Fabrication of Activated Carbon Fibers with Sheath-Core, Hollow, or Porous Structures via Conjugated Melt Spinning of Polyethylene Precursor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
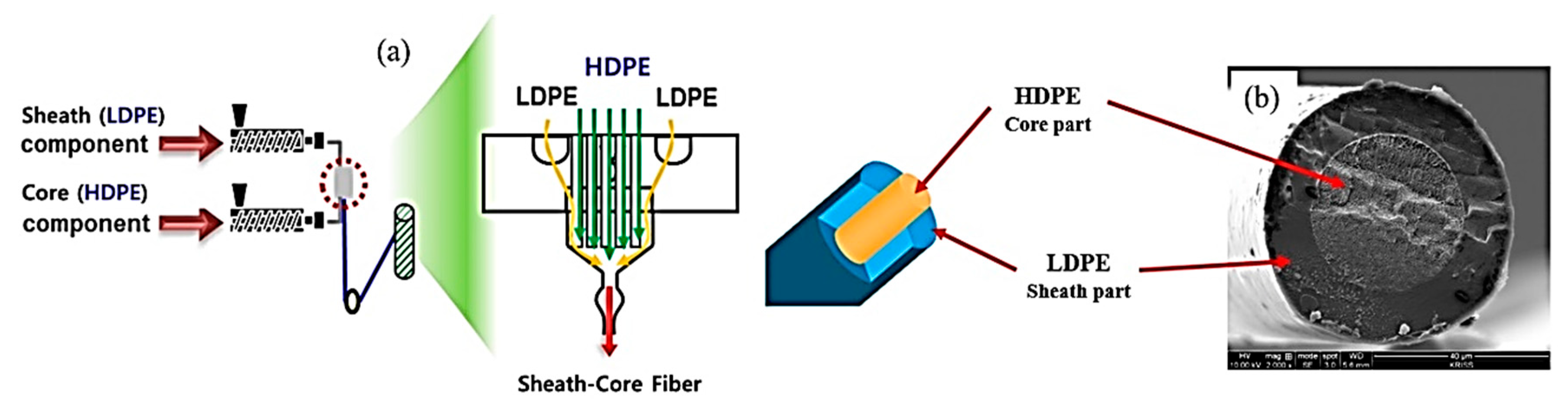
2.2. Preparation of Sheath-Core Fibers
2.3. Cross-Linking, Carbonization, and Activation of Sheath-Core Fibers
2.4. Characterization of Sheath-Core Fibers
3. Results and Discussion
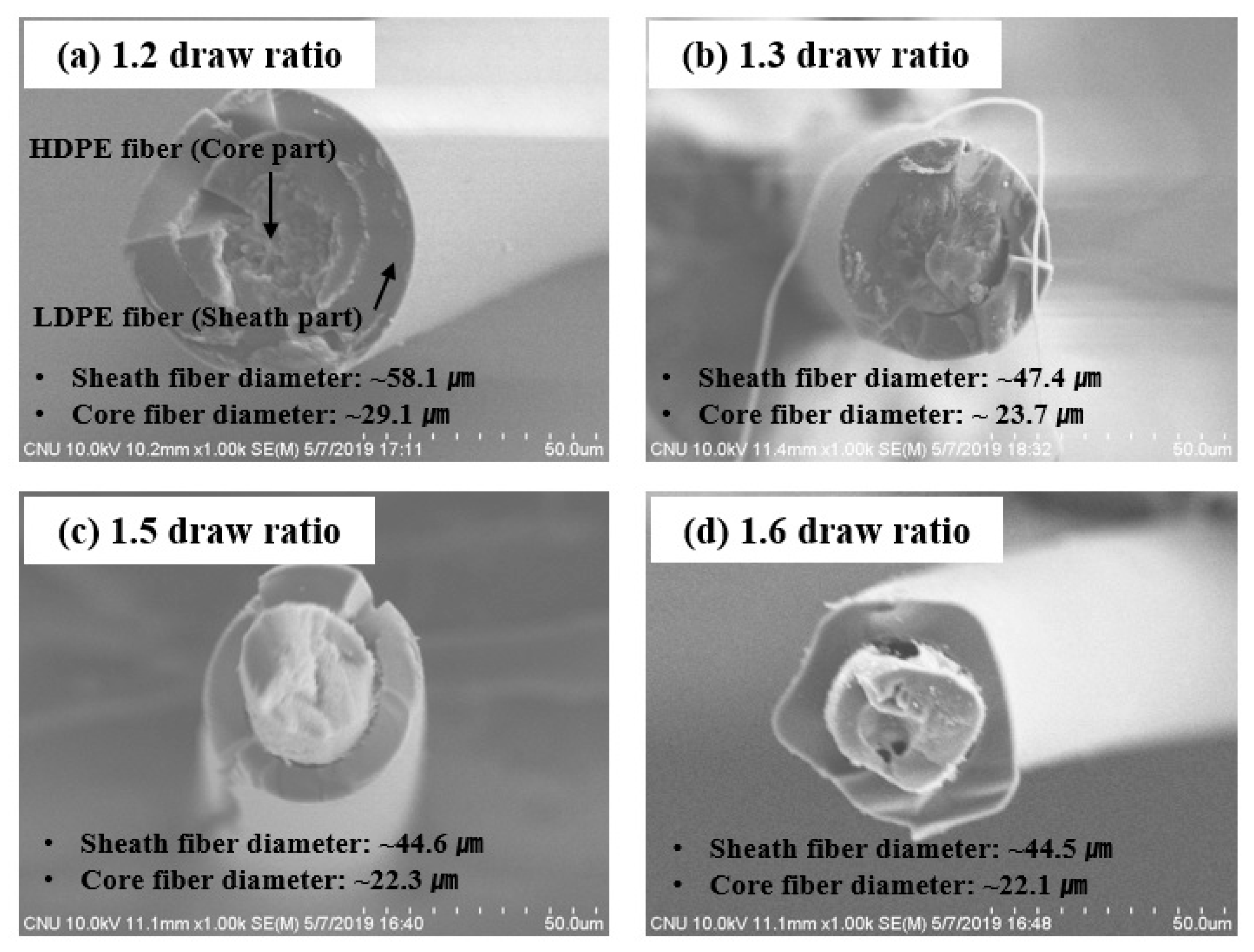
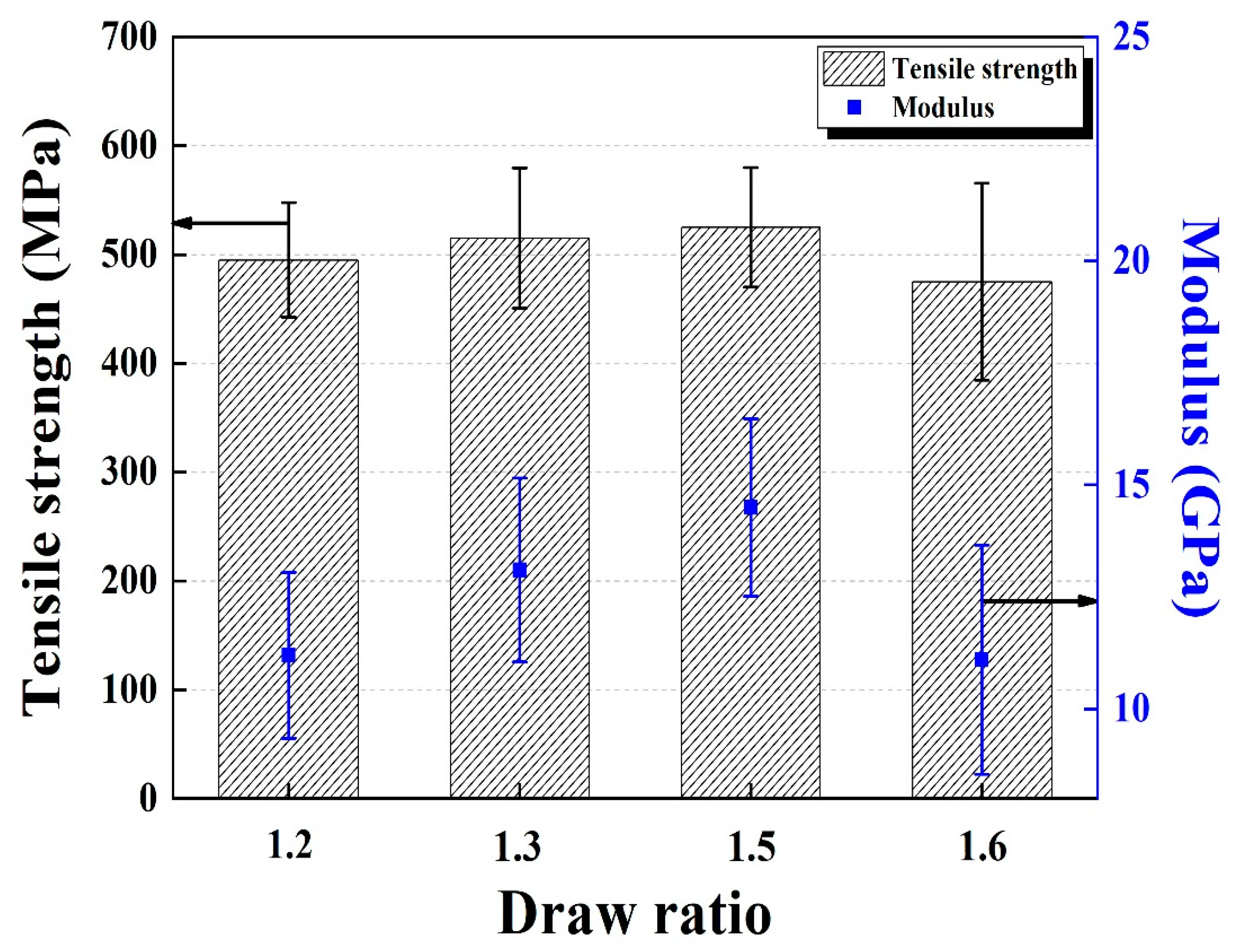
3.1. Characterization of LDPE/HDPE Sheath-Core Fibers
3.2. Characterization of Cross-Linked LDPE/HDPE Sheath-Core Fibers
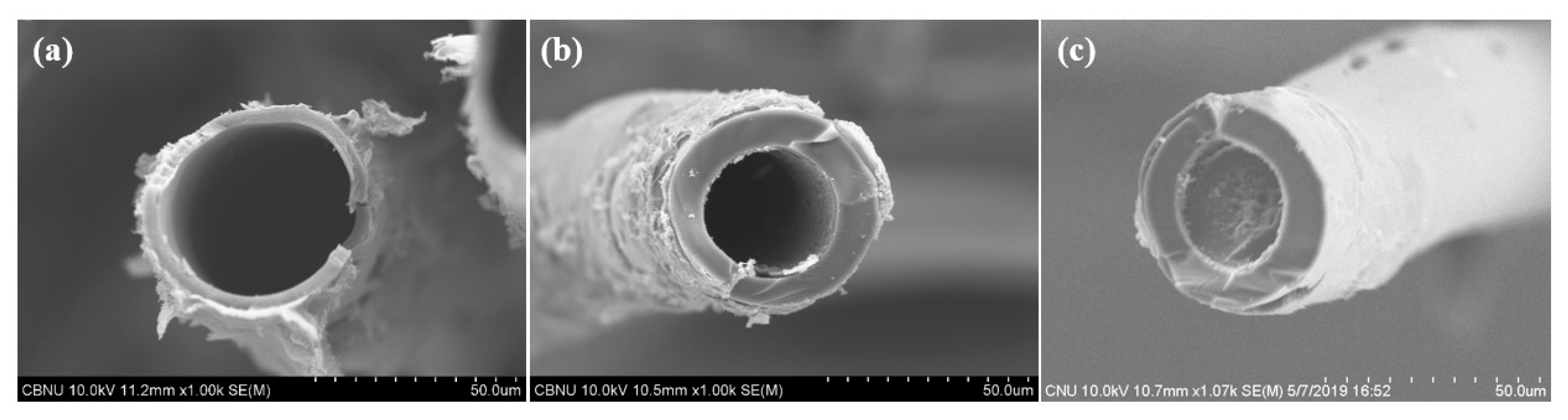
3.3. Characterization of Carbonized and Activated LDPE/HDPE Sheath-Core Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bagheri, S.; Julkapli, N.M. Effect of hybridization on the value-added activated carbon material. Int. J. Ind. Chem. 2016, 7, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Mochida, I.; Korai, Y.; Shirahama, M.; Kawano, S.; Hada, T.; Seo, Y.; Yoshikawa, M.; Yasutake, A. Removal of SOx and NOx over activated carbon fibers. Carbon 2000, 38, 227–239. [Google Scholar] [CrossRef]
