Electrical Conductivity Based Ammonia Sensing Properties of Polypyrrole/MoS2 Nanocomposite
Abstract
1. Introduction
2. Materials, Methods and Instrumentation
2.1. Materials
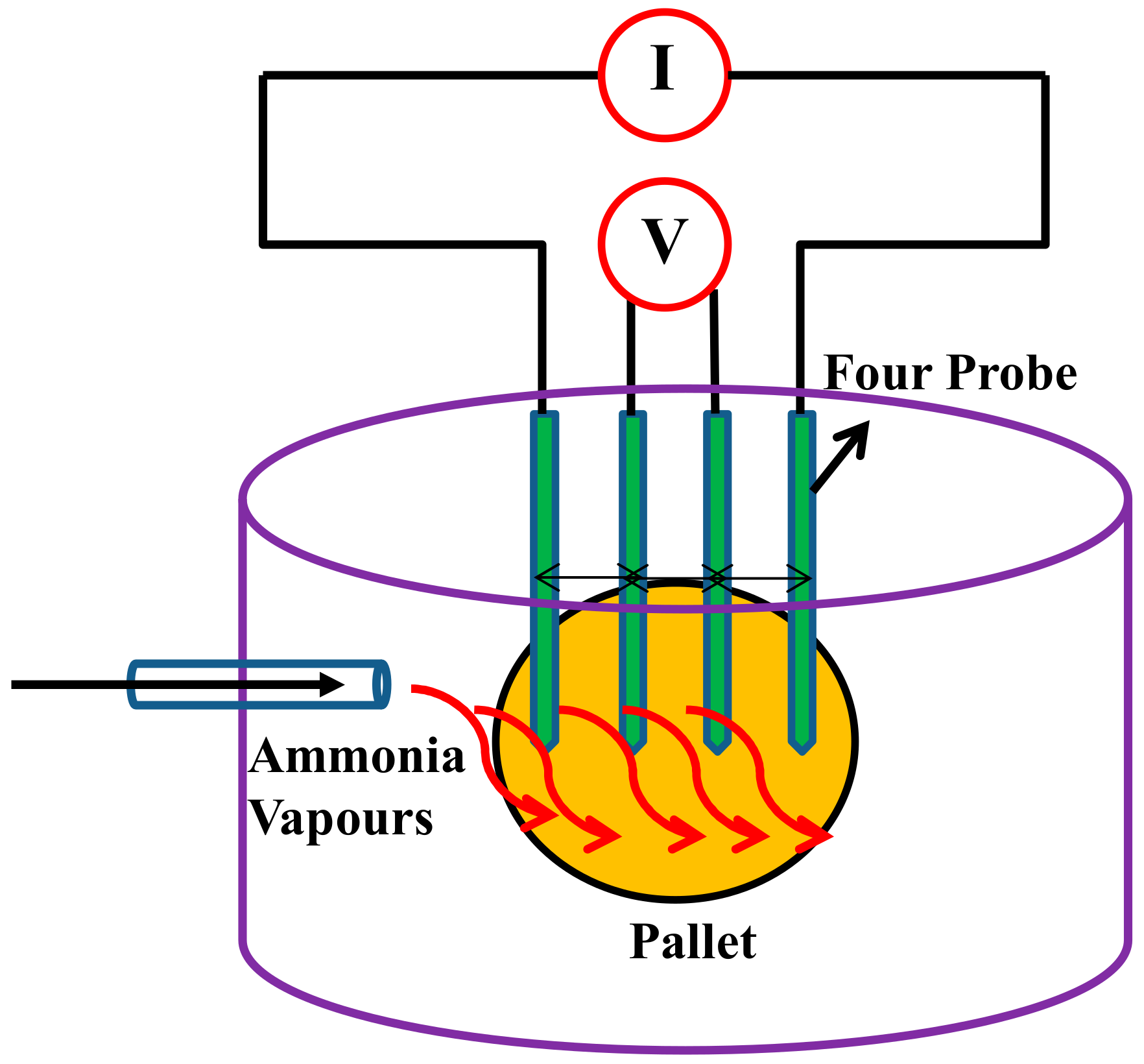
2.2. Instrumentation
2.3. Preparation of PPy and PPy/MoS2 Nanocomposite
2.4. Characterization
3. Results and Discussions
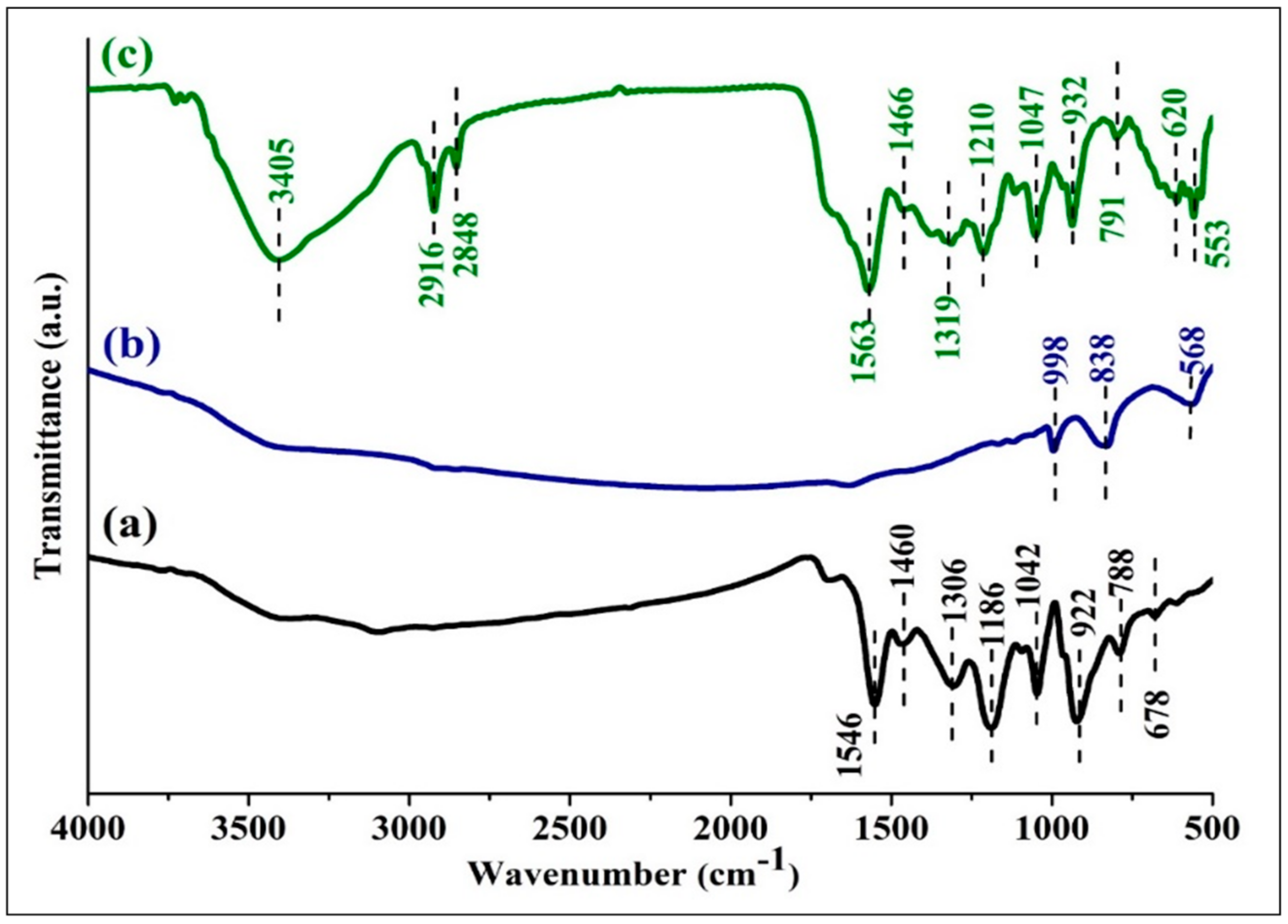
3.1. Fourier Transform Infrared Spectroscopic (FTIR) Studies
3.2. X-ray Diffraction (XRD) Studies
3.3. Morphological Studies
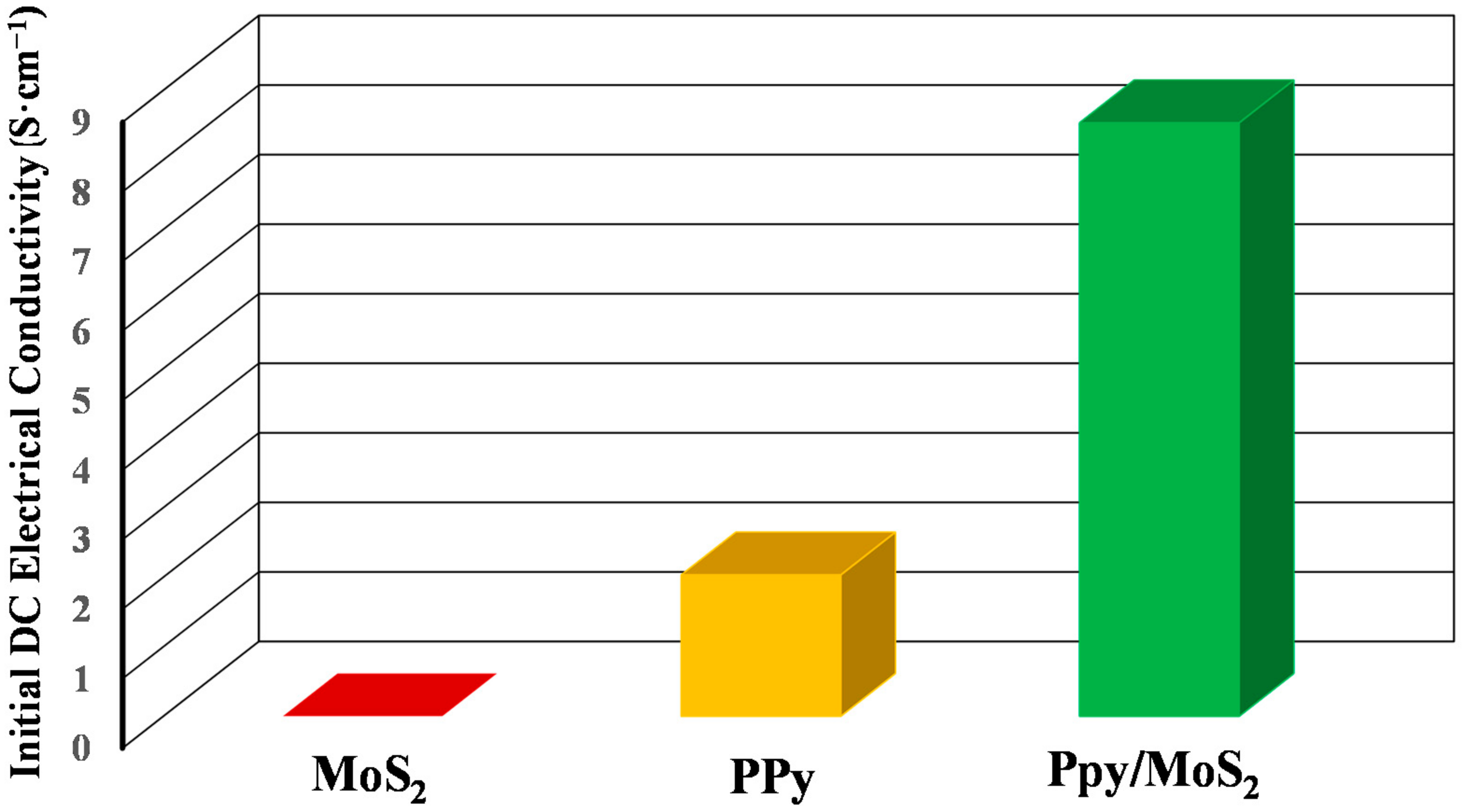
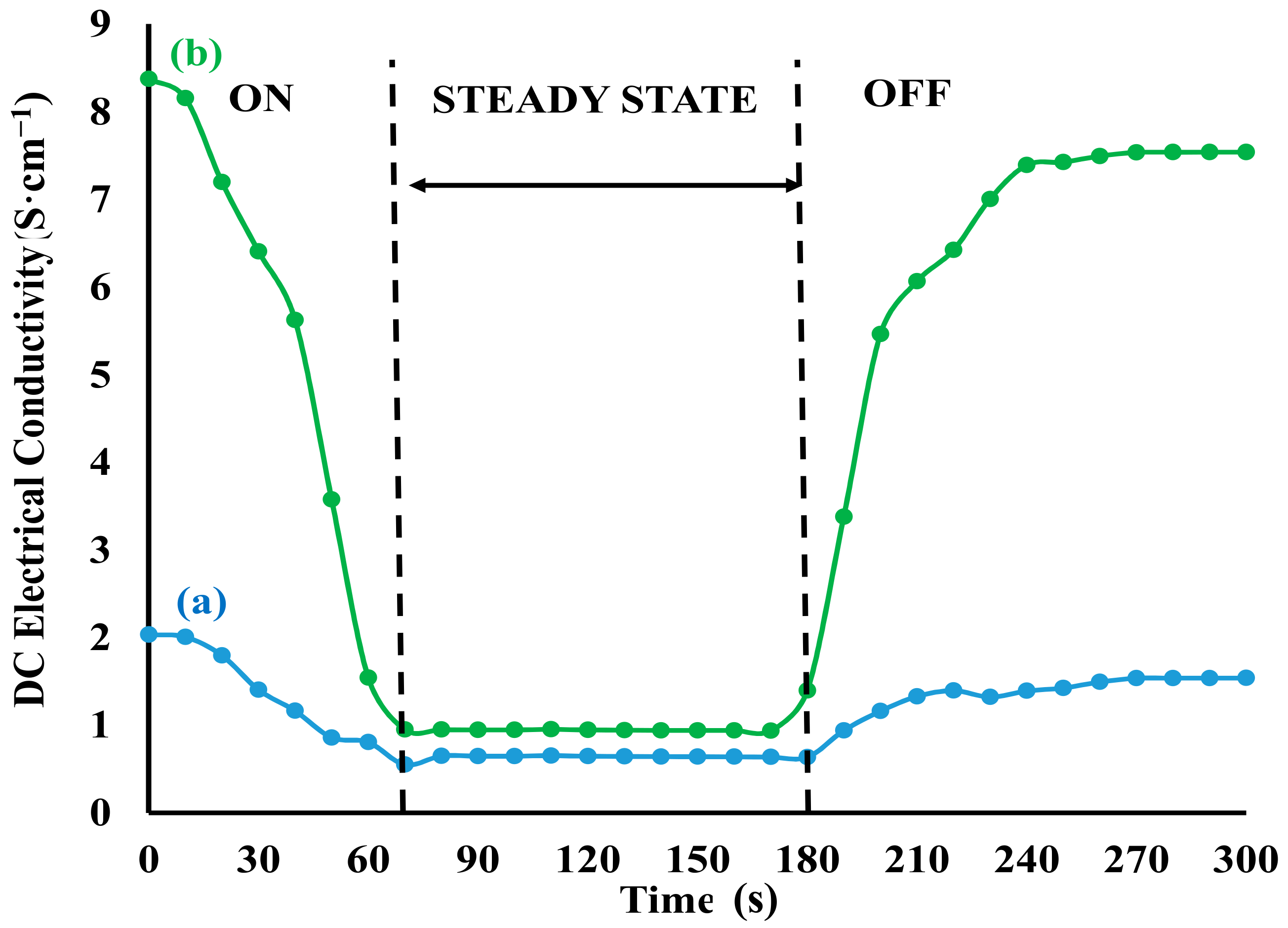
3.4. DC Electrical Conductivity
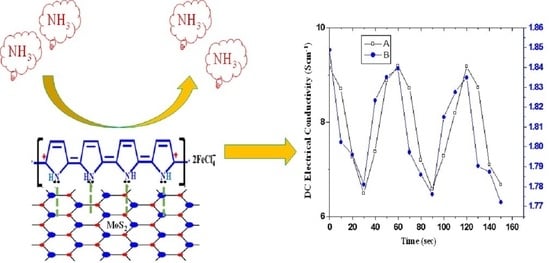
3.5. Sensitivity
3.6. Limit of Detection
3.7. Reversibility
3.8. Selectivity
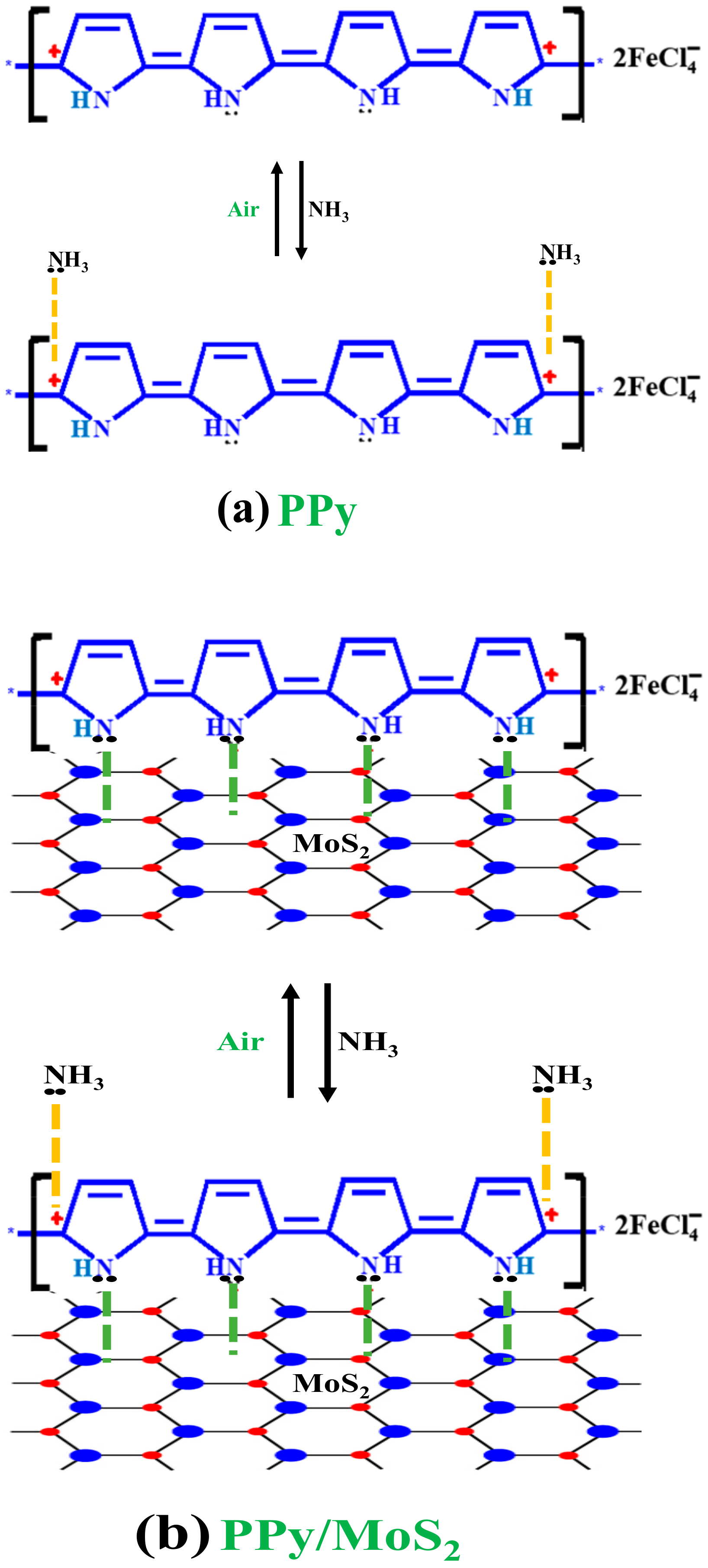
3.9. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Wang, S.R.; Kang, Y.F.; Wang, L.W.; Zhang, H.X.; Wang, Y.S.; Wang, Y. Organic/inorganic hybrid sensors: A review. Sens. Actuators. B. Chem. 2013, 182, 467–481. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Pandey, S. Highly Sensitive and Selective Chemiresistor Gas/Vapor Sensors based on Polyaniline Nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices. 2016, 1, 431–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Wen, F.; Huang, C.; Wang, H. Construction