Conducting Polymer-Based Nanohybrids for Fuel Cell Application
Abstract
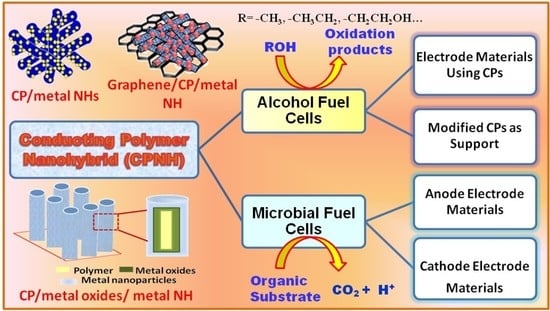
:1. Introduction
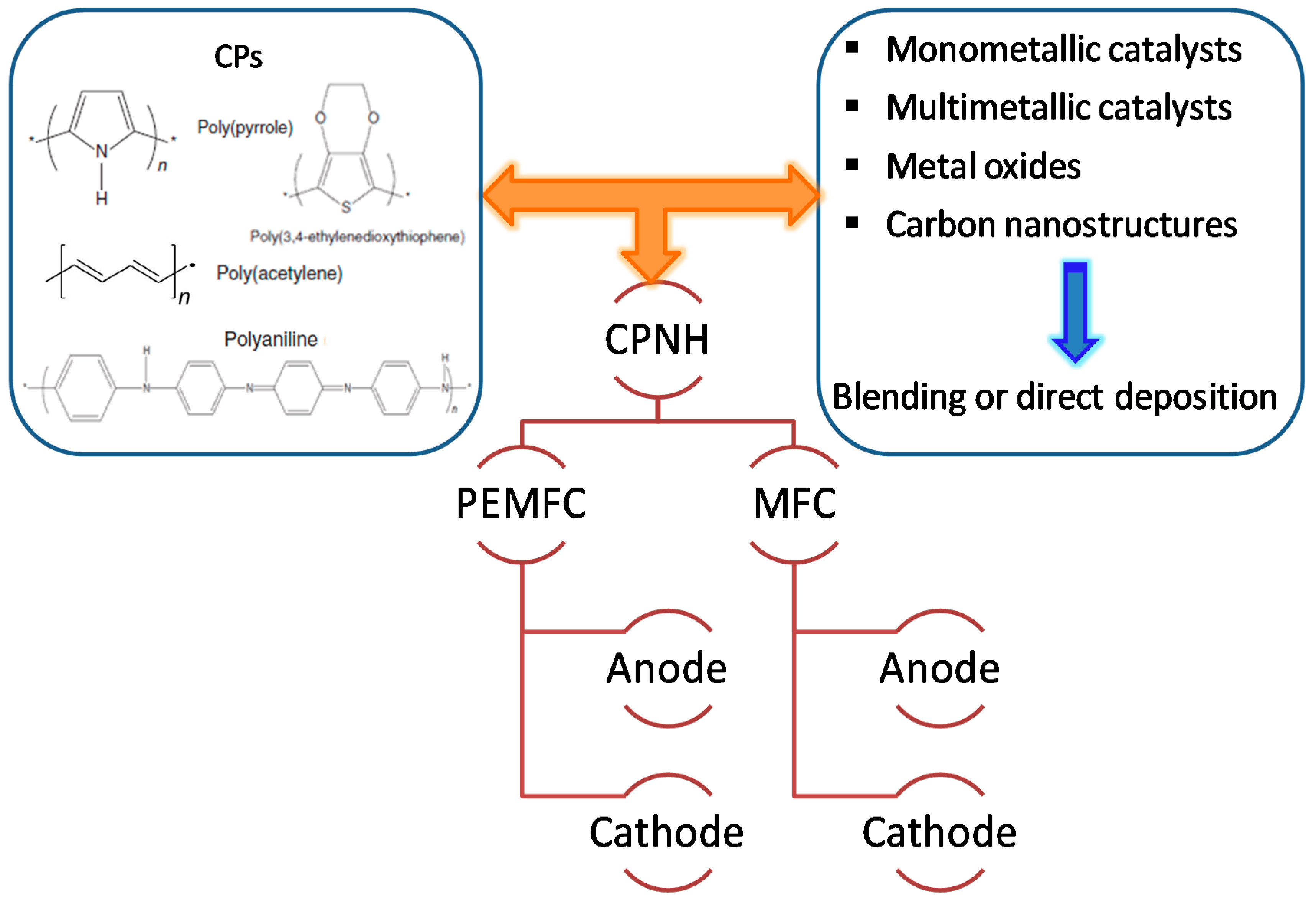
2. CPNH-Based Electrode Materials
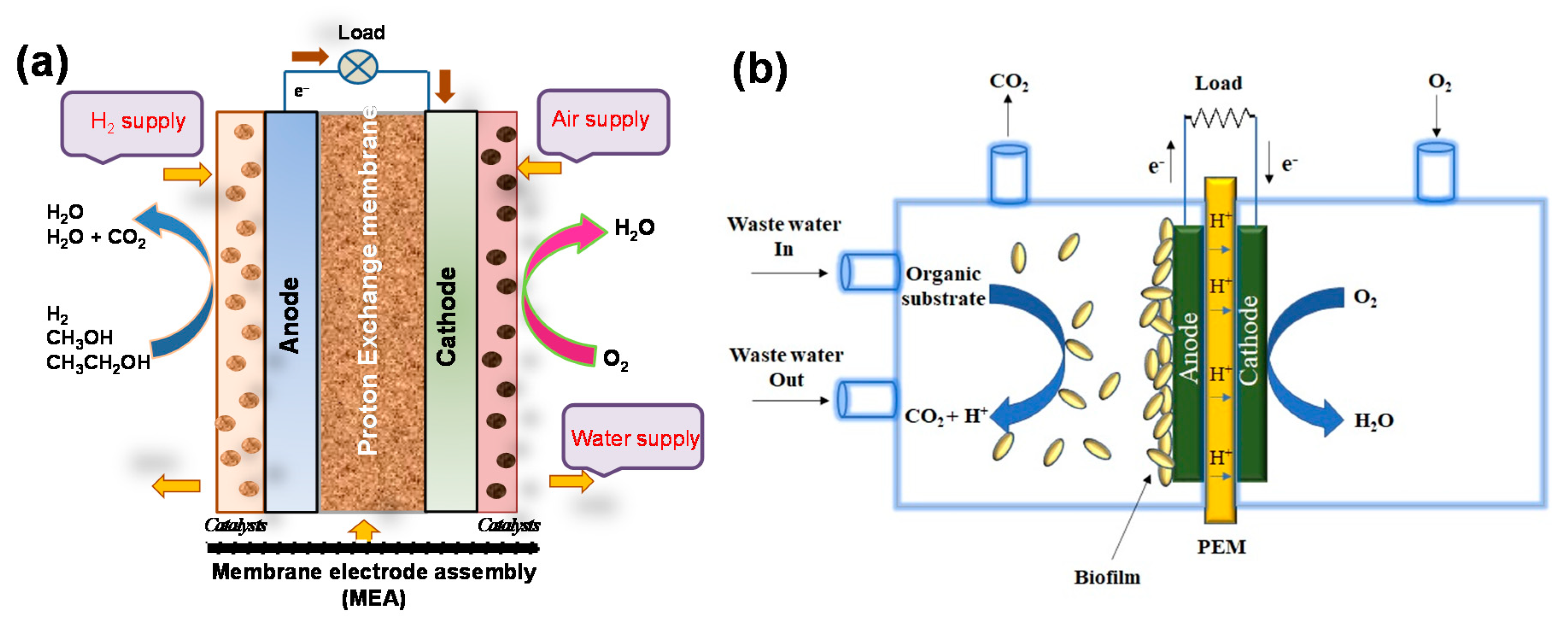
2.1. CPNH-Based Electrode for Alcohol Fuel Cells
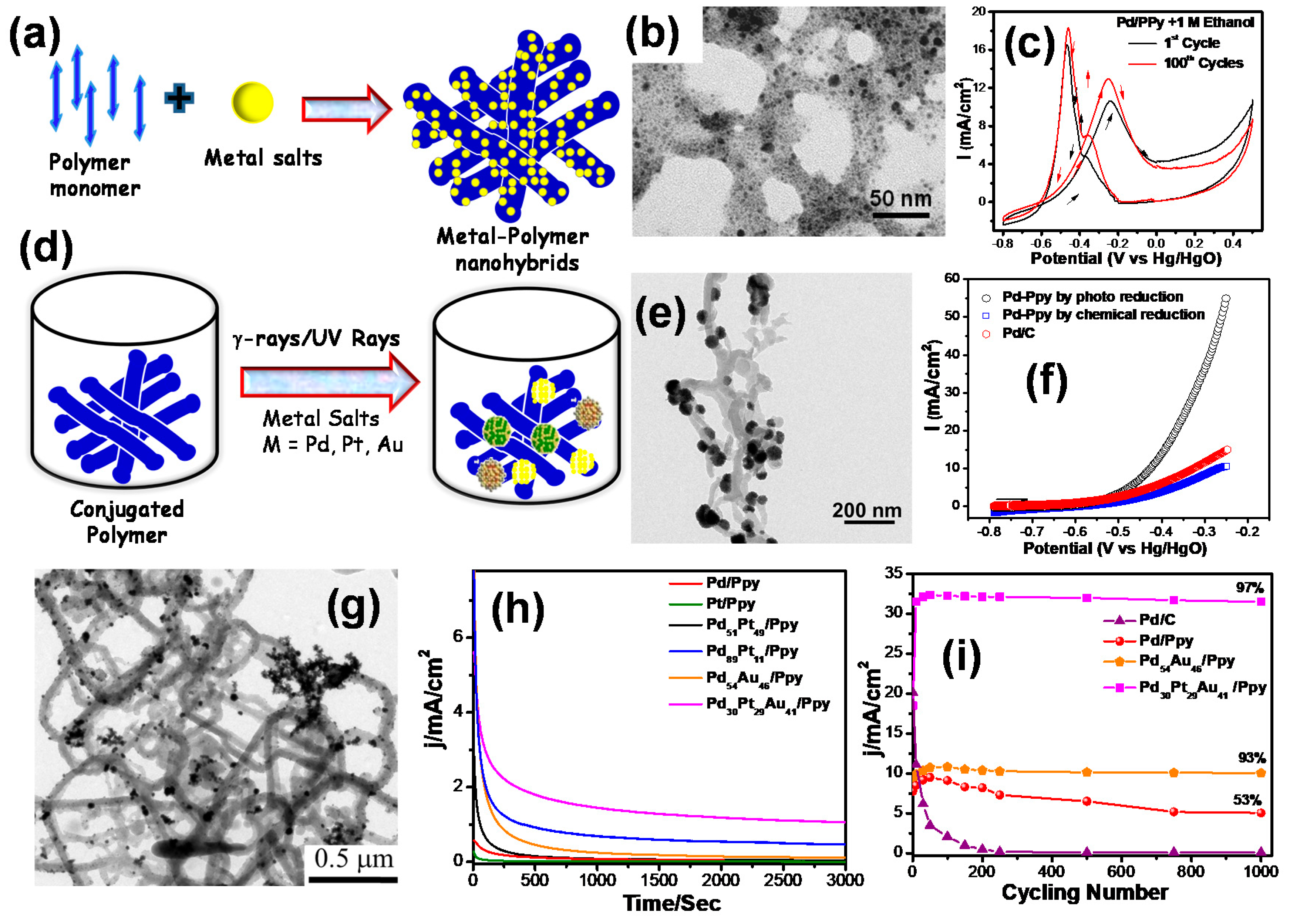
2.1.1. Nanohybrid Electrode Materials Using CPs
2.1.2. Nanohybrid Electrode Materials Using Modified CPs as Support
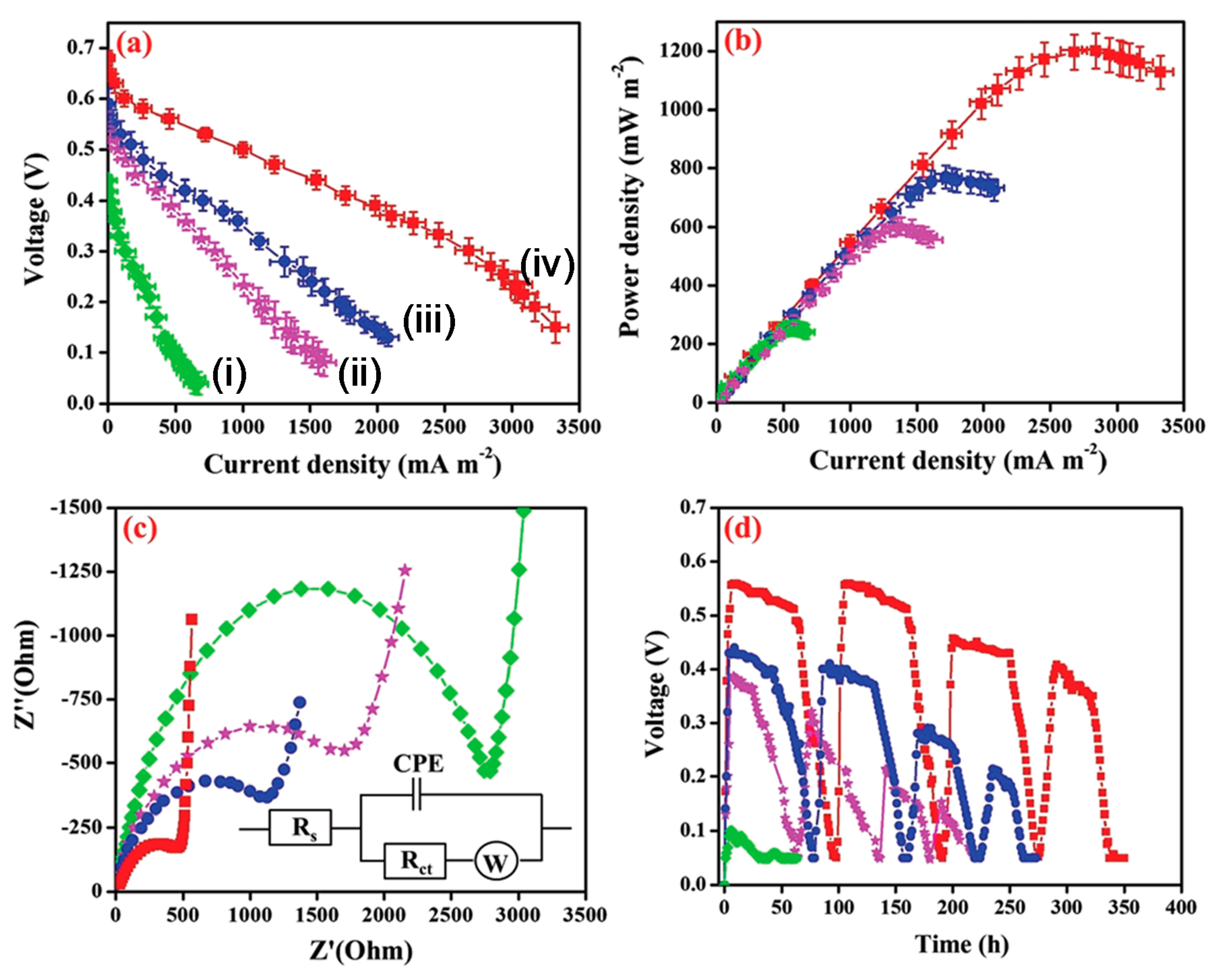
3. CPNH-Based Electrode for Microbial Fuel Cells
3.1. CPNH-Based Anode for MFCs
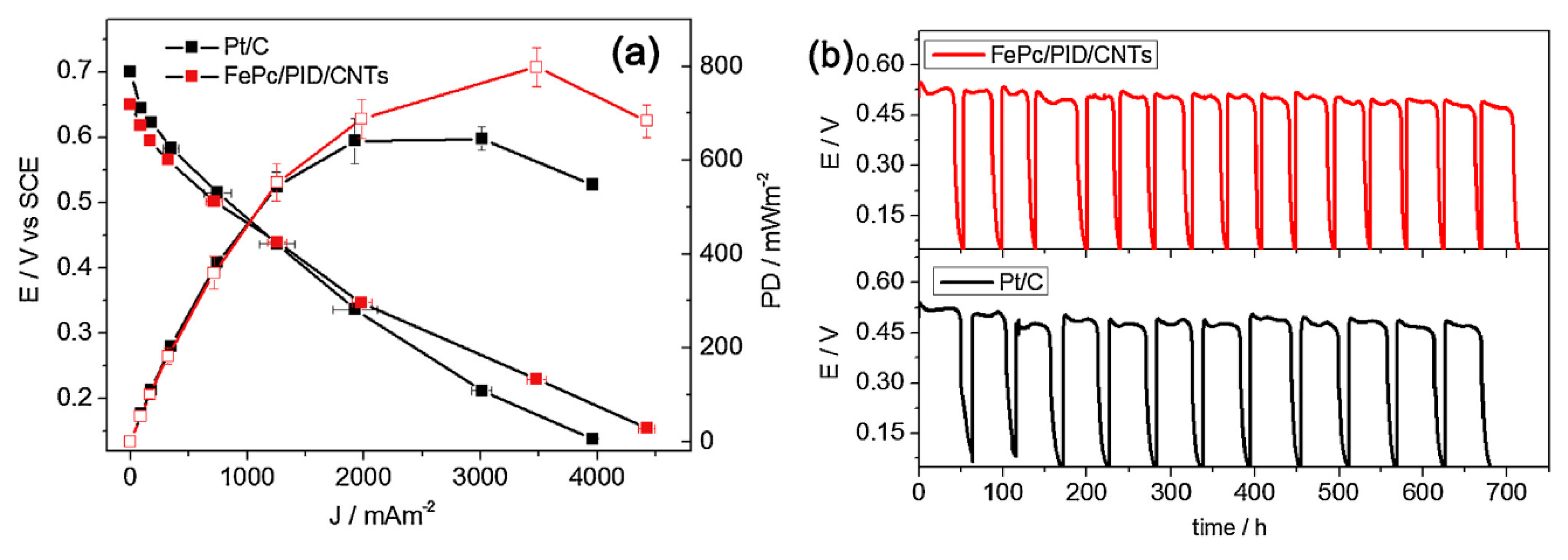
3.2. CPNH-Based Cathode for MFCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gasteiger, H.A.; Markovic, N.M. Just a dream or future reality. Science 2009, 324, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, J.P. Reimagine fuel cells. Nature 2015, 525, 447–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.H.; Wang, X.; Krewer, U.; Li, L.; Scott, K. Direct oxidation alkaline fuel cells: From materials to systems. Energy Environ. Sci. 2012, 5, 5668–5680. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Shen, P.K. Recent development of Polymer Electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Saleem, F.; Ni, B.; Yong, Y.; Gu, L.; Wang, X. Ultra-small Tetrametallic Pt-Pd-Rh-Ag Nanoframes with tunable behavior for direct formic acid/methanol oxidation. Small 2016, 12, 5261–5268. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode Catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef]
- Santoroa, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 2006, 403, 388–3394. [Google Scholar] [CrossRef]
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular microbial fuel cells for efficient electricity generation. Environ. Sci. Technol. 2005, 39, 8077–8082. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Schmittinger, W.; Vahidi, A. A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J. Power Sources 2008, 180, 1–14. [Google Scholar] [CrossRef]
- Long, Z.; Gao, L.; Li, Y.; Kang, B.; Lee, J.Y.; Ge, J.; Liu, C.; Ma, S.; Jin, Z.; Ai, H. Micro galvanic cell to generate PtO and extend the triple-phase boundary during self-assembly of Pt/C and nafion for catalyst layers of PEMFC. ACS Appl. Mater. Interfaces 2017, 9, 38165–38169. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef] [Green Version]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Holade, Y.; Morais, C.; Servat, K.; Napporn, T.W.; Kokoh, K.B. Enhancing the available specific surface area of carbon supports to boost the electroactivity of nanostructured Pt Catalysts. Phys. Chem. Chem. Phys. 2014, 16, 25609–25620. [Google Scholar] [CrossRef]
- Mardle, P.; Ji, X.; Wu, J.; Guan, S.; Dong, H.; Du, S. Thin film electrodes from Pt nanorods supported on aligned N-CNTs for proton exchange membrane fuel cells. Appl. Catal. B 2020, 260, 118031. [Google Scholar] [CrossRef]
- Du, H.; Gan, L.; Li, B.; Wu, P.; Qiu, Y.; Kang, F.; Fu, R.; Zeng, Y. Influences of mesopore size on oxygen reduction reaction catalysis of Pt carbon aerogels. J. Phys. Chem. C 2007, 11, 2040–2043. [Google Scholar] [CrossRef]
- Park, Y.C.; Tokiwa, H.; Kakinuma, K.; Watanabe, M.; Uchida, M. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J. Power Sources 2016, 315, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.B.; Zhao, T.S. Mesoporous carbon with uniquely combined electrochemical and mass transport characteristics for polymer electrolyte membrane fuel cells. RSC Adv. 2013, 3, 16–24. [Google Scholar] [CrossRef]