- Amoros, D.C.; Monge, J.A.; Solano, A.L. Characterization of Activated Carbon Fibers by CO2 Adsorption. Langmuir 1996, 12, 2820–2824. [Google Scholar] [CrossRef]
- Mangun, C.L.; Benak, K.R.; Economy, J.; Foster, K.L. Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 2001, 39, 1809–1820. [Google Scholar] [CrossRef]
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon fibers: Precursors, manufacturing, and properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Sedghi, A.; Farsani, R.E.; Shokuhfar, A. The effect of commercial polyacrylonitrile fibers characterizations on the produced carbon fibers properties. J. Mater. Process. Technol. 2008, 198, 60–67. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Sporl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Jain, M.K.; Abhiraman, A.S. Conversion of acrylonitrile-based precursor fibres to carbon fibres. J. Mater. Sci. 1987, 22, 278–300. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Liu, L.; Dong, Y.; Liu, A. Mesophase pitch-based carbon fiber spinning through a filter assembly and the microstructure evolution mechanism. J. Mater. Sci. 2014, 49, 191–198. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lee, C.Y.; Lee, H.C. Surface chemistry of polyacrylonitrile- and rayon-based activated carbon fibers after post-heat treatment. Mater. Chem. Phys. 2007, 101, 199–210. [Google Scholar] [CrossRef]