of polyaniline/ molybdenum sulfide nanocomposite: Characterization and its electrocatalytic performance on nitrite. Ionics 2016, 22, 1095–1102. [Google Scholar] [CrossRef]
- Amirul, M.; Mohd, A.; Syahirah, N.; Razali, M.; Teng, P.; Kulandaivalu, S.; Sulaiman, Y. One-step potentiostatic electrodeposition of polypyrrole/graphene oxide/multi-walled carbon nanotubes ternary nanocomposite for supercapacitor. Mater. Chem. Phys. 2018, 219, 120–128. [Google Scholar] [CrossRef]
- Jamadade, S.; Jadhav, S.V.; Puri, V. Electromagnetic reflection, shielding and conductivity of polypyrrole thin film electropolymerized in P-Tulensulfonic acid. J. Non. Cryst. Solids. 2011, 357, 1177–1181. [Google Scholar] [CrossRef]
- Ahmad, S.; Sultan, A.; Mohammad, F. Rapid response and excellent recovery of a polyaniline/silicon carbide nanocomposite for cigarette smoke sensing with enhanced thermally stable DC electrical conductivity. RSC Adv. 2016, 6, 59728–59736. [Google Scholar] [CrossRef]
- Sultan, A.; Ahmad, S.; Anwer, T.; Mohammad, F. Binary doped polypyrrole and polypyrrole/boron nitride nanocomposite: Preparation, characterization and application in detection of liquefied petroleum gas leaks. RSC Adv. 2015, 5, 105980–105991. [Google Scholar] [CrossRef]