- Ghosh, S.; Bysakh, S.; Basu, R.N. Bimetallic Pd96Fe4Nanodendrites Embedded in Graphitic Carbon Nanosheets as Highly Efficient Anode Electrocatalysts. Nanoscale Adv. 2019, 1, 3929–3940. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Wang, X. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6266–6291. [Google Scholar] [CrossRef]
- Ghosh, S.; Remita, H.; Kar, P.; Choudhury, S.; Sardar, S.; Beaunier, P.; Roy, P.S.; Bhattacharya, S.K.; Pal, S.K. Facile synthesis of Pd nanostructures in hexagonal mesophases as a promising electrocatalyst for ethanol oxidation. J. Mater. Chem. A 2015, 3, 9517–9527. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Yang, F.; Rasouli, S.; Qiu, Y.; Li, Z.-F.; Uzunoglu, A.; Sun, C.-J.; Liu, Y.; Ferreira, P.; Li, W.; et al. Understanding Pt nanoparticle anchoring on graphene supports through surface functionalization. ACS Catal. 2016, 6, 2642–2653. [Google Scholar] [CrossRef]
- Franceschini, E.A.; Bruno, M.M.; Viva, F.A.; Williams, F.J.; Jobbágy, M.; Corti, H.R. Mesoporous Pt electrocatalyst for methanol tolerant cathodes of DMFC. Electrochim. Acta 2012, 71, 173–180. [Google Scholar] [CrossRef]
- Qi, Z.; Shan, J.; Pickup, P. Conducting polymer-supported fuel cell catalysts. ACS Symp. Ser. 2002, 832, 166–183. [Google Scholar]
- Fan, L.-P.; Gao, T. Applications of Nanoscale Polypyrrole Proton Exchange Membrane in Microbial Fuel Cells. Int. J. Electrochem. Sci. 2019, 14, 470–480. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Rana, D.; Kundu, P.P. Enhancements of catalyst distribution and functioning upon utilization of conducting polymers as supporting matrices in DMFCs: A review. Polym. Rev. 2015, 55, 1–56. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Rana, D.; Kundu, P.P. A review on aromatic conducting polymer based catalysts supporting martrices for application in microbial fuel cells. Polym. Rev. 2014, 54, 401–435. [Google Scholar] [CrossRef]
- Ghosh, S.; Thandavarayan, M.; Basu, R.N. Nanostructured conducting polymers for energy applications: Towards a sustainable platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.; Zhang, B.; Du, Y.; Wang, H.L. Multifunctional polymer–metal nanocomposites via direct chemical reduction by conjugated polymers. Chem. Soc. Rev. 2014, 43, 1349–1360. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef]
- Ghosh, S.; Remita, H.; Basu, R.N. Visible-light-induced reduction of Cr(VI) by PDPB-ZnO nanohybrids and its photo-electrochemical response. Appl. Catal. B 2018, 239, 362–372. [Google Scholar] [CrossRef]
- Ghosh, S.; Rashmi, D.; Bera, S.; Basu, R.N. Functionalized conjugated polymer with plasmonic Au nanoalloy for photocatalytic hydrogen generation under visible-NIR. Int. J. Hydrogen Energy 2019, 44, 13262–13272. [Google Scholar] [CrossRef]
- Tintula, K.K.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. A solid-polymer-electrolyte direct methanol fuel cell (DMFC) with Pt-Ru nanoparticles supported onto poly(3,4- ethylenedioxythiophene) and polystyrene sulphonic acid polymer composite as anode. J. Chem. Sci. 2010, 122, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Dutta, K.; Kundu, P.P.; Bhattacharya, S.K. Nanostructured polyaniline: An efficient support matrix for Platinum-Ruthenium anode Catalyst in direct Methanol fuel cell. Fuel Cell 2018, 18, 369–378. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energ. Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-based nanomaterials: Synthesis and electrochemical applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of Hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Microbial fuel cells—Challenges and applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef] [Green Version]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N.L. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sust. Energ. Rev. 2020, 132, 110041. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhandary, N.; Basu, S.; Basu, R.N. Synergistic Effects of Polypyrrole Nanofibers and Pd Nanoparticles for Improved Electrocatalytic Performance of Pd/PPy Nanocomposites for Ethanol Oxidation. Electrocatalysis 2017, 8, 329–339. [Google Scholar] [CrossRef]
- Pandey, R.K.; Lakshminarayanan, V. Enhanced electrocatalytic activity of Pd-Dispersed 3,4-Polyethylenedioxythiophene film in hydrogen evolution and Ethanol Electro-oxidation reactions. J. Phys. Chem. C 2010, 114, 8507–8514. [Google Scholar] [CrossRef]
- Ghosh, S.; Bera, S.; Karmakar, N.; Basu, R.N. Enhanced electrocatalytic activity of branched Pd nanostructures decorated conducting polymer nanofibers for Alkaline fuel cells. Mater. Today Proc. 2018, 5, 9733–9742. [Google Scholar] [CrossRef]
- Ghosh, S.; Bera, S.; Bysakh, S.; Basu, R.N. Highly active multimetallic palladium nanoalloys embedded in conducting polymer as anode catalysts for electrooxidation of ethanol. ACS Appl. Mater. Interfaces 2017, 9, 33775–33790. [Google Scholar] [CrossRef]
- Xu, H.; Ding, L.-X.; Liang, C.-L.; Tong, Y.-X.; Li, G.-R. High-performance polypyrrole functionalized PtPd electrocatalysts PtPd/PPy/PtPd three-layered nanotube arrays based on for the electrooxidation of small organic molecules. NPG Asia Mater. 2013, 5, e69–e72. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.-L.; Xu, H.; Feng, J.-X.; Ding, L.-X.; Tong, Y.-X.; Li, G.-R. Design of Pd/PANI/Pd sandwich-structured nanotube array catalysts with special shape effects and synergistic effects for ethanol electrooxidation. J. Am. Chem. Soc. 2013, 135, 10703–10709. [Google Scholar] [CrossRef] [PubMed]
- Kurbiel, M.G.; Drelinkiewicz, A.; Kosydar, R.; Dembińska, B.; Kulesza, P.J.; Gurgul, J. Palladium content effect on the electrocatalytic activity of Palladium–Polypyrrole nanocomposite for cathodic reduction of oxygen. Electrocatalysis 2014, 5, 23–40. [Google Scholar] [CrossRef]
- Bogdanović, U.; Pašti, I.; Marjanović, G.Ć.; Mitrić, M.; Ahrenkiel, S.P.; Vodnik, V. Interfacial synthesis of gold–Polyaniline nanocomposite and its electrocatalytic application. ACS Appl Mater Interfaces 2015, 7, 28393–30282. [Google Scholar] [CrossRef]
- Verma, C.J.; Kumar, A.; Ojha, R.P.; Prakash, R. Au-V2O5/Polyindole composite: An approach for ORR in different electrolytes. J. Electroanal. Chem. 2020, 861, 113959. [Google Scholar] [CrossRef]
- Vercelli, B.; Zotti, G.; Berlin, A. Mono- and multilayers of platinum nanoparticles and poly(3,4-ethylenedioxythiophene) as nanostructures for methanol electrooxidation. J. Phys. Chem. C 2009, 113, 3525–3529. [Google Scholar] [CrossRef]
- Yuan, X.; Ding, X.L.; Wang, C.-Y.; Ma, Z.F. Use of polypyrrole in catalysts for low temperature fuel cells. Energy Environ. Sci. 2013, 6, 1105–1124. [Google Scholar] [CrossRef]
- Rajesh, B.; Thampi, K.R.; Bonard, J.M.; Mathieu, H.J.; Xanthopoulos, N.; Viswanathan, B. Nanostructured Conducting Polyaniline tubules as Catalyst support for Pt particles for possible fuel cell applications. Electrochem. Solid-State Lett. 2004, 7, A404–A407. [Google Scholar] [CrossRef]
