, 3, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Osbeck, S.; Bradley, R.H.; Liu, C.; Idriss, H.; Ward, S. Effect of an ultraviolet/ozone treatment on the surface texture and functional groups on polyacrylonitrile carbon fibres. Carbon 2011, 49, 4322–4330. [Google Scholar] [CrossRef]
- Woo, L.; Sandford, S.L. Comparison of electron beam irradiation with gamma processing for medical packaging materials. Radiat. Phys. Chem. 2002, 63, 845–850. [Google Scholar] [CrossRef]
- Park, S.K.; Jung, S.Y.; Lee, D.Y.; Ghim, H.D.; Yoo, S.H. Effects of electron-beam irradiation and radiation cross-linker on tensile properties and thermal stability of polypropylene-based carbon fiber reinforced thermoplastic. Polym. Degrad. Stab. 2020, 181, 109301. [Google Scholar] [CrossRef]
- Zhenjuan, Z.; Yaungsheng, W.; Qinyl, D.; Siliang, W.; Te, H. Surface modification of carbon fiber via electron-beam irradiation grafting. Suf. Int. Anal. 2012, 45, 5. [Google Scholar] [CrossRef]
- Giovedi, C.; Machado, L.D.B.; Augusto, M.; Pino, E.S.; Raino, P. Evaluation of the mechanical properties of carbon fiber after electron beam irradiation. Nucl. Instr. Meth. Phys. Res. B 2005, 236, 526–530. [Google Scholar] [CrossRef]
- Pino, E.S.; Machado, L.D.B.; Giovedi, C. Improvement of carbon fiber surface properties using electron beam irradiation. Nucl. Sci. Tech. 2007, 18, 39–41. [Google Scholar] [CrossRef]