- Ansari, M.O.; Mohammad, F. Chemical Thermal stability, Electrical conductivity and ammonia sensing studies on p-toluenesulfonic acid doped polyaniline: Titanium dioxide (pTSA/Pani: TiO2) nanocomposites. Sens. Actuators. B. Chem. 2011, 157, 122–129. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Synthesis, characterisation and ethanol sensing application of polythiophene/graphene nanocomposite. Mater. Chem. Phys. 2020, 239, 122324. [Google Scholar] [CrossRef]
- Sultan, A.; Mohammad, F. Chemical sensing, thermal stability, electrochemistry and electrical conductivity of silver nanoparticles decorated and polypyrrole enwrapped boron nitride nanocomposite. Polymer 2017, 113, 221–232. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Thermally Stable and Highly Sensitive Ethene Gas Sensor Based on Polythiophene/Zirconium Oxide Nanocomposites. Mater. Today Commun. 2019, 20, 100574. [Google Scholar] [CrossRef]
- Husain, A.; Shariq, M.U.; Mohammad, F. DC electrical conductivity and liquefied petroleum gas sensing application of polythiophene/zinc oxide nanocomposite. Materialia 2020, 9, 100599. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Electrical conductivity and alcohol sensing studies on polythiophene/tin oxide nanocomposites. J. Sci. Adv. Mater. Devices. 