- Jin, W.; Huang, X.; Cheng, H.; Xu, T.; Wang, F.; Guo, X.; Wu, Y.; Ying, Y.; Wen, Y.; Yang, H. Polyaniline hollow tubes loading tiny platinum nanoparticles for boosting methanol oxidation. Appl. Surf. Sci. 2019, 483, 489–495. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, X. Preparation of three-dimensional composite of poly(N-acetylaniline) nanorods/platinum nanoclusters and electrocatalytic oxidation of methanol. J. Power Sources 2007, 164, 49–55. [Google Scholar] [CrossRef]
- Kim, K.; Ahn, H.; Park, M.J. Highly Catalytic Pt Nanoparticles Grown in Two-Dimensional Conducting Polymers at the Air–Water Interface. ACS Appl. Mater. Interfaces 2017, 9, 30278–30282. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, B.; Thampi, B.; Bonard, K.R.; Mathieu, J.M.; Xanthopoulos, H.J.; Viswanathan, N.B. Conducting polymeric nanotubules as high performance methanol oxidation catalyst support. Chem. Commun. 2003, 2022–2023. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Polyaniline/Pt Hybrid nanofibers: High-efficiency nanoelectrocatalysts for electrochemical devices. Small 2009, 5, 1869–1876. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, N.; Huang, X. Polyaniline-coated platinum nanocube assemblies as enhanced methanol oxidation electrocatalysts. ChemCatChem 2016, 8, 3436–3440. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P. Nickel nanocatalysts supported on sulfonated polyaniline: Potential toward methanol oxidation and as anode materials for DMFCs. J. Mater. Chem. A 2015, 3, 11349–11357. [Google Scholar] [CrossRef]
- Choi, S.-H.; Gopalan, A.I.; Ryu, J.-H.; Lee, K.-P. Hollow spherical nanocapsules of poly(pyrrole) as a promising support for Pt/Ru nanoparticles based catalyst. Mater. Chem. Phys. 2010, 120, 18–22. [Google Scholar] [CrossRef]
- Gajendran, P.; Saraswathi, R. Electrocatalytic performance of poly(o-phenylenediamine)-Pt–Ru nanocomposite for methanol oxidation. J Solid State Electrochem. 2013, 17, 2741–2747. [Google Scholar] [CrossRef]
- Rajathi, P.M.; Sivakumar, C.; Berchmans, S. Methanol electro-oxidation by nanostructured Pt/Cu bimetallic on poly 3, 4 ethylenedioxythiophene (PEDOT). Electrochim. Acta 2018, 282, 163–170. [Google Scholar]
- Zhao, H.; Li, L.; Yang, J.; Zhang, Y.; Li, H. Synthesis and characterization of bimetallic Pt–Fe/polypyrrole–carboncatalyst as DMFC anode catalyst. Electrochem. Commun. 2008, 10, 876–879. [Google Scholar] [CrossRef]
- Ghosh, S.; Bera, S.; Bysakh, S.; Basu, R.N. Conducting polymer nanofiber-supported Pt alloys: Unprecedented materials for methanol oxidation with enhanced electrocatalytic performance and stability. Sustain. Energy Fuels 2017, 1, 1148–1161. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of polymer structures in catalysis by transition metal and metal oxide nanoparticle composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Munichandraiah, N. Electrooxidation of methanol on pt-modified conductive polymer PEDOT. Langmuir 2009, 25, 1732–1738. [Google Scholar] [CrossRef]
- Dash, S.; Munichandraiah, N. Electrocatalytic oxidation of C3-aliphatic alcohols on electrodeposited Pd-PEDOT nanodendrites in alkaline medium. J. Electrochem. Soc. 2013, 160, 197–202. [Google Scholar] [CrossRef]
- Joice, E.K.; Anitha, V.; Sudhakar, Y.N.; Bala, G.; Joseph, S. Poly (aniline) decorated with nanocactus platinum on carbon fiber paper and its electrocatalytic behavior toward toluene oxidation. J. Electrochem. Soc. 2018, 165, H399–H406. [Google Scholar] [CrossRef]