- Jung, S.Y.; Park, S.K.; Ghim, H.D.; Lee, D.Y.; Yoo, S.H. Synergetic effect of cross-linking and interfacial interaction in carbon fiber reinforced thermoplastic to enhance its tensile strength by electron-beam irradiation. Carbon Lett. 2020, 30, 165–175. [Google Scholar] [CrossRef]
- Gill, Y.Q.; Jin, J.; Song, M. Melt flow behavior of high density polyethylene nanocomposites with 1D, 2D and 3D nanofillers. Nanocomposites 2015, 1, 160–169. [Google Scholar] [CrossRef]
- Uematsu, H.; Horisawa, N.; Horikida, T.; Tanoue, S.; Iemoto, Y. Effect of carbon fiber on the capillary extrusion behaviors of high-density polyethylene. Polym. J. 2013, 45, 449–456. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, H.M.; An, J.H.; Kim, B.S.; Min, B.G.; Kang, S.J.; An, K.H.; Kim, B.J. Effects of cross-linking methods for polyethylene-based carbon fibers: Review. Carbon Lett. 2015, 16, 147–170. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, H.; Hosoi, F. γ-Radiation-lnduced Cross-Linking of Polyethylene. Polym. J. 1973, 4, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Mizera, A.; Manas, M.; Holik, Z.; Manas, D.; Stanek, M.; Cerny, J.; Bednarik, M.; Ovsik, M. Properties of HDPE after Radiation Cross-linking. Int. J. Math. Comput. Simul. 2012, 6, 584–591. [Google Scholar]
- Ziaie, F.; Anvari, F.; Ghaffari, M.; Borhani, M. Dose rate effect on LDPE cross-linking induced by electron beam irradiation. Nukleonika 2005, 50, 125–127. [Google Scholar]
