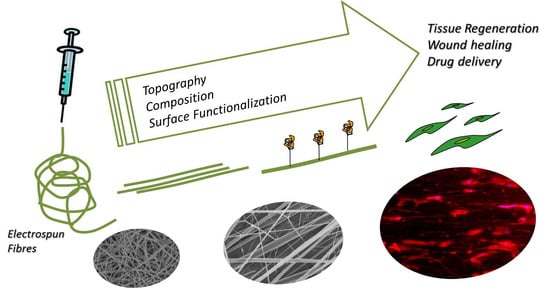
Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications
Abstract
1. Topographical and Biochemical Guidance: Electrospinning Potentialities for Production of Fibers
1.1. Electrospinning Basic Principles
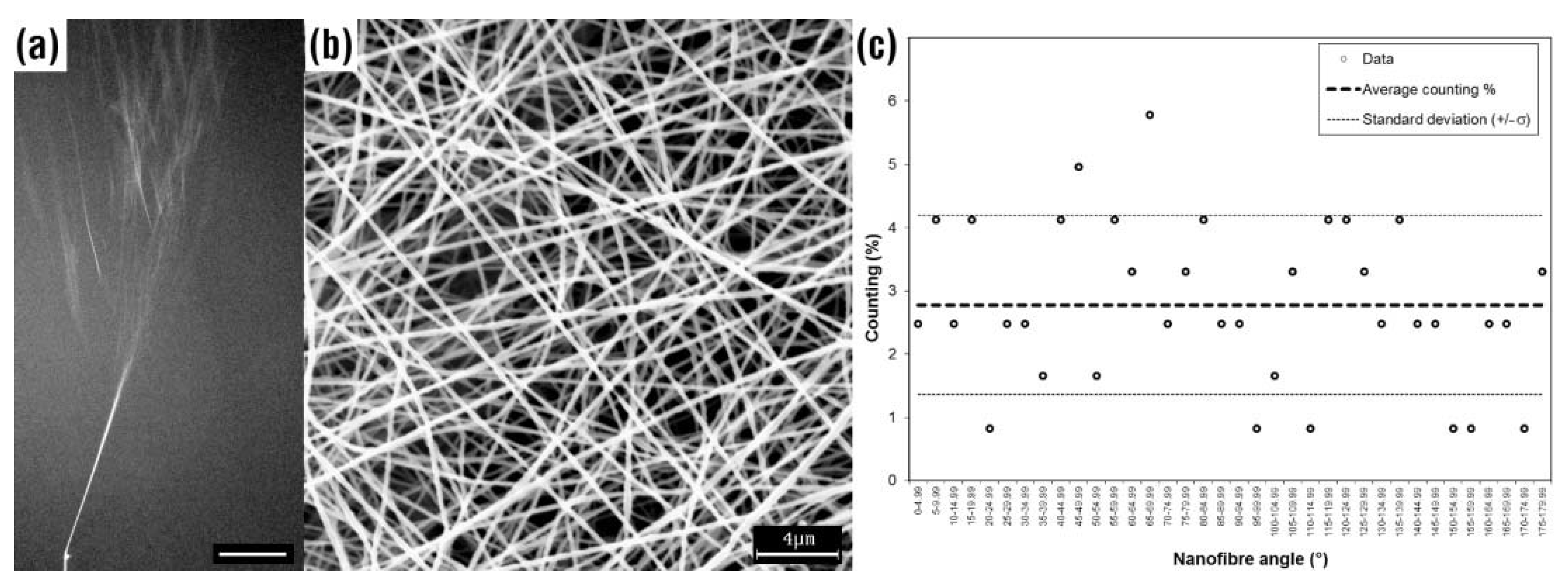
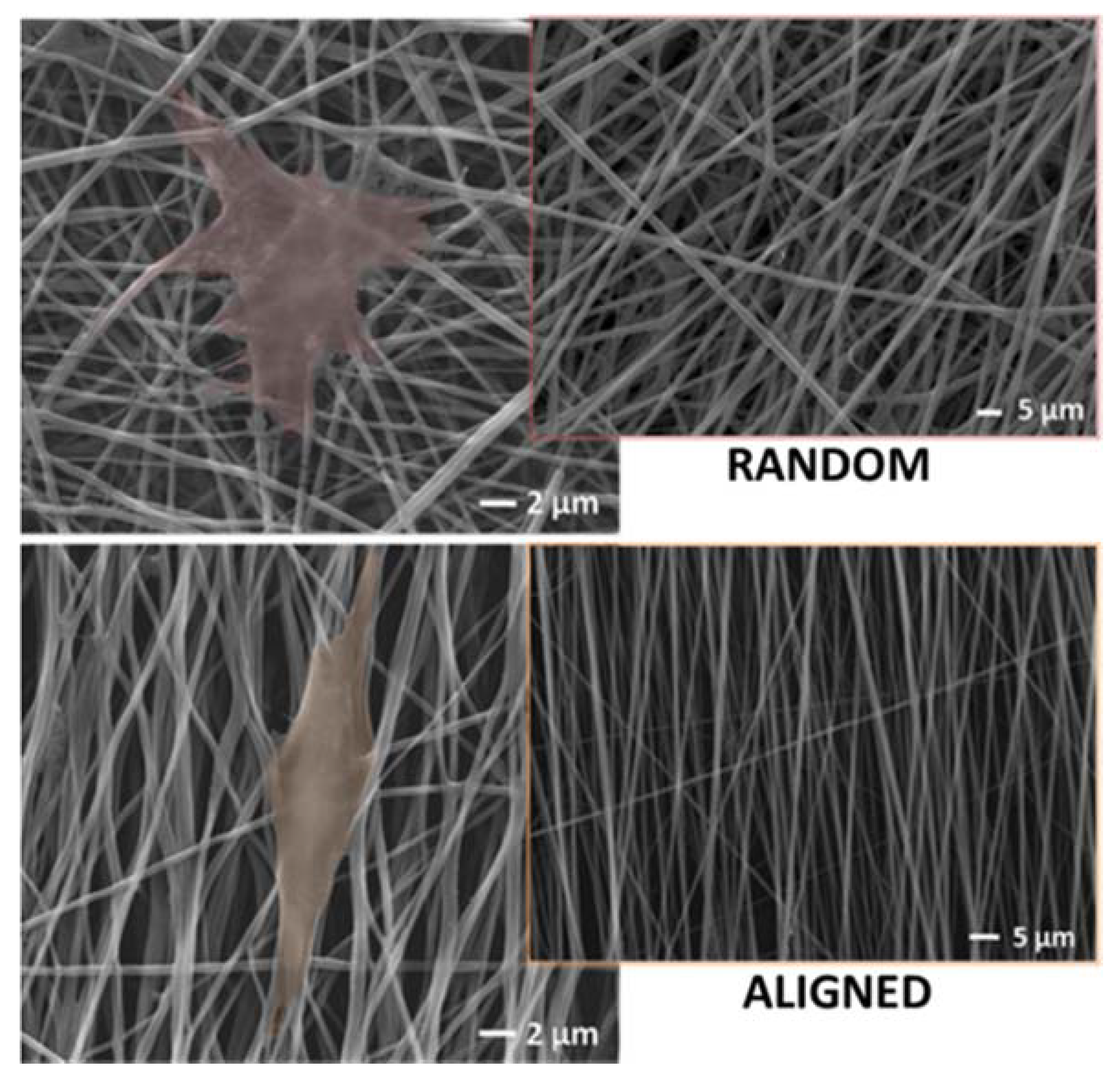
1.2. Fiber Alignment
1.3. Natural vs. Synthetic Fibers
1.4. Composite Nanofibers
2. Topographical and Biomechanical Guidance: The Biological Standpoint
2.1. Contact Guidance
2.2. Biomechanical Guidance
2.3. Combinatory Stimulation of Topographical and Biochemical Guidance
2.4. Biological Advantages of Cytocompatible Nanofibers-Based Biomaterials
3. Topographical and Biochemical Guidance: A Focus on Electrospun Fibers in Implantable Devices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the Fiber Diameter during Electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Tao, J.; Shivkumar, S. Molecular weight dependent structural regimes during the electrospinning of PVA. Mater. Lett. 2007, 61, 2325–2328. [Google Scholar] [CrossRef]
- Liu, K.; Ertley, C.; Reneker, D.H. Interpretation and use of glints from an electrospinning jet of polymer solutions. Polymer 2012, 53, 4241–4253. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Grammatikos, K.N.; Deitzel, J.M.; Wagner, N.J. Theory and kinematic measurements of the mechanics of stable electrospun polymer jets. Polymer 2008, 49, 2924–2936. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Bending instability in electrospinning of nanofibers. J. Appl. Phys. 2001, 89, 3018–3026. [Google Scholar] [CrossRef]
- Lauricella, M.; Succi, S.; Zussman, E.; Pisignano, D.; Yarin, A.L. Models of polymer solutions in electrified jets and solution blowing. Rev. Mod. Phys. 2020, 92, 035004. [Google Scholar] [CrossRef]
- Varesano, A.; Aluigi, A.; Vineis, C.; Tonin, C. Study on the shear viscosity behavior of keratin/PEO blends for nanofibre electrospinning. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1193–1201. [Google Scholar] [CrossRef]
- Munir, M.M.; Suryamas, A.B.; Iskandar, F.; Okuyama, K. Scaling law on particle-to-fiber formation during electrospinning. Polymer 2009, 50, 4935–4943. [Google Scholar] [CrossRef]
- Aluigi, A.; Varesano, A.; Vineis, C.; Del Rio, A. Electrospinning of immiscible systems: The wool keratin/polyamide-6 case study. Mater. Des. 2017, 127, 144–153. [Google Scholar] [CrossRef]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat polymer ribbons and other shapes by electrospinning. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Nakano, A.; Miki, N.; Hishida, K.; Hotta, A. Solution parameters for the fabrication of thinner silicone fibers by electrospinning. Phys. Rev. E 2012, 86. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Branda, F.; Ausanio, G.; Iannotti, V.; Lanotte, L.; Ambrosio, L. Elastomagnetic NI-PDMS nanofibers via coaxial electrospinning. Mater. Res. Express 2018, 5, 085029. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymer 2019, 11, 2008. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs. Expert Rev. Med. Devices 2015, 13, 83–102. [Google Scholar] [CrossRef]
- Zahedi, E.; Esmaeili, A.; Eslahi, N.; Shokrgozar, M.A.; Simchi, A. Fabrication and Characterization of Core-Shell Electrospun Fibrous Mats Containing Medicinal Herbs for Wound Healing and Skin Tissue Engineering. Mar. Drugs 2019, 17, 27. [Google Scholar] [CrossRef]
- Guarino, V.; Ambrosio, L. Electrofluidodynamics: Exploring a new toolbox to design biomaterials for tissue regeneration and degeneration. Nanomedicine 2016, 11, 1515–1518. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Altobelli, R.; Ambrosio, L. Polymer-based platforms by electric field-assisted techniques for tissue engineering and cancer therapy. Expert Rev. Med. Devices 2014, 12, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.-E.; Inai, R.; RamaKrishnaan, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Lei, T.; Xu, Z.; Cai, X.; Xu, L.; Sun, D. New Insight into Gap Electrospinning: Toward Meter-long Aligned Nanofibers. Langmuir 2018, 34, 13788–13793. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Iannotti, V.; Ausanio, G.; Ambrosio, L.; Lanotte, L. Elastomagnetic nanofiber wires by magnetic field assisted electrospinning. Express Polym. Lett. 2019, 13, 419–428. [Google Scholar] [CrossRef]
- Cirillo, V.; Guarino, V.; Alvarez-Perez, M.A.; Marrese, M.; Ambrosio, L. Optimization of fully aligned bioactive electrospun fibers for “in vitro” nerve guidance. J. Mater. Sci. Mater. Med. 2014, 25, 2323–2332. [Google Scholar] [CrossRef]
- Chang, J.-C.; Fujita, S.; Tonami, H.; Kato, K.; Iwata, H.; Hsu, S.-H. Cell orientation and regulation of cell–cell communication in human mesenchymal stem cells on different patterns of electrospun fibers. Biomed. Mater. 2013, 8, 055002. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; Balachandran, K.; Capulli, A.K.; Golecki, H.M.; Agarwal, A.; Goss, J.A.; Kim, H.; Shin, K.; Parker, K.K. Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning. Biomaterials 2014, 35, 3188–3197. [Google Scholar] [CrossRef]
- Ding, M.; Andersson, H.; Martinsson, S.; Sabirsh, A.; Jonebring, A.; Wang, Q.-D.; Plowright, A.T.; Drowley, L. Aligned nanofiber scaffolds improve functionality of cardiomyocytes differentiated from human induced pluripotent stem cell-derived cardiac progenitor cells. Sci. Rep. 2020, 10, 13575. [Google Scholar] [CrossRef]
- Khodir, W.K.W.A.; Razak, A.H.A.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef]
- Patel, N.P.; Lyon, K.A.; Huang, J.H. An update–tissue engineered nerve grafts for the repair of peripheral nerve injuries. Neural Regen. Res. 2018, 13, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; Hurtado, A.; Oudega, M.; Trombley, M.T.; Gilbert, R.J. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng. 2009, 6, 016001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Clements, B.A.; Guarino, V.; Bushman, J.; Kohn, J.; Ambrosio, L. Mono and bi-component electrospun conduits: In Vivo response in Rat Sciatic model. Biomaterials 2014, 35, 8970–8982. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, S.; Karimi, A.; Razavi, S.; Zargar, E.N. Poly(hydroxybutyrate)/chitosan Aligned Electrospun Scaffold as a Novel Substrate for Nerve Tissue Engineering. Adv. Biomed. Res. 2018, 7, 44. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhang, E.; Yang, L.; Yuan, H.; Tu, W.; Zhang, H.; Yin, Z.; Shen, W.; Chen, X.; et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 2018, 66, 141–156. [Google Scholar] [CrossRef]