- Ghosh, S.; Teillout, A.L.; Floresyona, D.; Oliveira, P.D.; Hagège, A.; Remita, H. Conducting polymer-supported palladium nanoplates for applications in direct alcohol oxidation. Int. J. Hydrogen Energy 2015, 40, 4951–4959. [Google Scholar] [CrossRef]
- Gharibi, H.; Kakaei, K.; Zhiani, M. Platinum nanoparticles supported by a vulcan XC-72 and PANI doped with Trifluoromethane Sulfonic acid substrate as a new Electrocatalyst for direct methanol fuel cells. J. Phys. Chem. C 2010, 114, 5233–5240. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Huang, L.-M.; Wen, T.-C.; Gopalan, A. Enhanced electrocatalytic performance for methanol oxidation of a novel Pt-dispersed poly(3,4-ethylenedioxythiophene)–poly(styrene sulfonic acid) electrode. J. Power Sources 2006, 160, 65–72. [Google Scholar] [CrossRef]
- Liu, F.-J.; Huang, L.-M.; Wen, T.-C.; Li, C.-F.; Huang, S.-L.; Gopalan, A. Effect of deposition sequence of platinum and ruthenium particles into nanofibrous network of polyaniline–poly(styrene sulfonic acid) on electrocatalytic oxidation of methanol. Synth. Met. 2008, 158, 603–609. [Google Scholar] [CrossRef]
- Ye, B.; Cheng, K.; Li, W.; Liu, J.; Zhang, J.; Mu, S. Polyaniline and Perfluorosulfonic acid co-stabilized metal Catalysts for oxygen reduction reaction. Langmuir 2017, 33, 5353–5361. [Google Scholar] [CrossRef]
- Oliveira, R.C.P.; Milikić, J.; Daş, E.; Yurtcan, A.B.; Santos, D.M.F.; Šljukić, B. Platinum/polypyrrole-carbon electrocatalysts for direct borohydride-peroxide fuel cells. Appl. Catal. B: Environ. 2018, 238, 454–464. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Sivakumar, C.; Wen, T.-C. Nanoparticles of Pt/HxMoO3 electrodeposited in poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid) as the electrocatalyst for methanol oxidation. J. Power Sources 2008, 185, 807–814. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Kuo, Z.-Y.; Jow, J.-J.; Wu, T.-Y.; Chen, J.-Y.; Zhu, X.-X. Enhanced electrocatalytic performance for methanol oxidation via insertion of Ruthenium Oxide particles into Pt and Polyaniline-Poly(Acrylic Acid-co-Maleic Acid) composite electrode. Inter. J. Electrochem. Sci. 2012, 7, 4974–4987. [Google Scholar] [CrossRef]
- Xu, H.; Wang, A.L.; Tong, Y.X.; Li, G.R. Enhanced catalytic activity and stability of Pt/CeO2/PANI hybrid hollow nanorod arrays for methanol electro-oxidation. ACS Catal. 2016, 6, 5198–5206. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Zhang, W.; Sherrell, P.; Minett, A.I.; Razal, J.M.; Chen, J. Carbon nanotube architectures as catalyst supports for proton exchange membrane fuel cells. Energy Environ. Sci. 2010, 3, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- Jha, N.; Ramesh, P.; Bekyarova, E.; Tian, X.; Wang, F.; Itkis, M.E.; Haddon, R.C. Functionalized single-walled carbon nanotube-based fuel cell benchmarked against US DOE 2017 technical targets. Sci. Rep. 2013, 3, 2257. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, V.; Alagar, M. Pt and Pt–Ru nanoparticles decorated polypyrrole/multiwalled carbon nanotubes and their catalytic activity towards methanol oxidation. Electrochem. Commun. 2007, 9, 1145–1153. [Google Scholar] [CrossRef]
- Selvaraj, V.; Alagar, M. Ethylene glycol oxidation on Pt and Pt–Ru nanoparticle decorated polythiophene/multiwalled carbon nanotube composites for fuel cell applications. Nanotechnology 2008, 19, 045504. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Rajalakshmi, N.; Ramaprabhu, S. Cobalt-polypyrrole-multiwalled carbon nanotube catalysts for hydrogen and alcohol fuel cells. Carbon 2008, 46, 2–11. [Google Scholar] [CrossRef]