- Wang, H.; Xu, L.; Hu, J.; Wang, M.; Wu, G. Radiation-induced oxidation of ultra-high molecular weight poly-ethylene(UHMWPE) powder by gamma rays and electron beams: A clear dependence of dose rate. Radiat. Phys. Chem. 2015, 115, 88–96. [Google Scholar] [CrossRef]
- Buttafuva, A.; Tavares, A.; Arimondi, M.; Zaopo, A.; Nesti, S.; Dondi, D.; Mariani, M.; Fauciatano, A. Dose rate effects on the radiation induced oxidation of polyethylene. Nucl. Instr. Meth. Phys. Res. B 2007, 265, 221–226. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. J. Mar. Pol. Bul. 2017, 127, 704–716. [Google Scholar] [CrossRef]
- Becerril, J.J.; López, A.M.; Reyes, M.J. Radiocatalytic degradation of dissolved organic compounds in wastewater. Nukleonika 2016, 61, 473–476. [Google Scholar] [CrossRef] [Green Version]
- Caër, S.L. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, L.; Li, D.Y.; Yi, R.B.; Mo, J.W.; Wu, M.H.; Xu, G. Controllable reduction of graphene oxide by electron-beam irradiation. RSC Adv. 2019, 9, 3597. [Google Scholar] [CrossRef] [Green Version]
- Gardette, M.; Perthue, A.; Gardette, J.L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef] [Green Version]
- Carpentieri, I.; Brunella, V.; Bracco, P.; Paganini, M.C.; Prever, E.M.B.D.; Luda, M.P.; Bonomi, S.; Costa, L. Post-irradiation oxidation of different polyethylenes. Polym. Degrad. Stab. 2011, 96, 624–629. [Google Scholar] [CrossRef]