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Electrical conductivity and ammonia sensing studies on polythiophene/MWCNTs nanocomposites. Materialia 2020, 14, 100868. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Urooj, M.; Khan, M.M.A. Ultra-sensitive, highly selective and completely reversible ammonia sensor based on polythiophene/SWCNT nanocomposite. Materialia 2020, 10, 100704. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Polythiophene/graphene/zinc tungstate nanocomposite: Synthesis, characterization, DC electrical conductivity and cigarette smoke sensing application. Polym. Polym. Compos. 2020, 1–12. [Google Scholar] [CrossRef]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of WS2. Nature 1992, 360, 444–446. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano. 2014, 8, 5297–5303. [Google Scholar]
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A promising building block for bio sensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, S.V.P.; Byon, C. Synthesis and Characterization of Molybdenum Disulfide Nanoflowers and Nanosheets. Nanotribol. J. Nanomater 2015, 1–11. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Shanmugapriya, K.; Sriram, S. Photocatalytic activity of MoS2 nanoparticles: An experimental and DFT analysis. Appl. Phys. A Mater. Sci. Process. 2019, 125, 1–8. [Google Scholar] [CrossRef]
- Ahmad, H.; Ismail, M.A.; Suthaskumar, M.; Tiu, Z.C.; Harun, S.W.; Zulkifli, M.Z.; Samikannu, S.; Sivaraj, S. S-band Q-switched fiber laser using molybdenum disulfide (MoS2) saturable absorber. Laser. Phys. Lett. 2016, 13. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Bissessur, R. Nanocomposites based on graphene analogous materials and conducting polymers: A review. J. Mater. Sci. 2020, 55, 6721–6753. [Google Scholar] [CrossRef]
- Lyle, E.S.; McAllister, C.; Dahn, D.C.; Bissessur, R. Exfoliated MoS2–Polyaniline Nanocomposites: Synthesis and Characterization. J. Inorg. Organomet. Polym. Mater. 2020, 30, 206–213. [Google Scholar] [CrossRef]
- Lin, B.Z.; Ding, C.; Xu, B.H.; Chen, Z.J.; Chen, Y.L. Preparation and characterization of polythiophene/molybdenum disulfide intercalation material. Mater. Res. Bull. 2009, 44, 719–723. [Google Scholar] [CrossRef]
- Ahmad, S.; Mujahid, M.; Mohammad, F. Graphene/Nickel Oxide-Based Nanocomposite of Polyaniline with Special Reference to Ammonia Sensing. ACS Omega 2018, 3, 9378–9387. [Google Scholar] [CrossRef]