- Kharaziha, M.; Fathi, M.; Edris, H. Development of novel aligned nanofibrous composite membranes for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2013, 24, 9–20. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Chiera, S.; Gershovich, P.; Motta, A.; Reis, R.L.; Gomes, M.E. Enhancing the Biomechanical Performance of Anisotropic Nanofibrous Scaffolds in Tendon Tissue Engineering: Reinforcement with Cellulose Nanocrystals. Adv. Healthc. Mater. 2016, 5, 1364–1375. [Google Scholar] [CrossRef]
- Saracino, E.; Cirillo, V.; Marrese, M.; Guarino, V.; Benfenati, V.; Zamboni, R.; Ambrosio, L. Structural and functional properties of astrocytes on PCL based electrospun fibres. Mater. Sci. Eng. C 2021, 118, 111363. [Google Scholar] [CrossRef]
- Wan, S.; Fu, X.; Ji, Y.; Li, M.; Shi, X.; Wang, Y. FAK- and YAP/TAZ dependent mechanotransduction pathways are required for enhanced immunomodulatory properties of adipose-derived mesenchymal stem cells induced by aligned fibrous scaffolds. Biomaterials 2018, 171, 107–117. [Google Scholar] [CrossRef]
- De Falco, F.; Guarino, V.; Gentile, G.; Cocca, M.; Ambrogi, V.; Ambrosio, L.; Avella, M. Design of functional textile coatings via non-conventional electrofluidodynamic processes. J. Colloid Interface Sci. 2019, 541, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Giachet, F.T.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S.M. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C 2017, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Guarino, V.; Cochis, A.; Varesano, A.; Maya, I.C.; Vineis, C.; Rimondini, L.; Spriano, S. Aligned keratin submicrometric-fibers for fibroblasts guidance onto nanogrooved titanium surfaces for transmucosal implants. Mater. Lett. 2018, 229, 1–4. [Google Scholar] [CrossRef]
- Meinel, A.J.; Germershaus, O.; Luhmann, T.; Merkle, H.P.; Meinel, L. Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications. Eur. J. Pharm. Biopharm. 2012, 81, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, M.; Khorrami, M.; Yi, N.; Majd, S.; Abidian, M.R. Electrospinning of highly aligned fibers for drug delivery applications. J. Mater. Chem. B 2019, 7, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Romo, I.L.; Gutiérrez, H.M.L.; Barrera, E.V.; Elías-Zuñiga, A. Development of forcespun fiber-aligned scaffolds from gelatin–zein composites for potential use in tissue engineering and drug release. MRS Commun. 2018, 8, 885–892. [Google Scholar] [CrossRef]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.S.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef]
- Guarino, V.; Gloria, A.; Raucci, M.G.; De Santis, R.; Ambrosio, L. Bio-inspired cell instructive composite platforms for bone regeneration. Int. Mater. Rev. 2012, 57, 256–275. [Google Scholar] [CrossRef]
- Lu, H.H.; Jiang, J. Interface Tissue Engineeringand the Formulation of Multiple-Tissue Systems BT. In Tissue Engineering I; Lee, K., Kaplan, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 91–111. ISBN 978-3-540-31948-1. [Google Scholar]
- Guarino, V.; Gloria, A.; Raucci, M.G.; Ambrosio, L. Hydrogel-Based Platforms for the Regeneration of Osteochondral Tissue and Intervertebral Disc. Polymers 2012, 4, 1590–1612. [Google Scholar] [CrossRef]
- Shamsah, A.H.; Cartmell, S.H.; Richardson, S.M.; Bosworth, L.A. Mimicking the Annulus Fibrosus Using Electrospun Polyester Blended Scaffolds. Nanomaterials 2019, 9, 537. [Google Scholar] [CrossRef]
- Holy, C.E.; Fialkov, J.A.; Davies, J.E.; Shoichet, M.S. Use of a biomimetic strategy to engineer bone. J. Biomed. Mater. Res. 2003, 65, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Guarino, V.; Flores, A.A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly polydisperse keratin rich nanofibers: Scaffold design and in vitro characterization. J. Biomed. Mater. Res. Part A 2019, 107, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Yu, H.-S.; Lee, H.-H. Nanofibrous matrices of poly(lactic acid) and gelatin polymeric blends for the improvement of cellular responses. J. Biomed. Mater. Res. Part A 2008, 87, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Maya, I.C.; Varesano, A.; Vineis, C.; Guarino, V. Comparative Study on Protein-Rich Electrospun Fibers for in Vitro Applications. Polymers 2020, 12, 1671. [Google Scholar] [CrossRef]
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. In Natural and Synthetic Biomedical Polymers; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 351–371. [Google Scholar]
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N.; Vacanti, J.P. Preparation and characterization of poly(l-lactic acid) foams. Polymers 1994, 35, 1068–1077. [Google Scholar] [CrossRef]
- Liu, F.; Guo, R.; Shen, M.; Wang, S.; Shi, X. Effect of Processing Variables on the Morphology of Electrospun Poly[(lactic acid)-co-(glycolic acid)] Nanofibers. Macromol. Mater. Eng. 2009, 294, 666–672. [Google Scholar] [CrossRef]
- Denchai, A.; Tartarini, D.; Mele, E. Cellular response to surface morphology: Electrospinning and computational modeling. Front. Bioeng. Biotechnol. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Marrese, M.; Cirillo, V.; Guarino, V.; Ambrosio, L. Short-Term Degradation of Bi-Component Electrospun Fibers: Qualitative and Quantitative Evaluations via AFM Analysis. J. Funct. Biomater. 2018, 9, 27. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Taddei, P.; Alvarez-Perez, M.A.; Ambrosio, L. Tuning STuning size scale and crystallinity of PCL electrospun fibres via solvent permittivity to address hMSC response. Macromol. Biosci. 2011, 11, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Mikos, A.G. Polymer Scaffold Fabrication. In Principles of Tissue Engineering; Elsevier BV: Amsterdam, The Netherlands, 2007; pp. 309–321. [Google Scholar]