- Costa, L.; Bracco, P. Mechanisms of crosslynking, oxidative degradation and stabilization of UHMWPE. In UHMWPE Biomaterials Handbook; Kurtz, S.M., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2009; p. 309e23. [Google Scholar]
- Jiang, T.; Qi, Y.; Wu, Y.; Zhang, J. Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation. E Polymers 2019, 19, 499–510. [Google Scholar] [CrossRef]
- Gottlieb, R.; Schmidt, T.; Arndt, K.F. Synthesis of temperature-sensitive hydrogel blends by high-energy irradiation. Nucl. Instr. Meth. Phys. Res. B 2005, 236, 371–376. [Google Scholar] [CrossRef]
- Sonnier, R.; Taguet, A.; Rouif, S. Chapter 9 Modification of polymer blends by E-beam and Gamma irradiation. In Functional Polymer Blends: Synthesis, Properties, and Performance; Mittal, V., Ed.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2017; pp. 261–304. [Google Scholar]
- Forster, A.L.; Tsinas, Z.; Al-Sheikhly, M. Effect of Irradiation and Detection of Long-Lived Polyenyl Radicals in Highly Crystalline Ultra-High Molar Mass Polyethylene (UHMMPE) Fibers. Polymers 2019, 11, 924. [Google Scholar] [CrossRef] [Green Version]
- Tadao, S.; Yasuaki, Y. Diffusion and solubility of oxygen in γ-ray irradiated polymer insulation materials. JAERI 1986, 1299, 68. [Google Scholar]