- Niaz, N.A.; Shakoor, A.; Imran, M.; Khalid, N.R.; Hussain, F.; Kanwal, H.; Maqsood, M.; Afzal, S. Enhanced electrochemical performance of MoS2/PPy nanocomposite as electrodes material for supercapacitor applications. J. Mater. Sci. Mater. Electron 2020, 31, 11336–11344. [Google Scholar] [CrossRef]
- Lian, M.; Wu, X.M.; Wang, Q.G.; Zhang, W.Z.; Wang, Y. Hydrothermal synthesis of Polypyrrole/MoS2 intercalation composites for supercapacitor electrodes. Ceram. Int. 2017, 43, 9877–9883. [Google Scholar] [CrossRef]
- Lei, J.Y.; Lu, X.F.; Nie, G.D.; Jiang, Z.Q.; Wang, C. One-Pot Synthesis of Algae-Like MoS2/PPy Nanocomposite: A Synergistic Catalyst with Superior Peroxidase-Like Catalytic Activity for H2O2 Detection. Part. Part. Syst. Charact. 2015, 32, 886–892. [Google Scholar] [CrossRef]
- Evie, L.P.; Davide, M.; Mirko, P.; Alessandro, B.; Athanassia, A.; Ilker, S.B. An efficient pure polyimide ammonia sensor. J. Mater. Chem. C. 2016, 4, 7790–7797. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Zhang, R.; Zhou, T.; Deng, J.; Zhang, T. Hybrid Co3O4/SnO2 Core−Shell Nanospheres as Real-Time Rapid-Response Sensors for Ammonia Gas. ACS Appl. Mater. Interfaces 2016, 8, 6539–6545. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Sun, Y. Room-temperature high-performance ammonia gas sensor based on layer-by-layer self-assembled molybdenum disulfide/zinc oxide nanocomposite film. J. Alloys Compd. 