- Wei, L.; Fan, Y.J.; Ma, J.H.; Tao, L.H.; Wang, R.X.; Zhong, J.-P.; Wang, H. Highly dispersed Pt nanoparticles supported on manganese oxide-poly(3,4-ethylenedioxythiophene) carbon nanotubes composite for enhanced methanol electrooxidation. J. Power Sources 2013, 238, 157–164. [Google Scholar] [CrossRef]
- Fard, L.A.; Ojani, R.; Raoof, J.B. Electrodeposition of three-dimensional Pd nanoflowers on a PPy@MWCNTs with superior electrocatalytic activity for methanol electrooxidation. Int. J. Hydrogen Energy 2016, 41, 17987–17994. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, L.; Tian, J.; Nie, S.; Ning, Z. Enhanced electrocatalytic oxidation of methanol on Pd/polypyrrole–graphene in alkaline medium. Electrochim. Acta 2011, 56, 1967–1972. [Google Scholar] [CrossRef]
- Yang, L.; Tang, Y.; Yan, D.; Liu, T.; Liu, C.; Luo, S. Polyaniline-reduced Graphene Oxide Hybrid nanosheets with nearly vertical orientation anchoring Palladium nanoparticles for highly active and stable electrocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 169–176. [Google Scholar] [CrossRef]
- Yue, R.; Wang, H.; Bin, D.; Xu, J.; Du, Y.; Lu, W.; Guo, J. Facile one-pot synthesis of Pd–PEDOT/graphene nanocomposites with hierarchical structure and high electrocatalytic performance for Ethanol oxidation. J. Mater. Chem. A 2015, 3, 1077–1088. [Google Scholar] [CrossRef]
- Choe, J.E.; Ahmed, M.S.; Jeon, S. 3, 4-Ethylenedioxythiophene functionalized graphene with palladium nanoparticles for enhanced electrocatalytic oxygen reduction reaction. J. Power Sources 2015, 281, 211–218. [Google Scholar] [CrossRef]
- Eswaran, M.; Dhanusuraman, R.; Tsai, P.C.; Ponnusamy, V.K. One-step preparation of graphitic carbon nitride/Polyaniline/Palladium nanoparticles based nanohybrid composite modified electrode for efficient methanol electro-oxidation. Fuel 2019, 251, 91–97. [Google Scholar] [CrossRef]
- Peng, X.; Yu, H.; Wang, X.; Zhou, Q.; Zhang, S.; Geng, L.; Sun, J.; Cai, Z. Enhanced performance and capacitance behavior of anode by rolling Fe3O4 into activated carbon in microbial fuel cells. Bioresour. Technol. 2012, 121, 450–453. [Google Scholar] [CrossRef]
- Nakamura, R.; Kai, F.; Okamoto, A.; Newton, G.J.; Hashimoto, K. Self-constructed electrically conductive bacterial networks. Angew. Chem. Int. Ed. 2009, 48, 508–511. [Google Scholar] [CrossRef]
- Reddy, K.R.; Park, W.; Sin, B.C.; Noh, J.; Lee, Y. Synthesis of electrically conductive and superparamagnetic monodispersed iron oxide-conjugated polymer composite nanoparticles by in situ chemical oxidative polymerization. J. Colloid Interface Sci. 2009, 335, 34–39. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Pannipara, M.; Al-Sehemi, A.G.; Kumar, G.G. PEDOT/NiFe2O4 nanocomposites on biochar as a free-standing anode for high-performance and durable microbial fuel cells. New J. Chem. 2019, 43, 7743–7750. [Google Scholar] [CrossRef]
- Qiao, Y.; Bao, S.-J.; Li, C.M.; Cui, X.-Q.; Lu, Z.-S.; Guo, J. Nanostructured Polyaniline/Titanium Dioxide composite anode for microbial fuel cells. ACS Nano 2008, 2, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Stadlhofer, A.; Hacker, V.; Squadrito, G.; Schröder, U.; Li, B. Activated carbon nanofibers (ACNF) as cathode for single chamber microbial fuel cells (SCMFCs). J. Power Sources 2013, 243, 499–507. [Google Scholar] [CrossRef]