- Jansen, E.J.P.; Sladek, R.E.J.; Bahar, H.; Yaffe, A.; Gijbels, M.J.; Kuijer, R.; Bulstra, S.K.; Guldemond, N.A.; Binderman, I.; Koole, L.H. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomaterials 2005, 26, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Vallés-Lluch, A.; Fombuena, V.; Napiwocki, B.; Lih-Sheng, T. Influence of the Hydrophobic-Hydrophilic Nature of Biomedical Polymers and Nanocomposites on In Vitro Biological Development. Macromol. Mater. Eng. 2017, 302, 1–10. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251. [Google Scholar] [CrossRef]
- Salerno, A.; Guarino, V.; Oliviero, O.; Ambrosio, L.; Domingo, C. Bio-safe processing of polylactic-co-caprolactone and polylactic acid blends to fabricate fibrous porous scaffolds for in vitro mesenchymal stem cells adhesion and proliferation. Mater. Sci. Eng. C 2016, 63, 512–521. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Hu, W.; Shi, Y.; Cartmell, S.H. Enhancing Biocompatibility without Compromising Material Properties: An Optimised NaOH Treatment for Electrospun Polycaprolactone Fibres. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Guida, P.; Piscitelli, E.; Marrese, M.; Martino, V.; Cirillo, V.; Guarino, V.; Angeli, E.; Cocola, C.; Lo Savio, R.; Repetto, L.; et al. Integrating Microstructured Electrospun Scaffolds in an Open Microfluidic System for in Vitro Studies of Human Patient-Derived Primary Cells. ACS Biomater. Sci. Eng. 2020, 6, 3649–3663. [Google Scholar] [CrossRef]
- Yildirimer, L.; Seifalian, A.M. Three-dimensional biomaterial degradation—Material choice, design and extrinsic factor considerations. Biotechnol. Adv. 2014, 32, 984–999. [Google Scholar] [CrossRef]
- Pires, L.R.; Guarino, V.; Oliveira, M.J.; Ribeiro, C.C.; Barbosa, M.A.; Ambrosio, L.; Pêgo, A.P. Ibuprofen-loaded poly(trimethylene carbonate-co-ε-caprolactone) electrospun fibres for nerve regeneration. J. Tissue Eng. Regen. Med. 2016, 10, E154–E166. [Google Scholar] [CrossRef]
- Dias, J.C.; Ribeiro, C.M.O.; Sencadas, V.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S. Influence of fiber diameter and crystallinity on the stability of electrospun poly(l-lactic acid) membranes to hydrolytic degradation. Polym. Test. 2012, 31, 770–776. [Google Scholar] [CrossRef]
- Guarino, V.; Cruz-Maya, I.; Altobelli, R.; Khodir, W.K.A.; Ambrosio, L.; Pèrez, M.A.A.; Flores, A.A. Electrospun polycaprolactone nanofibres decorated by drug loaded chitosan nano-reservoirs for antibacterial treatments. Nanotechnology 2017, 28, 505103. [Google Scholar] [CrossRef] [PubMed]
- Ramdhanie, L.I.; AuBuchon, S.R.; Boland, E.D.; Knapp, D.C.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Thermal and Mechanical Characterization of Electrospun Blends of Poly(lactic acid) and Poly(glycolic acid). Polym. J. 2006, 38, 1137–1145. [Google Scholar] [CrossRef]
- Washburn, N.R.; Yamada, K.M.; Simon, C.G.; Kennedy, S.B.; Amis, E.J. High-throughput investigation of osteoblast response to polymer crystallinity: Influence of nanometer-scale roughness on proliferation. Biomaterials 2004, 25, 1215–1224. [Google Scholar] [CrossRef]
- Metwally, S.; Ferraris, S.; Spriano, S.; Krysiak, Z.J.; Łukasz, K.; Marzec, M.M.; Kim, S.K.; Szewczyk, P.K.; Gruszczyński, A.; Wytrwal-Sarna, M.; et al. Surface potential and roughness controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration. Mater. Des. 2020, 194, 108915. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for SkinWound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Yıldız, A.; Kara, A.A.; Acartürk, F. Peptide-protein based nanofibers in pharmaceutical and biomedical applications. Int. J. Biol. Macromol. 2020, 148, 1084–1097. [Google Scholar] [CrossRef]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Bharathi, V.A.; Lim, K.H.C. Ramakrishna SElectrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Naskar, D.; Ghosh, A.K.; Mandal, M.; Das, P.; Nandi, S.; Kundu, S.C. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials 2017, 136, 67–85. [Google Scholar] [CrossRef]
- Hu, J.; Kai, D.; Ye, H.; Tian, L.; Ding, X.; Ramakrishna, S.; Loh, X.J. Electrospinning of poly(glycerol sebacate)-based nanofibers for nerve tissue engineering. Mater. Sci. Eng. C 2017, 70, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.; Lotfi, A.S.; Barzin, J.; Hatam, M.; Adibi, B.; Khalaj, Z.; Massumi, M. Human Bone Marrow Mesenchymal Stem Cell Behaviors on PCL/Gelatin Nanofibrous Scaffolds Modified with A Collagen IV-Derived RGD-Containing Peptide. Cell J. 2014, 16, 1–10. [Google Scholar] [PubMed]
- Yang, G.; Yao, Y.; Wang, X. Comparative study of kerateine and keratose based composite nanofibers for biomedical applications. Mater. Sci. Eng. C 2018, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Figoli, A.; Ursino, C.; Sanchez Ramirez, D.O.; Carletto, R.A.; Tonetti, C.; Varesano, A.; De Santo, M.P.; Cassano, A.; Vineis, C. Fabrication of Electrospun Keratin Nano fiber Membranes for Air and Water Treatment. Polym. Eng. Sci. 2019, 59, 1472–1478. [Google Scholar] [CrossRef]
- Tomaselli, S.; Ramirez, D.O.S.; Carletto, R.A.; Varesano, A.; Vineis, C.; Zanzoni, S.; Molinari, H.; Ragona, L. Electrospun Lipid Binding Proteins Composite Nanofibers with Antibacterial Properties. Macromol. Biosci. 2017, 17, 1600300. [Google Scholar] [CrossRef]
- Esparza, Y.; Ullah, A.; Boluk, Y.; Wu, J. Preparation and characterization of thermally crosslinked poly(vinyl alcohol)/feather keratin nanofiber scaffolds. Mater. Des. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Suarato, G.; Contardi, M.; Perotto, G.; Heredia-Guerrero, J.A.; Fiorentini, F.; Ceseracciu, L.; Pignatelli, C.; Debellis, D.; Bertorelli, R.; Athanassiou, A. From fabric to tissue: Recovered wool keratin/polyvinylpyrrolidone biocomposite fibers as artificial scaffold platform. Mat. Sci. Eng. C 2020, 116, 111151. [Google Scholar] [CrossRef]
- Jain, A.; Ravi, V.; Muhamed, J.; Chatterjee, K.; Sundaresan, N.R. A simplified protocol for culture of murine neonatal cardiomyocytes on nanoscale keratin coated surfaces. Int. J. Cardiol. 2017, 232, 160–170. [Google Scholar] [CrossRef]