2017, 698, 476–483. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Jiang, C.; Liu, A.; Xia, B. Quantitative detection of formaldehyde and ammonia gas via metal oxide-modified graphene-based sensor array combining with neural network model. Sens. Sctuator B Chem. 2017, 240, 55–65. [Google Scholar] [CrossRef]
- Siciliano, T.; Di Giulio, M.; Tepore, M.; Filippo, E.; Micocci, G.; Tepore, A. Ammonia sensitivity of rf sputtered tellurium oxide thin films. Sens. Actuators B Chem. 2009, 138, 550–555. [Google Scholar] [CrossRef]
- Duc Hoa, N.; Van Quy, N.; Suk Cho, Y.; Kim, D. Nanocomposite of SWNTs and SnO2 fabricated by soldering process for ammonia gas sensor application. Phys. Status Solidi (A) Appl. Mater. 2007, 6, 1820–1824. [Google Scholar] [CrossRef]
- Bandgar, D.K.; Navale, S.T.; Naushad, M.; Mane, R.S.; Stadler, F.J.; Patil, V.B. Ultra-sensitive polyaniline–iron oxide nanocomposite room temperature flexible ammonia sensor. RSC. Adv. 2015, 5, 68964–68971. [Google Scholar] [CrossRef]





| Ammonia Sensing Properties | Co3O4/SnO2 [35] | MoS2/ZnO [36] | MOx (SnO2, CuO) Modified rGO [37] | TeO2 [38] | SnO2/SWNTs [39] | PANI/α-Fe2O3 [40] | PPy/MoS2 (This Work) |
|---|---|---|---|---|---|---|---|