- Feng, L.; Yan, Y.; Chen, Y.; Wang, L. Nitrogen-doped carbon nanotubes as efficient and durable metal-free cathodic catalysts for oxygen reduction in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1892–1899. [Google Scholar] [CrossRef]
- Santoro, C.; Artyushkova, K.; Babanova, S.; Atanassov, P.; Ieropoulos, I.; Grattieri, M.; Cristiani, P.; Trasatti, S.; Li, B.; Schuler, A.J. Parameters characterization and optimization of activated carbon (AC) cathodes for microbial fuel cell application. Bioresour. Technol. 2014, 163, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-T.; Mecheri, B.; Iannaci, A.; Epifanio, A.D.; Licoccia, S. Iron/Polyindole-based electrocatalysts to enhance oxygen reduction in microbial fuel cells. Electrochim. Acta 2016, 190, 388–395. [Google Scholar]
- Zhao, F.; Harnisch, F.; Schröder, U.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Application of pyrolysediron(II) phthalocyanine and CoTMPP based oxygen reduction catalysts as cathode materials in microbial fuel cells. Electrochem. Commun. 2005, 1405–1410. [Google Scholar] [CrossRef]
- Lu, M.; Guo, L.; Kharkwal, S.; Wu, H.; Ng, H.Y.; Li, S.F.Y. Manganese–polypyrrole–carbon nanotube, a new oxygen reduction catalyst for air-cathode microbial fuel cells. J. Power Sources 2013, 221, 381–386. [Google Scholar] [CrossRef]
- Pattanayak, P.; Papiya, F.; Kumar, V.; Pramanik, N.; Kundu, P.P. Deposition of Ni–NiO nanoparticles on the reduced graphene oxide filled polypyrrole: Evaluation as cathode catalyst in microbial fuel cells. Sustain. Energy Fuels 2019, 3, 1808–1826. [Google Scholar] [CrossRef] [Green Version]
- Yuana, Y.; Ahmed, J.; Kim, S. Polyaniline/carbon black composite-supported iron phthalocyanine as an oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2011, 196, 1103–1106. [Google Scholar] [CrossRef]
- Ansari, S.A.; Parveen, N.; Han, T.H.; Ansari, M.O.; Cho, M.H. Fibrous polyaniline@manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 9053–9060. [Google Scholar] [CrossRef]
- Zakaria, Z.; Shaari, N.; Kamarudin, S.K.; Bahru, R.; Musa, M.T. A review of progressive advanced polymer nanohybrid membrane in fuel cell application. Int. J. Energy Res. 2020, 1–41. [Google Scholar] [CrossRef]
- Han, J.; Wang, M.; Hu, Y.; Zhou, C.; Guo, R. Conducting polymer-noble metal nanoparticle hybrids: Synthesis mechanism application. Prog. Polym. Sci. 2017, 70, 52–91. [Google Scholar] [CrossRef]

| Electrode | Synthesis Method | Fuel Cell Reaction | Eonset mV | If, mA.cm−2 | Reference |
|---|---|---|---|---|---|
| Pd/PPy | Chemical polymerization | EOR | −628 | 7.05 | [47] |
| Pd/PEDOT | Electrochemical method | EOR | −562 | 137 | [48] |
| Pd/PPy | Chemical polymerization followed by photo reduction | EOR | −708 | 53.8 | [49] |
| Pd/PPy | Chemical polymerization followed by radiolysis | EOR | −640 | 9.50 | [50] |
| Pd30Pt29Au41/PPy | Chemical polymerization followed by radiolysis | EOR | −630 | 32.45 | [50] |
| Pd/PPy | ‘water-in-oil’ microemulsion for chemical polymerization | ORR | - | - | [53] |
| Au/PANI | Interfacial chemical polymerization | ORR | −100 | - | [54] |
| Au-V2O5/Polyindole | Wet chemical method followed by chemical polymerization | −400 | - | [55] | |
| Pt/PPy nanofibers | Interfacial polymerization | MOR | - | 14.1 | [62] |
| Pt/PPy nanofibers | Iinterfacial polymerization | MOR | - | - | [63] |
| Pt nanocube assemblies/PANI | Wet-chemical approach followed by chemical polymerization | MOR | - | 0.85 | [64] |