- Ferraris, S.; Prato, M.; Vineis, C.; Varesano, A.; Di Confiengo, G.G.; Spriano, S. Coupling of keratin with titanium: A physico-chemical characterization of functionalized or coated surfaces. Surf. Coat. Technol. 2020, 397, 126057. [Google Scholar] [CrossRef]
- Tachibana, A.; Furuta, Y.; Takeshima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- Yamauchi, K.; Maniwa, M.; Mori, T. Cultivation of fibroblast cells on keratin-coated substrata. J. Biomater. Sci. 1998, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Kaneko, S.; Tanabe, T.; Yamauchi, K. Rapid fabrication of keratin?hydroxyapatite hybrid sponges toward osteoblast cultivation and differentiation. Biomaterials 2005, 26, 297–302. [Google Scholar] [CrossRef]
- Cochis, A.; Ferraris, S.; Sorrentino, R.; Azzimonti, B.; Novara, C.; Geobaldo, F.; Giachet, F.T.; Vineis, C.; Varesano, A.; Abdelgeliel, A.S.; et al. Silver-doped keratin nanofibers preserve a titanium surface from biofilm contamination and favor soft-tissue healing. J. Mater. Chem. B 2017, 5, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.O.S.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Packag. Shelf Life 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Varesano, A.; Vineis, C.; Tonetti, C.; Ramirez, D.O.S.; Mazzuchetti, G. Chemical and Physical Modifications of Electrospun Keratin Nanofibers Induced by Heating Treatments. J. Appl. Polym. Sci. 2014, 131, 40532. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R.; Chaudhari, A.A.; Vig, K.; et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Dias Antunes, M.; da Silva Dannenberg, G.; Fiorentini Ângela, M.; Pinto, V.Z.; Lim, L.-T.; da Rosa Zavareze, E.; Dias, A.R.G. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef]
- Heydari-Majda, M.; Rezaeiniab, H.; Reza Shadanc, M.; Ghoranib, B.; Tuckerd, N. Enrichment of zein nanofibre assemblies for therapeutic delivery of Barije (Ferula gummosa Boiss) essential oil. J. Drug Deliv. Sci. Technol. 2019, 54, 101290. [Google Scholar] [CrossRef]
- Guarino, V.; Bonadies, I.; Ambrosio, L. Nanotubes and Nanofibers for Tissue Repair and Regeneration. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair, 1st ed.; Barbosa, M., Martins, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Chen, H.; Wang, N.; Di, J.; Zhao, Y.; Song, Y.; Jiang, L. Nanowire-in-Microtube Structured Core/Shell Fibers via Multifluidic Coaxial Electrospinning. Langmuir 2010, 26, 11291–11296. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Guarino, V.; Schiavo, L.; Alvarez-Perez, M.A.; Ambrosio, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Electron. 2011, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhahebi, A.M. Subash Chandra Bose Gopinath & Mohamed Shuaib Mohamed Saheed Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 27. [Google Scholar]
- Zadeh, Z.E.; Solouk, A.; Shafieian, M.; Nazarpak, M.H. Electrospun polyurethane/carbon nanotube composites with different amounts of carbon nanotubes and almost the same fiber diameter for biomedical applications. Mater. Sci. Eng. C 2021, 118, 111403. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, H.S.; Ranjbar-Mohammadi, M.; Milan, P. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef]
- Guarino, V.; Cruz-Maya, I.; Reineck, P.; Abe, H.; Ohshima, T.; Fox, K.; Greentree, A.D.; Gibson, B.C.; Ambrosio, L. Fluorescent Nanodiamonds Embedded in Poly-ε-Caprolactone Fibers as Biomedical Scaffolds. ACS Appl. Nano Mater. 2020, 3, 10814–10822. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Von der Mark, K.; Park, J. Engineering biocompatible implant surfacesPart II: Cellular recognition of biomaterial surfaces: Lessonsfrom cell–matrix interactions. Prog. Mater. Sci. 2013, 58, 327–381. [Google Scholar] [CrossRef]
- Sales, A.; Holle, A.W.; Kemkemer, R. Initial contact guidance during cell spreading is contractility-independent. Soft Matter 2017, 13, 5158–5167. [Google Scholar] [CrossRef]
- Sander, L.M. Modeling Contact Guidance and Invasion by Cancer Cells. Modeling contact guidance and invasion by cancer cells. Cancer Res. 2014, 74, 4588–4596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mejillano, M.R.; Kojima, S.-I.; Applewhite, D.A.; Gertler, F.B.; Svitkina, T.; Borisy, G.G. Lamellipodial Versus Filopodial Mode of the Actin Nanomachinery: Pivotal Role of the Filament Barbed End. Cell 2004, 118, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.D.; Isenberg, B.C.; Chua, S.G.; Wong, J.Y. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc. Natl. Acad. Sci. USA 2016, 113, 11190–11195. [Google Scholar] [CrossRef] [PubMed]
- Ballestrem, C.; Wehrle-Haller, B.; Hinz, B.; Imhof, B.A. Actin-dependent Lamellipodia Formation and Microtubule-dependent Tail Retraction Control-directed Cell Migration. Mol. Biol. Cell 2000, 11, 2999–3012. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Garcia, P.A.; De Jesus, J.K.; Williams, S.P.; Nowicki, M.O.; Chiocca, E.A.; Liyanarachchi, S.; Li, P.-K.; Lannutti, J.J.; Johnson, J.K.; Lawler, S.E.; et al. Glioma Cell Migration on Three-dimensional Nanofiber Scaffolds Is Regulated by Substrate Topography and Abolished by Inhibition of STAT3 Signaling. Neoplasia 2011, 13, 831-IN22. [Google Scholar] [CrossRef] [PubMed]
- Pourfarhangi, K.E.; De La Hoz, E.C.; Cohen, A.R.; Gligorijevic, B. Contact guidance is cell cycle-dependent. APL Bioeng. 2018, 2, 031904. [Google Scholar] [CrossRef]
- Juan, G.R.R.-S.; Oakes, P.W.; Gardel, M.L. Contact guidance requires spatial control of leading-edge protrusion. Mol. Biol. Cell 2017, 28, 1043–1053. [Google Scholar] [CrossRef]
- Novoseletskaya, E.S.; Grigorieva, O.A.; Efimenko, A.Y.; Kalinina, N.I. Extracellular Matrix in the Regulation of Stem Cell Differentiation. Biochemistry 2019, 84, 232–240. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr. Top. Dev. Biol. 2018, 130, 1–37. [Google Scholar] [CrossRef]
- Gehler, S.; Baldassarre, M.; Lad, Y.; Leight, J.L.; Wozniak, M.A.; Riching, K.M.; Eliceiri, K.W.; Weaver, V.M.; Calderwood, D.A.; Keely, P.J. Filamin A–β1 Integrin Complex Tunes Epithelial Cell Response to Matrix Tension. Mol. Biol. Cell 2009, 20, 3224–3238. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, Y.; Hu, D.; Zhu, P.; Wang, N.; Zhang, Q.; Wang, M.; Aldeewan, A.; Xia, H.; Qu, X.; et al. Nuclear SIPA1 activates integrin β1 promoter and promotes invasion of breast cancer cells. Oncogene 2014, 34, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Kurpakus, M.A.; Cooper, H.M.; Quaranta, V. A function for the integrin alpha 6 beta 4 in the hemidesmosome. Cell Regul. 1991, 2, 427–438. [Google Scholar] [CrossRef] [PubMed]