- Seguchi, T.; Hashimoto, S.; Arakawa, K.; Hayakawa, N.; Kawakami, W.; Kuriyama, I. Radiation induced oxidative degradation of polymers I-Oxidation region in polymer films irradiated in oxygen under pressure. Radiat. Phys. Chem. 1981, 17, 195–201. [Google Scholar] [CrossRef]
- Yu, Q.; Zheng, Y.; Wang, Y.; Shen, L.; Wang, H.; Zheng, Y.; He, N.; Li, Q. Highly selective adsorption of phosphate by pyromellitic acid intercalated ZnAl-LDHs: Assembling hydrogen bond acceptor sites. Chem. Eng. J. 2015, 260, 809–817. [Google Scholar] [CrossRef]
- Flamia, R.; Lanza, G.; Salvi, A.M.; Castle, J.E.; Tamburro, A.M. Conformational Study and Hydrogen Bonds Detection on Elastin-Related Polypeptides Using X-ray Photoelectron Spectroscopy. Biomacromolcules 2005, 6, 1299–1309. [Google Scholar] [CrossRef]
- Stevens, J.S.; Coultas, S.; Jaye, C.; Fischer, D.A.; Schreder, S.L.M. Core level spectroscopies locate hydrogen in the proton transfer pathway–identifying quasisymmetrical hydrogen bonds in the solid state. Phys. Chem. Chem. Phys. 2020, 22, 4916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
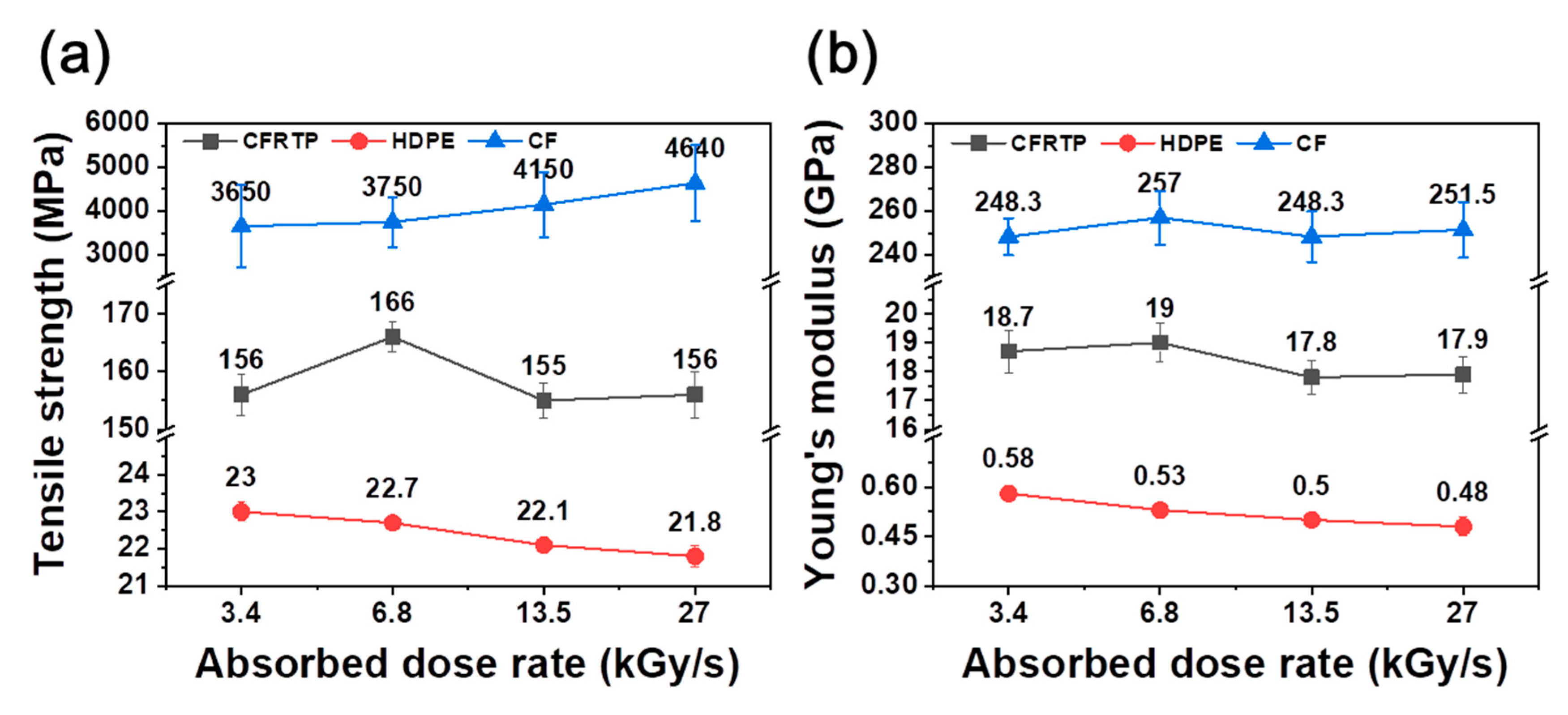

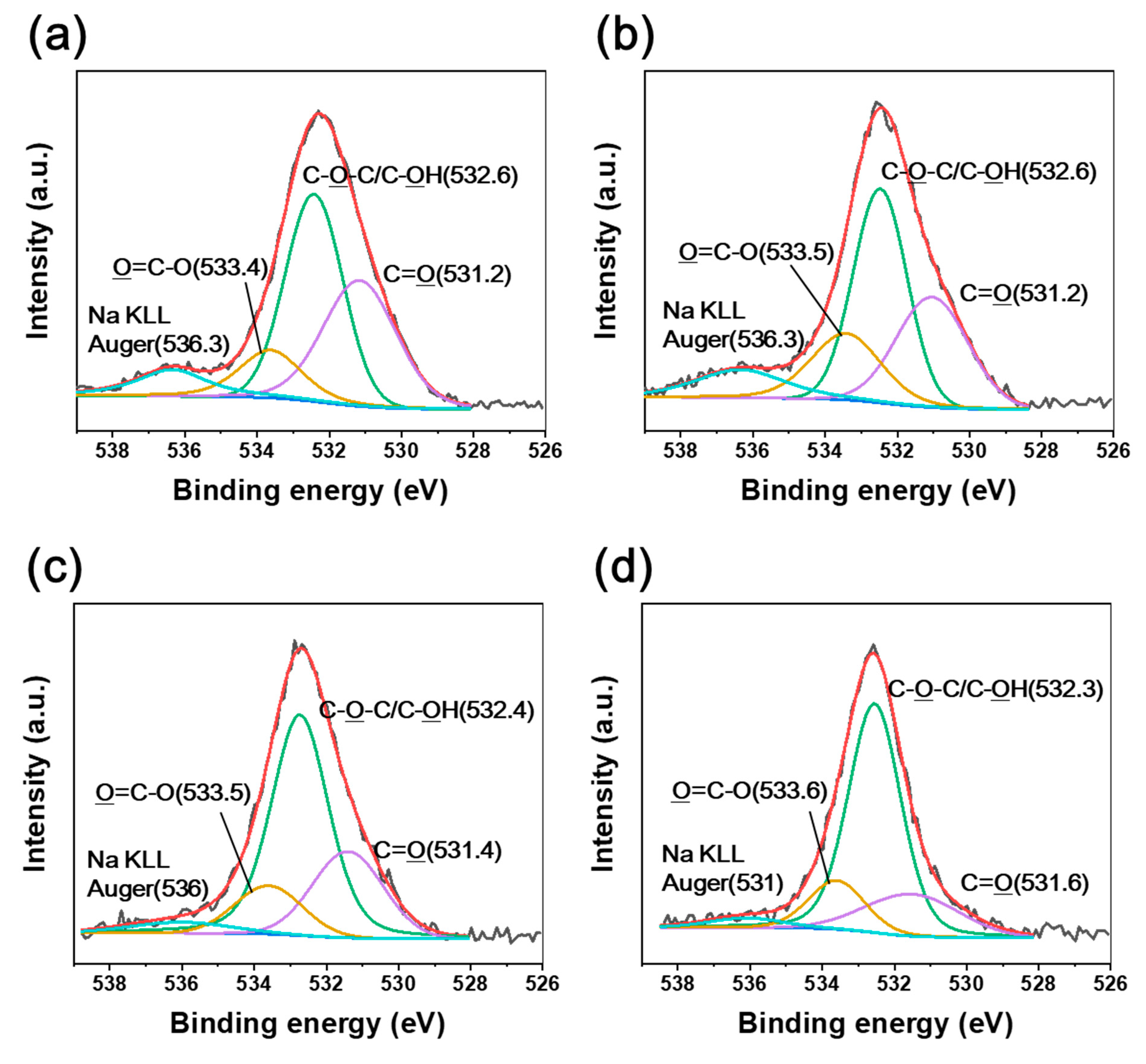

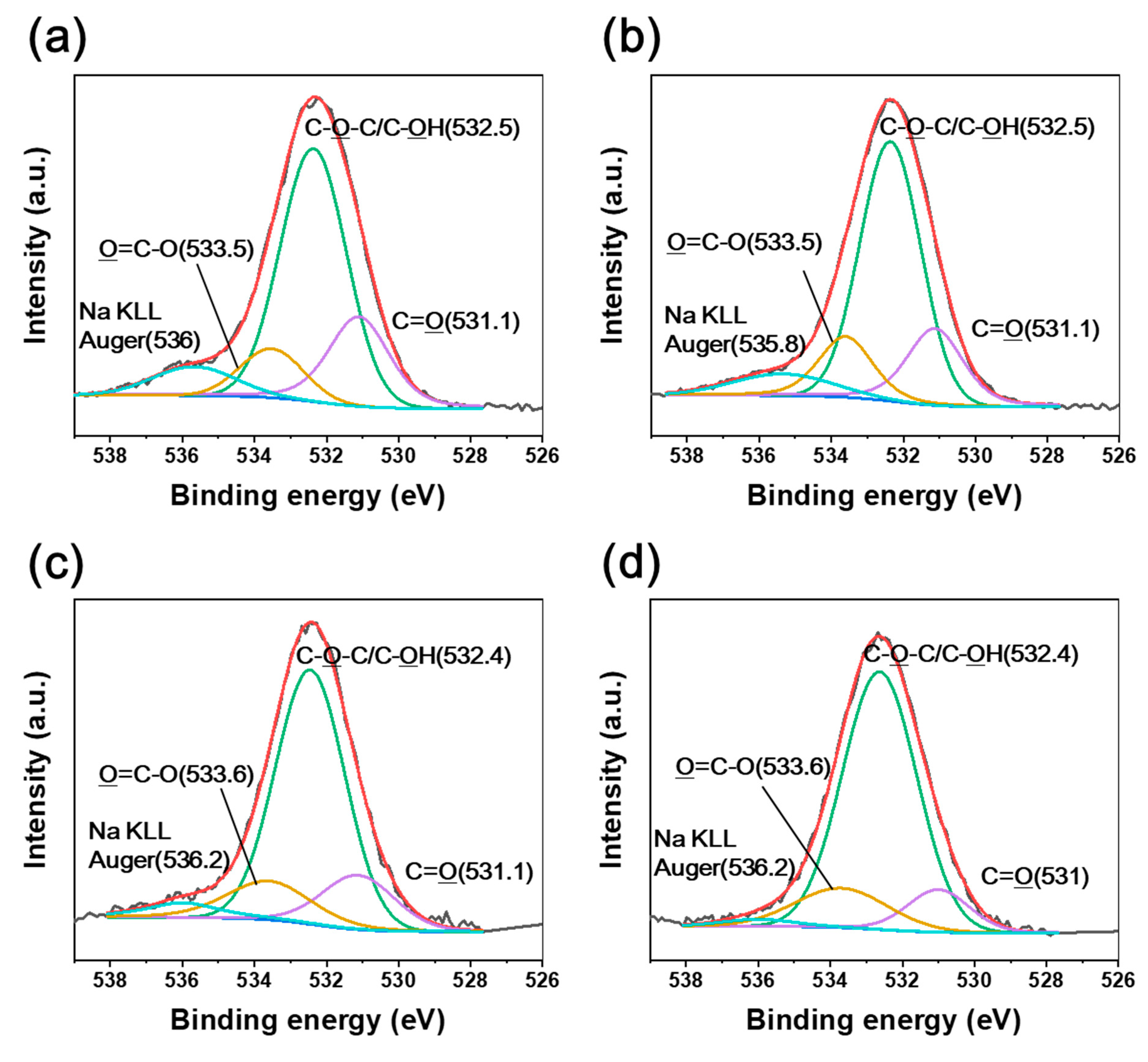


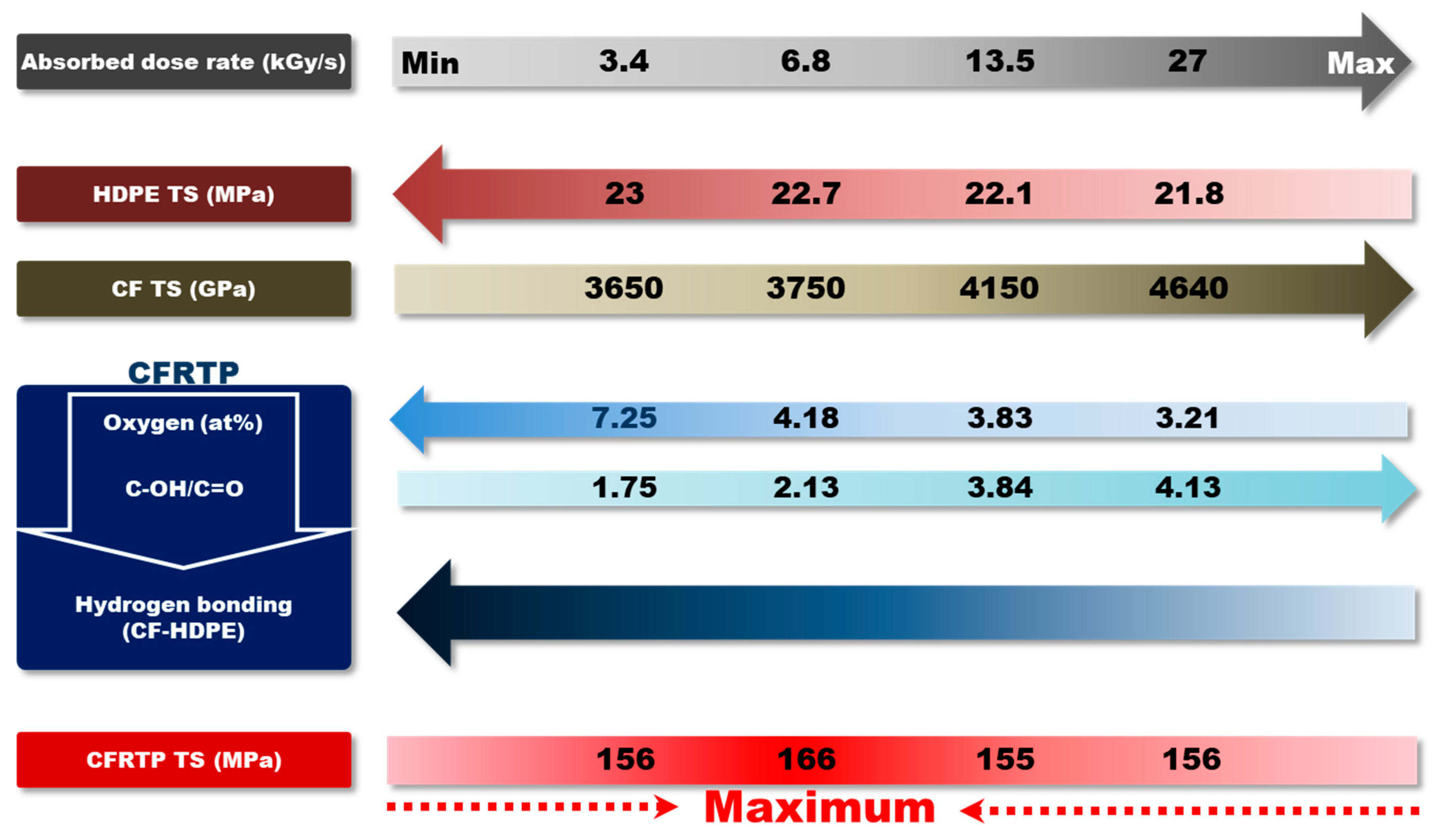
| Absorbed Dose Rate (kGy/s) | Tensile Strength (MPa) | Young’s Modulus (GPa) | |
|---|---|---|---|