| Ni/SPAni | Chemical polymerization followed by chemical reducing agent | MOR | - | 2.15 | [65] |
| PtPd/PPy/PtPd | Electrochemical synthesis via galvanostatic electrodeposition | MOR | 250 | 0.9 | [51] |
| Pt–Fe/PPy | In situ interfacial polymerization | MOR | 170 | - | [60] |
| Pt66Pd34/PPy | Chemical polymerization followed by radiolysis | MOR | 222 | 8.14 | [70] |
| Pt24Pd26Au50/PPy | Chemical polymerization followed by radiolysis | MOR | 227 | 6.76 | [70] |
| Electrode | Functional Unit as Support | Fuel Cells Reaction | Ref. |
|---|---|---|---|
| Pt/C/PEDOT | Carbon paper coated 3,4-polyethylenedioxythiophene | MOR | [72] |
| Pd nanodendritic /C/PEDOT | Carbon paper coated 3,4-polyethylenedioxythiophene | Alcohols oxidation | [73] |
| Pt nanocactus /PANI/ CFP | Poly (aniline) decorated with platinum on carbon fiber paper | Toluene oxidation | [74] |
| Pd nanoplates /PDPB/Nafion | Nafion modified poly(diphenylbutadyine) nanofiber | EOR | [75] |
| Pt/C-PANI | Vulcan XC-72 and PANI-doped with trifluoromethane sulfonic acid | MOR | [76] |
| Pt/PEDOT/PSS | 3,4-polyethylenedioxythiophene modified with poly(styrene sulfonic acid) (PSS) | MOR | [77] |
| Pt-Ru/PANI/PSS | Polyaniline–poly(styrene sulfonic acid) (PSS) | MOR | [78] |
| Pt-PFSA/C/PANI | Perfluorosulfonic acid, PFSA and polyaniline | ORR | [79] |
| Pt/PPy-C | Polypyrrole-carbon | borohydride oxidation and hydrogen peroxide reduction | [80] |
| Pt/HxMoO3/PEDOT-PSS | Pt NPs and HxMoO3deposited in poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid) | MOR | [81] |
| PANI-PAMA-Pt-RuO2 | Polyaniline doped with poly(acrylic acid-co-maleic acid) (PAMA) and electrodeposition of RuO2 | MOR | [82] |
| Pt/CeO2/PANI | Polyaniline with CeO2 as multilayered supporting material | MOR | [83] |
| ZnO/Pt/CeO2/PANI | Polyaniline with ZnO-CeO2 as multilayered supporting material | MOR | [83] |
| Pt–Ru/PPy–CNT | Polypyrrole/multiwalled carbon nanotubes | MOR | [87] |
| Pt–Ru/PTh-CNTs | Polythiophene/CNT composites (PTh-CNTs) | Ethylene glycol oxidation | [88] |
| Cobalt-PPy/MWCNT | Polypyrrole-multiwalled carbon nanotube | ORR | [89] |
| Pt/MnOx–PEDOT–MWCNTs | Manganese oxide-poly(3,4-ethylenedioxythiophene)-carbon nanotubes composite | MOR | [90] |
| Pd nanoflowers /PPy/MWCNTs | Polypyrrole-multiwalled carbon nanotube | MOR | [91] |
| Pd /PANI/GNS | polyaniline-reduced graphene oxide hybrid nanosheets | MOR and EOR | [93] |
| Pd/PEDOT/graphene | Poly(3,4-ethylenedioxythiophene) nanosphere-graphene nanosheets | EOR | [94] |
| Pd/PEDOT/rGO | Poly(3,4-ethylenedioxythiophene) functionalized graphene | ORR | [95] |
| Pd/graphitic carbon nitride/PANI | Graphitic carbon nitride-polyaniline | MOR | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Das, S.; Mosquera, M.E.G. Conducting Polymer-Based Nanohybrids for Fuel Cell Application. Polymers 2020, 12, 2993. https://doi.org/10.3390/polym12122993
Ghosh S, Das S, Mosquera MEG. Conducting Polymer-Based Nanohybrids for Fuel Cell Application. Polymers. 2020; 12(12):2993. https://doi.org/10.3390/polym12122993
Chicago/Turabian StyleGhosh, Srabanti, Suparna Das, and Marta E. G. Mosquera. 2020. "Conducting Polymer-Based Nanohybrids for Fuel Cell Application" Polymers 12, no. 12: 2993. https://doi.org/10.3390/polym12122993
APA StyleGhosh, S., Das, S., & Mosquera, M. E. G. (2020). Conducting Polymer-Based Nanohybrids for Fuel Cell Application. Polymers, 12(12), 2993. https://doi.org/10.3390/polym12122993