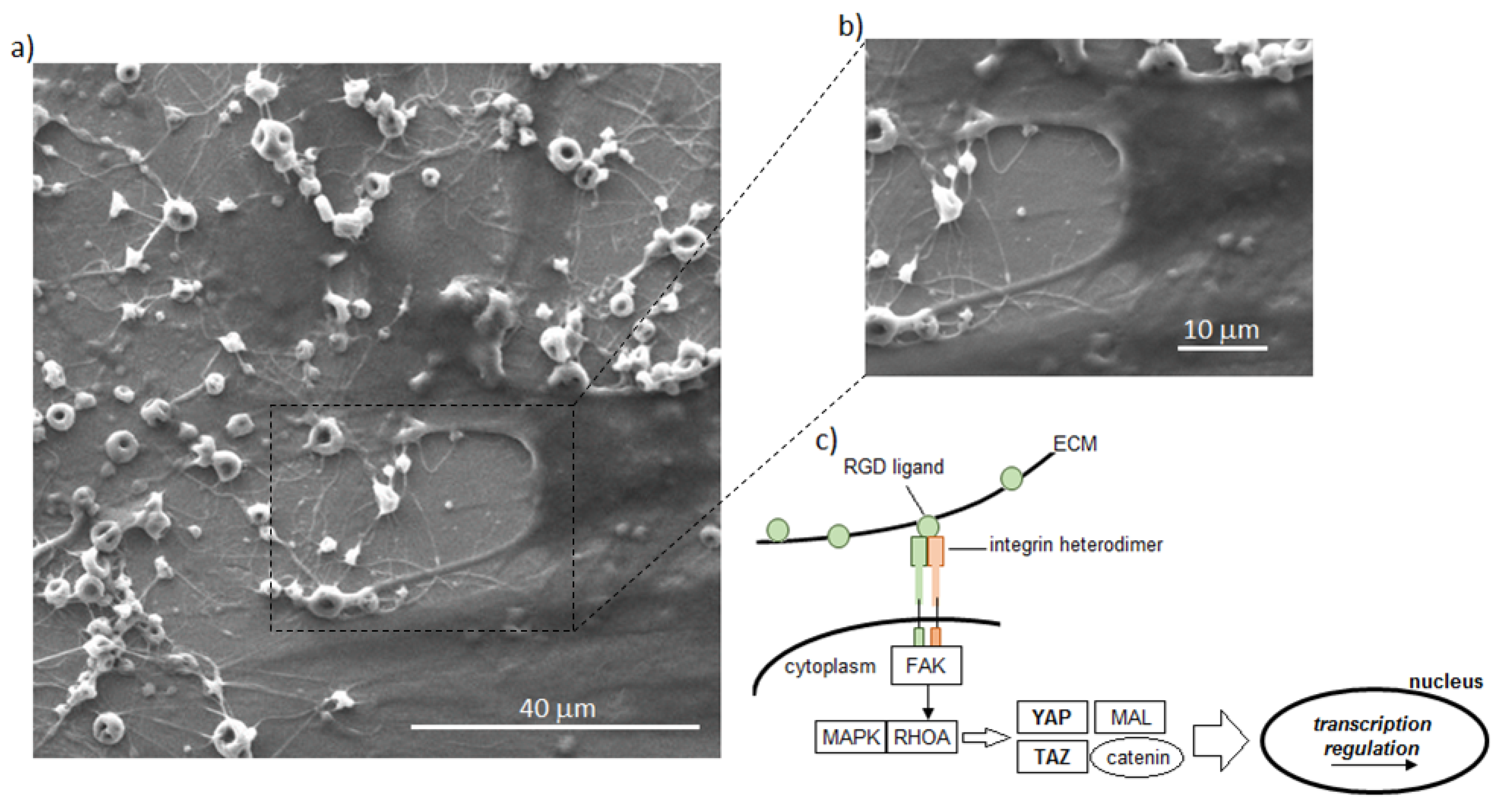
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Cabodi, S.; Di Stefano, P.; Leal, M.D.P.C.; Tinnirello, A.; Bisaro, B.; Morello, V.; Damiano, L.; Aramu, S.; Repetto, D.; Tornillo, G.; et al. Integrins and Signal Transduction. Adv. Exp. Med. Biol. 2010, 674, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nat. Cell Biol. 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.-Y.; Li, B.; Tian, X.Y.; Chen, L.-J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S.; et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nat. Cell Biol. 2016, 540, 579–582. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Fu, H.-L.; Valiathan, R.R.; Arkwright, R.; Sohail, A.; Mihai, C.; Kumarasiri, M.; Mahasenan, K.V.; Mobashery, S.; Huang, P.; Agarwal, G.; et al. Discoidin Domain Receptors: Unique Receptor Tyrosine Kinases in Collagen-mediated Signaling. J. Biol. Chem. 2013, 288, 7430–7437. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin domain receptor tyrosine kinases: New players in cancer progression. Cancer Metastasis Rev. 2012, 31, 295–321. [Google Scholar] [CrossRef] [PubMed]
- Kawai, I.; Hisaki, T.; Sugiura, K.; Naito, K.; Kano, K. Discoidin domain receptor 2 (DDR2) regulates proliferation of endochondral cells in mice. Biochem. Biophys. Res. Commun. 2012, 427, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Ouhtit, A.; Rizeq, B.; Saleh, H.A.; Rahman, M.; Zayed, H. Novel CD44-downstream signaling pathways mediating breast tumor invasion. Int. J. Biol. Sci. 2018, 14, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Gong, Q.; Cheng, Y.; Su, G. Regulatory mechanisms of Robo4 and their effects on angiogenesis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Smith-Berdan, S.; Nguyen, A.; Hassanein, D.; Zimmer, M.; Ugarte, F.; Ciriza, J.; Li, D.; García-Ojeda, M.E.; Hinck, L.; Forsberg, E.C. Robo4 Cooperates with Cxcr4 to Specify Hematopoietic Stem Cell Localization to Bone Marrow Niches. Cell Stem Cell 2011, 8, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Warchomicka, F.; Ramskogler, C.; Tortello, M.; Cochis, A.; Scalia, A.; Di Confiengo, G.G.; Keckes, J.; Rimondini, L.; Spriano, S. Surface structuring by Electron Beam for improved soft tissues adhesion and reduced bacterial contamination on Ti-grade 2. J. Mater. Process. Technol. 2019, 266, 518–529. [Google Scholar] [CrossRef]
- Chung, K.; DeQuach, J.A.; Christman, K.L. Nanopatterned Interfaces for Controlling Cell Behavior. Nano LIFE 2010, 1, 63–77. [Google Scholar] [CrossRef]
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; García, A.J.; Guldberg, R.E. Nanofiber orientation and surface functionalization modulate human mesenchymal stem cell behavior in vitro. Tissue Eng. Part A 2014, 20, 398–409. [Google Scholar] [CrossRef]
- Di Cio, S.; Gautrot, J. Cell sensing of physical properties at the nanoscale: Mechanisms and control of cell adhesion and phenotype. Acta Biomater. 2016, 30, 26–48. [Google Scholar] [CrossRef]
- Spriano, S.; Chandra, V.S.; Cochis, A.; Uberti, F.; Rimondini, L.; Bertone, E.; Vitale, A.; Scolaro, C.; Ferrari, M.; Cirisano, F.; et al. How do wettability, zeta potential and hydroxylation degree affect the biological response of biomaterials? Mater. Sci. Eng. C 2017, 74, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.M.A.; Van Der Ham, I.; Brinker, M.G.; Van Rijn, P.; Harmsen, M.C. Topography-driven alterations in endothelial cell phenotype and contact guidance. Heliyon 2020, 6, e04329. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Blanquer, S.B.G.; Haimi, S.P.; Korus, G.; Dunlop, J.W.C.; Duda, G.N.; Grijpma, D.W.; Petersen, A. Surface Curvature Differentially Regulates Stem Cell Migration and Differentiation via Altered Attachment Morphology and Nuclear Deformation. Adv. Sci. 2017, 4, 1600347. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.K.; Yarin, A.L.; Megaridis, C.M.; Cho, M. Chondrogenic Differentiation of Human Mesenchymal Stem Cells on Oriented Nanofibrous Scaffolds: Engineering the Superficial Zone of Articular Cartilage. Tissue Eng. Part A 2009, 15, 913–921. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, M.; Wu, G.; Lai, Y.; Greber, B.; Schöler, H.R.; Chi, L. Topographic effect on human induced pluripotent stem cells differentiation towards neuronal lineage. Biomaterials 2013, 34, 8131–8139. [Google Scholar] [CrossRef]
- Da Cruz, M.B.; Marques, J.F.; Peñarrieta-Juanito, G.M.; Costa, M.; Souza, J.C.; Magini, R.S.; Miranda, G.; Silva, F.S.; Da Mata, A.D.S.P.; Caramês, J.M.M. Hard and Soft Tissue Cell Behavior on Polyetheretherketone, Zirconia, and Titanium Implant Materials. Int. J. Oral Maxillofac. Implant. 2019, 34, 39–46. [Google Scholar] [CrossRef]
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef]
- Sahoo, S.; Ang, L.T.; Goh, J.C.-H.; Toh, S.-L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res. Part A 2009, 93, 1539–1550. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Nerurkar, N.L.; Jacobs, N.T.; Elliott, D.M.; Mauck, R.L. Fiber angle and aspect ratio influence the shear mechanics of oriented electrospun nanofibrous scaffolds. J. Mech. Behav. Biomed. Mater. 2011, 4, 1627–1636. [Google Scholar] [CrossRef]
- Shin, M.; Yoshimoto, H.; Vacanti, J.P. In Vivo Bone Tissue Engineering Using Mesenchymal Stem Cells on a Novel Electrospun Nanofibrous Scaffold. Tissue Eng. 2004, 10, 33–41. [Google Scholar] [CrossRef]
- Li, W.-J.; Danielson, K.G.; Alexander, P.G.; Tuan, R.S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(ϵ-caprolactone) scaffolds. J. Biomed. Mater. Res. Part A 2003, 67, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Raic, A.; Friedrich, F.; Kratzer, D.; Bieback, K.; Lahann, J.; Lee-Thedieck, C. Potential of electrospun cationic BSA fibers to guide osteogenic MSC differentiation via surface charge and fibrous topography. Sci. Rep. 2019, 9, 20003–20015. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, L.; Tabrizian, M. Sensing surfaces: Challenges in studying the cell adhesion process and the cell adhesion forces on biomaterials. IRBM 2008, 29, 77–88. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Song, K.; Liu, M.; Wang, J. Effect of surface charge on osteoblastic proliferation and differentiation on a poly(ethylene glycol)-diacrylate hydrogel. J. Mater. Sci. 2017, 53, 908–920. [Google Scholar] [CrossRef]