- Holmes, M. Lowering the cost of carbon fiber. Reinf. Plast. 2017, 61, 279–283. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Zhang, J. Surface treatment of LLDPE and LDPE blends by nitric acid, sulfuric acid, and chromic acid etching. Colloid Polym. Sci. 2009, 287, 541–548. [Google Scholar] [CrossRef]
- Kaneko, M.; Kumagai, S.; Nakamura, T.; Sato, H. Study of sulfonation mechanism of low-density polyethylene films with fuming sulfuric acid. J. Appl. Polym. Sci. 2004, 91, 2435–2442. [Google Scholar] [CrossRef]
- Younker, J.M.; Saito, T.; Hunt, M.A.; Naskar, A.K.; Beste, A. Pyrolysis pathways of sulfonated polyethylene, an alternative carbon fiber precursor. J. Am. Chem. Soc. 2013, 135, 6130–6141. [Google Scholar] [CrossRef]
- Hunt, M.A.; Saito, T.; Brown, R.H.; Kumbhar, A.S.; Naskar, A.K. Patterned functional carbon fibers from polyethylene. Adv. Mater. 2012, 24, 2386–2389. [Google Scholar] [CrossRef]
- Jin, Z.; Yan, X.; Yu, Y.; Zhao, G. Sustainable activated carbon fibers from liquefied wood with controllable porosity for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 11706–11715. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J. Preparation of carbon fibers from linear low density polyethylene. Carbon 2015, 94, 524–530. [Google Scholar] [CrossRef]