| Response Time | 4 s | 10 s | 100–120 s and 108–132 s | 3.1 min | 100 s | 75 s | 60–70 s |
| Reversibility | 17 s | 11 s | 98–109 s and 98–138 s | 5.6 min | 3.2 min | 5 s | 50–60 s |
| Operating Temperature | 200 °C | Room Temperature(RT) | RT | 170 °C | RT | RT | RT |
| Selectivity | Very good | Excellent | Not reported | Not reported | Not reported | Good | Very good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Khan, I.; Husain, A.; Khan, A.; Asiri, A.M. Electrical Conductivity Based Ammonia Sensing Properties of Polypyrrole/MoS2 Nanocomposite. Polymers 2020, 12, 3047. https://doi.org/10.3390/polym12123047
Ahmad S, Khan I, Husain A, Khan A, Asiri AM. Electrical Conductivity Based Ammonia Sensing Properties of Polypyrrole/MoS2 Nanocomposite. Polymers. 2020; 12(12):3047. https://doi.org/10.3390/polym12123047
Chicago/Turabian StyleAhmad, Sharique, Imran Khan, Ahmad Husain, Anish Khan, and Abdullah M. Asiri. 2020. "Electrical Conductivity Based Ammonia Sensing Properties of Polypyrrole/MoS2 Nanocomposite" Polymers 12, no. 12: 3047. https://doi.org/10.3390/polym12123047
APA StyleAhmad, S., Khan, I., Husain, A., Khan, A., & Asiri, A. M. (2020). Electrical Conductivity Based Ammonia Sensing Properties of Polypyrrole/MoS2 Nanocomposite. Polymers, 12(12), 3047. https://doi.org/10.3390/polym12123047