| CFRTP | 3.4 | 156 ± 3.6 | 18.7 ± 0.74 |
| 6.8 | 166 ± 2.5 | 19.0 ± 0.66 | |
| 13.5 | 155 ± 3.0 | 17.8 ± 0.60 | |
| 27 | 156 ± 4.0 | 17.9 ± 0.63 | |
| HDPE | 3.4 | 23.0 ± 0.25 | 0.58 ± 0.019 |
| 6.8 | 22.7 ± 0.03 | 0.53 ± 0.015 | |
| 13.5 | 22.1 ± 0.12 | 0.50 ± 0.015 | |
| 27 | 21.8 ± 0.29 | 0.48 ± 0.028 | |
| CF | 3.4 | 3650 ± 950 | 248.3 ± 8.4 |
| 6.8 | 3750 ± 580 | 257.0 ± 12.3 | |
| 13.5 | 4150 ± 740 | 248.3 ± 11.6 | |
| 27 | 4640 ± 880 | 251.5 ± 12.7 |
| Absorbed Dose Rate (kGy/s) | at% | ||||||
|---|---|---|---|---|---|---|---|
| C1s | O1s | Na1s | N1s | Si2p | C/O Ratio | ||
| CFRTP | 3.4 | 89.3 | 7.25 | 1.23 | 1.12 | 1.09 | 12.31 |
| 6.8 | 93.33 | 4.18 | 1.02 | 0.71 | 0.76 | 22.34 | |
| 13.5 | 94.38 | 3.83 | 0.68 | 0.63 | 0.47 | 24.62 | |
| 27 | 96.41 | 3.21 | 0.02 | 0.17 | 0.19 | 30.04 | |
| HDPE | 3.4 | 84.51 | 10.28 | 1.7 | 2.2 | 1.3 | 8.22 |
| 6.8 | 83.35 | 11.91 | 1.34 | 2.03 | 1.38 | 7 | |
| 13.5 | 91.22 | 5.77 | 0.66 | 1.52 | 0.82 | 15.82 | |
| 27 | 92.74 | 6.48 | 0 | 0.77 | 0 | 14.3 | |
| CF | 3.4 | 78.51 | 16.92 | – | 2.01 | 2.56 | 4.64 |
| 6.8 | 80.48 | 17.29 | – | – | 2.23 | 4.66 | |
| 13.5 | 80.1 | 15.09 | – | 2.31 | 2.5 | 5.31 | |
| 27 | 80.51 | 16.31 | – | 1.35 | 1.83 | 4.94 | |
| Orbital | C1s | O1s | |||||
|---|---|---|---|---|---|---|---|
| Chemical Bond | C–OH | C=O | O=C–O | C–OH | C=O | O=C–O | |
| Hydrogen bond interaction | Donor (δ+) | Acceptor (δ−) | Donor (δ+) | Acceptor (δ−) | |||