- Shi, D. Cancer Cell Surface Negative Charges: A Bio-Physical Manifestation of the Warburg Effect. Nano LIFE 2017, 7, 1771001. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; Yang, P.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospun fibers: An innovative delivery method for the treatment of bone diseases. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structuredpolymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, J.-J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Rashid, R.; Amn, T.; Hamid, R.; Sheikh, F.A. Recent advances in formulating electrospun nanofiber membranes: Delivering active phytoconstituents. J. Drug Deliv. Sci. Technol. 2020, 60, 102038. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Y.; Guan, J.; Guan, J.; Ye, T.; Chen, Y.; Zhu, Y.; Zhou, P.; Cui, W. Electrospun fibers: An innovative delivery method for the treatment of bone diseases. Expert Opin. Drug Deliv. 2020, 17, 993–1005. [Google Scholar] [CrossRef]
- Pogorielov, M.; Hapchenko, A.; Deineka, V.; Rogulska, L.; Oleshko, O.; Vodseďálková, K.; Berezkinová, L.; Vysloužilová, L.; Klápšťová, A.; Erben, J. In vitrodegradation andin vivotoxicity of NanoMatrix3D®polycaprolactone and poly(lactic acid) nanofibrous scaffolds. J. Biomed. Mater. Res. A 2018, 106, 2200–2212. [Google Scholar] [CrossRef]
- Liu, H.; Ding, X.; Zhou, G.; Li, P.; Wei, X.; Fan, Y. Electrospinning of Nanofibers for Tissue Engineering Applications. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Berton, F.; Porrelli, D.; Di Lenarda, R.; Turco, G. A Critical Review on the Production of Electrospun Nanofibres for Guided Bone Regeneration in Oral Surgery. Nanomaterials 2019, 10, 16. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Salehi, M.M.; Hakkak, F.; Tilebon, S.M.S.; Ataeefard, M.; Rafizadeh, M. Intelligently optimized electrospun polyacrylonitrile/poly(vinylidene fluoride) nanofiber: Using artificial neural networks. Express Polym. Lett. 2020, 14, 1003–1017. [Google Scholar] [CrossRef]
- Vimal, S.K.; Ahamad, N.; Katti, D.S. A simple method for fabrication of electrospun fibers with controlled degree of alignment having potential for nerve regeneration applications. Mater. Sci. Eng. C 2016, 63, 616–627. [Google Scholar] [CrossRef]
- Xia, H.; Xia, Y. An in vitro study of non-aligned or aligned electrospun poly(methylmethacrylate) nanofibers as primary rat astrocytes-loading scaffold. Mat. Sci. Eng. C 2018, 91, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Ao, Q.; Wang, L.; Huang, Z.; Cai, Q.; Chen, G.-Q.; Yang, X.; Zhong, W. Constructing conductive conduit with conductive fibrous infilling for peripheral nerve regeneration. Int. J. Chem. Eng. 2018, 345, 566–577. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Huang, Z.; Yin, G.; Pu, X. Fabrication of aligned, porous and conductive fibers and their effects on cell adhesion and guidance. Colloids Surf. B Biointerfaces 2015, 134, 469–474. [Google Scholar] [CrossRef]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 386–395. [Google Scholar] [CrossRef]
- Lau, Y.-T.; Kwok, L.-F.; Tam, K.-W.; Chan, Y.-S.; Shum, D.K.-Y.; Shea, G.K.-H. Genipin-treated chitosan nanofibers as a novel scaffold for nerveguidance channel design. Colloid Surf. B 2018, 162, 126–134. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials 2008, 29, 653–661. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of aligned and random fibers with different diameter on cell behaviors. Colloids Surf. B Biointerfaces 2018, 171, 461–467. [Google Scholar] [CrossRef]
- Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Zhang, Y. Stiffness of the aligned fibers affects structural and functional integrity of the oriented endothelial cells. Acta Biomater. 2020, 108, 237–249. [Google Scholar] [CrossRef]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL–PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, M.; Kumar, S.; Świeszkowski, W.; Ivanovski, S.; Vaquette, C. The effect of melt electrospun writing fiber orientation onto cellular organization and mechanical properties for application in Anterior Cruciate Ligament tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103631. [Google Scholar] [CrossRef] [PubMed]
- Chaurey, V.; Block, F.; Su, Y.-H.; Chiang, P.-C.; Botchwey, E.; Chou, C.-F.; Swami, N.S. Nanofiber size-dependent sensitivity of fibroblast directionality to the methodology for scaffold alignment. Acta Biomater. 2012, 8, 3982–3990. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Sun, Y.-C.; Chen, Y.-H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, Y.; Chang, J.; Li, L.; He, B.; Gu, Z. Guidance of myoblast migration on aligned electrospun PLGA nanofibrous meshes. Mater. Lett. 2012, 68, 218–221. [Google Scholar] [CrossRef]
- Tallawi, M.; Dippold, D.; Rai, R.; D’Atri, D.; Roether, J.; Schubert, D.; Rosellini, E.; Engel, F.B.; Boccaccini, A.R. Novel PGS/PCL electrospun fiber mats with patterned topographical features for cardiac patch applications. Mater. Sci. Eng. C 2016, 69, 569–576. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Liao, M.; Dai, L.; Tang, Y.; Zhang, H.; Coates, P.; Sefat, F.; Zheng, L.; Song, J.; et al. Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 2019, 213, 27–38. [Google Scholar] [CrossRef]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.-H.; Cho, H.-J.; Kim, H.K.; Yoon, T.R.; Shin, H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials 2013, 34, 5059–5069. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Ciardelli, G.; Zanetti, M.; Geuna, S.; Perroteau, I. The influence of electrospun fibre size on Schwann cell behaviour and axonal outgrowth. Mater. Sci. Eng. C 2015, 48, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Galusková, D.; Kaňková, H.; Zheng, K.; Michálek, M.; Liverani, L.; Galusek, D.; Boccaccini, A.R. Electrospun PCL Fiber Mats Incorporating Multi-Targeted B and Co Co-Doped Bioactive Glass Nanoparticles for Angiogenesis. Materials 2020, 13, 4010. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; An, Q.; Li, D.; Wang, J.; He, L.; Huang, C.; Li, Y.; Zhu, W.; Mo, X. A novel heparin loaded poly(l-lactide-co-caprolactone) covered stent for aneurysm therapy. Mater. Lett. 2014, 116, 39–42. [Google Scholar] [CrossRef]
- Sekar, M.P.; Roopmani, P.; Krishnan, U.M. Development of a novel porous polyvinyl formal (PVF) microfibrous scaffold for nerve tissue engineering. Polymer 2018, 142, 170–182. [Google Scholar] [CrossRef]
- Pavlova, E.R.; Bagrov, D.V.; Monakhova, K.Z.; Piryazev, A.A.; Sokolova, A.I.; Ivanov, D.A.; Klinov, D.V. Tuning the properties of electrospun polylactide mats by ethanol treatment. Mater. Des. 2019, 181, 108061. [Google Scholar] [CrossRef]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Kumar, N.; Bansal, M.; Mishra, B. Tinidazole functionalized homogeneous electrospun chitosan/poly (ε-caprolactone) hybrid nanofiber membrane: Development, optimization and its clinical implications. Int. J. Biol. Macromol. 2017, 103, 1311–1326. [Google Scholar] [CrossRef]
- Arenberger, P.; Arenberger, P.; Bednar, M.; Kubat, P.; Mosinger, J. Light-activated nanofibre textiles exert antibacterial effects in the setting of chronic wound healing. Exp. Dermatol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Fulco, I.; Erba, P.; Valeri, R.C.; Vournakis, J.; Schaefer, D.J.; Valeri, C.R. Poly-N-acetyl glucosamine nanofibers for negative-pressure wound therapies. Wound Repair Regen. 2015, 23, 197–202. [Google Scholar] [CrossRef]
- Kelechi, T.J.; Mueller, M.; Hankin, C.S.; Bronstone, A.; Samies, J.; Bonham, P.A. A randomized, investigator-blinded, controlled pilot study to evaluate the safety and efficacy of a poly-N-acetyl glucosamine–derived membrane material in patients with venous leg ulcers. J. Am. Acad. Dermatol. 2012, 66, e209–e215. [Google Scholar] [CrossRef]



| Subunit(s) α | Subunit(s) β | ECM Target Substrates |
|---|---|---|
| α1; α2; α3; α4; α5; α6; α7; α8; αv | β1 | Collagen; laminin; fibronectin; VCAM-1; RGD |
| αL, αM, αX | β2 | ICAM-1,2; C3b complement; fibrinogen; factor X; ICAM-1 |
| αIIb, αV | β3 | Fibrinogen, fibronectin, vWF, vitronectin, thrombospondin; osteopontin; collagen IV |
| α6 | β4 | Laminin |
| αV | β5 | Vitronectin |
| αV | β6 | Fibronectin, TGF- β1 |
| α4, αIEL | β7 | V25, VCAM-1 |
| αV | β8 | TGF- β1 |
| Material | Construct Type | Fiber Orientation | Additional Cue | Application | Main Results | Ref. |
|---|---|---|---|---|---|---|
| Polystyrene (PS) | Sub-micron fibers (0.7–0.9 µm diameter) | Different degree of alignment (from random to 95% alignment) | - | Neural regeneration | Human astrocytoma epithelial-like cells exhibited elongated and aligned morphology (suitable for nerve regeneration) on aligned fibers. | [174] |
| Poly-Methyl-Methacrylate (PMMA) | Nanofibers (0.5 µm diameter) | Aligned and random | - | Neural regeneration | Astrocytes aligned and proliferated in suitable way for neural regeneration on aligned fibers. | [175] |
| Poly(lactide-co-glycolide) (PLGA) | Sub-micron fibers (0.7–0.9 µm diameter) in conduits of the same material | Aligned fibers | Conductive coating (polypyrrole (PPY)) | Peripheral nerve regeneration | In vitro promotion of growth and differentiation of neural cells, in vivo (rat model) promotion of sciatic nerve regeneration. | [176] |
| Polyvinyl formal (PVF) | Scaffold of submicrometric fibers | Random | Nanoporous surface Optional curcumin loading | Neural regeneration | Good adhesion, growth, extension, and viability of hippocampal neurons. | [198] |
| Poly(L-lactic acid) (PLLA) | Nano (300 nm fiber diameter)/micro (1.5 µm fiber diameter) fibrous scaffold | Random or aligned | - | Neural regeneration | Fiber alignment affects neural stem cells alignment, elongation, and neurite outgrowth, while fiber diameter affects cell differentiation (higher on nanofibers, despite orientation). | [177] |
| Poly(L-lactic acid) (PLLA) | Porous fibers (0.8 µm fiber diameter, with 200 nm pores) | Random or aligned | Conductive coating (polypyrrole (PPY)). Final fiber diameter 1.24 µm with 100 nm pores) | Neural regeneration (applicable also to other tissues) | Alignment of fibroblasts and neurites increases on aligned, porous, and conductive fibers. | [178] |
| Gelatin | Nanofibers (200–300 nm diameter) | Random or aligned | Ceria nanoparticles doping | Neural regeneration | Aligned fibers promote neuronal alignment. Ceria nanoparticles improved mechanical properties and introduce antioxidant ability. | [179] |
| Chitosan (75–85% deacetylated) crosslinked with genipin | Nanofibers (200–300 nm) | Aligned | - | Neural regeneration | Crosslinking effectively limit swelling and degradation. Aligned fibers promote neuronal cells growth and alignment. | [180] |
| poly(lactic-co-glycolic acid) (PLGA) | Nanofibers (250–360 nm diameter) | Random or aligned | Conductive coating (polypyrrole (PPY)) | Neural regeneration | Electrical stimulation increases neurite outgrowth on all fibers, the same stimulation on aligned fibers also increases neurite length. | [181] |
| Poly(-caprolactone) (PCL) | Scaffold of micrometric (1–2 µm diameter) | Random or aligned | - | Neural regeneration | Aligned fibers promote cellular alignment and more pronounced maturation on human Swann cells. | [182] |
| Gelatin crosslinked with γ-glycidoxypropyltrimethoxysilane (GPTMS) | Nano (300–600 nm diameter) or micro (1–1.3 µm diameter) fibers | Random | - | Neural regeneration | Micro-fibers promote Swann cells migration and axonal outgrowth, while nanofibers promote Swann cells adhesion and proliferation. The combination of both sizes allows proper nerve regeneration. | [195] |
| Cellulose with cellulose nanocrystals | Nanofibers scaffolds (300 nm fiber diameter) | Random or aligned | Fiber doping with recombinant human Bone Morphogenetic Protein 2 (rh-BMP2) | Bone regeneration | BMP-2 doping increases ALP production, calcium content and alizarin red in bone marrow stromal cells with no dependence on fiber orientation. On aligned fibers, cell growth and calcium deposition follow fiber direction. Bone formation in vivo (rabbit model). | [192] |