| BE | |||||||
| Absorbed dose rate (kGy/s) | 3.4 | 285.9 | 287.7 | 288.7 | 532.6 | 531.2 | 533.4 |
| 6.8 | 285.9 | 287.7 | 288.7 | 532.6 | 531.2 | 533.5 | |
| 13.5 | 285.7 | 287.9 | 288.8 | 532.4 | 531.4 | 533.5 | |
| 27 | 285.6 | 288 | 288.9 | 532.3 | 531.6 | 533.6 | |
| BE shift | Down-shift | Up-shift | Down-shift | Up-shift | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Choi, D.Y.; Choi, D.Y.; Lee, D.Y.; Yoo, S.H. Influences of Absorbed Dose Rate on the Mechanical Properties and Fiber–Matrix Interaction of High-Density Polyethylene-Based Carbon Fiber Reinforced Thermoplastic Irradiated by Electron-Beam. Polymers 2020, 12, 3012. https://doi.org/10.3390/polym12123012
Park SK, Choi DY, Choi DY, Lee DY, Yoo SH. Influences of Absorbed Dose Rate on the Mechanical Properties and Fiber–Matrix Interaction of High-Density Polyethylene-Based Carbon Fiber Reinforced Thermoplastic Irradiated by Electron-Beam. Polymers. 2020; 12(12):3012. https://doi.org/10.3390/polym12123012
Chicago/Turabian StylePark, Se Kye, Dong Yun Choi, Du Young Choi, Dong Yun Lee, and Seung Hwa Yoo. 2020. "Influences of Absorbed Dose Rate on the Mechanical Properties and Fiber–Matrix Interaction of High-Density Polyethylene-Based Carbon Fiber Reinforced Thermoplastic Irradiated by Electron-Beam" Polymers 12, no. 12: 3012. https://doi.org/10.3390/polym12123012