| PLLA, PDLLA, PEG-PDLLA, PEG-PLLA | Fibers with diameter ranging 0.14–2.1 µm on glass substrate spin coated with the fiber polymer | Random or aligned | - | Bone regeneration | In presence of osteogenic factors in osteoprogenitor cells culture, an increase in cellular spreading and filopodia extension is obtained on fibers of higher diameter. | [193] |
| Poly(lactide-co-glycolic acid) (PLGA) | Porous membranes (fibers diameter 1 µm) | Random | Dopamine mediated functionalization with bone forming peptide derived from BMP-7 | Guided bone regeneration | Increase in ALP production and Ca deposition for bone marrow stromal cells. Increase in bone growth in vivo (mouse model). | [194] |
| Poly(D-L-lactic-co-glycolic acid) (PLGA) | Mesh of fibers (0.14–3.16 µm diameter) | Different degree of orientation | - | Ligament regeneration | Fibroblasts spreading increased with fibers diameter, spreading, and orientation of fibroblasts increased with alignment. | [185] |
| Poly(-caprolactone)-poly(ethylene-glycol) | Mats embedded in porous chitosan scaffolds | Random and oriented | - | Periodontal ligament regeneration | Chitosan acts as a glue to keep together fibers layers and induces anti-inflammatory action. Fiber orientation induces tendon/ligament cellular differentiation of bone marrow stromal cells, while random fibers promote osteogenic differentiation. In vivo (rat model) regeneration of periodontal ligament. | [186] |
| Polycaprolactone | Porous scaffold. Fibers diameter 20 µm Proposal of complex structures with 2 bone compartments and 1 ligament compartment with different fibers alignment. | Aligned fibers | - | Ligament regeneration | Fiber alignment induces alignment in mesenchymal stem cells. | [187] |
| Poly(caprolactone) | Micro (1.3–2.4 µm diameter) and nano (300–400 nm) fibers | Random and aligned | - | Cardiovascular | Nanofibers enhance endothelial cells adhesion and proliferation, while alignment is enhanced on aligned micro-fibers. Aligned endothelial cells mimic natural vessel orient by blood flow. | [183] |
| Poly(glycerol sebacate)-poly(caprolactone) | Fibers (1 µm diameter) on patterned substrate (grooves, squares…) | Random | - | Cardiac patch | Surface topography affects cell orientation and morphology, but not viability | [191] |
| (poly-L-lactide-co-caprolactone)/(poly-L-lactic acid) PLCL/PLLA | Core-shell fibers | Aligned | - | cardiovascular | Endothelial cell adhesion increases with fibers stiffness, however a loss in endothelium integrity is observed (pathological remodeling). Cellular alignment is observed on aligned fibers. | [184] |
| Poly(L-lactide-co-caprolactone) | Nanofibers on metallic stent | Random | Heparin doping | Aneurysm treatment | Effective aneurysm treatment in rabbit model | [197] |
| Poly(-caprolactone)-PCL + polyaniline (PANi) blend | Nanofibers (350–500 nm) | Aligned | Muscle regeneration | Fiber alignment and electrical conductivity stimulate myoblasts orientation and differentiation as in physiological muscle | [189] | |
| Poly(L-lactide-r-glycolide) (PLGA) | Mesh of nanofibers (500 nm diameter) | Random and aligned | Muscle regeneration | Myoblasts grow well on all the meshes, but align (as in physiological muscle) only on the oriented ones | [190] | |
| Poly(L-lactide-r-glycolide) (PLGA) | Nanofibers (80–740 nm diameter) | Aligned | Fiber polarization (electrical alignment) | Soft tissue regeneration (fibroblast orientation) | For fiber diameter >300 nm, all fibers promote fibroblasts alignment, when d > 300 nm electrical alignment significantly improves fibroblast alignment (fiber polarization parallel to geometrical alignment, higher directional signal to molecular scale receptor). When d < 100 nm nanoimprinting (perfect alignment at the nanoscale) becomes fundamental. | [186] |
| PLA | mats | Random | Stretching in ethanol for alignment | Soft tissues (test on keratinocytes) | Ethanol stretching induces alignment and contact guidance on keratinocytes, increases elasticity and molecular orientation without crystallization. | [199] |
| Polycaprolactone | Doping with bioactive glass nanoparticles doped with B and Co ions | Soft tissues with high vascularization (e.g., wounds) | Release of cobalt and boron increases cellular vascular endothelial growth factor. | [196] | ||
| Keratin | Submicrometric fibers on titanium (polished/grooved) | Random | Soft tissue adhesion and regeneration | Keratin fibers promote gingival fibroblast adhesion and proliferation. Random fibers effect is higher than the one of substrate grooves (loosening of alignment). | [37] | |
| Keratin | Submicrometric fibers on grooved titanium substrate | Aligned | Soft tissue adhesion and regeneration | Gingival fibroblasts align on keratin fibers parallel to substrate grooves. | [41] | |
| Keratin | Submicrometric fibers on titanium | Random | Ag ion doping | Soft tissue adhesion and regeneration | Keratin fibers promote gingival fibroblast adhesion and proliferation and significantly reduce S aureus adhesion (dose-dependent manner with respect to Ag content) maintaining biocompatibility. | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. https://doi.org/10.3390/polym12122896
Ferraris S, Spriano S, Scalia AC, Cochis A, Rimondini L, Cruz-Maya I, Guarino V, Varesano A, Vineis C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers. 2020; 12(12):2896. https://doi.org/10.3390/polym12122896
Chicago/Turabian StyleFerraris, Sara, Silvia Spriano, Alessandro Calogero Scalia, Andrea Cochis, Lia Rimondini, Iriczalli Cruz-Maya, Vincenzo Guarino, Alessio Varesano, and Claudia Vineis. 2020. "Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications" Polymers 12, no. 12: 2896. https://doi.org/10.3390/polym12122896
APA StyleFerraris, S., Spriano, S., Scalia, A. C., Cochis, A., Rimondini, L., Cruz-Maya, I., Guarino, V., Varesano, A., & Vineis, C. (2020). Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers, 12(12), 2896. https://doi.org/10.3390/polym12122896