- Meola, C.; Carlomagno, G. Cross-linked polyethylene. Encycl. Chem. Process. 2006, 1, 577–588. [Google Scholar]
- Malaika, S.A.; Riasat, S.; Lewucha, C. Reactive antioxidants for peroxide crosslinked polyethylene. Polym. Degrad. Stab. 2017, 145, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Ciuprina, F.; Teissedre, G.; Filippini, J. Polyethylene crosslinking and water treeing. Polymer 2001, 42, 7841–7846. [Google Scholar] [CrossRef]
- Li, C.; Zhu, H.; Salim, N.; Fox, B.; Hameed, N. Preparation of microporous carbon materials via in-depth sulfonation and stabilization of polyethylene. Polym. Degrad. Stab. 2016, 134, 272–283. [Google Scholar] [CrossRef]
- Postema, A.R.; Groot, H.D.; Pennings, A.J. Amorphous carbon fibres from linear low density polyethylene. J. Mater. Sci. 1990, 25, 4216–4222. [Google Scholar] [CrossRef]
- Zhang, D.; Bhat, G.S. Carbon fibers from polyethylene-based precursors. Mater. Manuf. Process. 1994, 9, 221–235. [Google Scholar] [CrossRef]
- Zhang, D. Carbon fibers from oriented polyethylene precursors. J. Thermoplast. Compos. Mater. 1993, 6, 38–48. [Google Scholar] [CrossRef]
- Penning, J.P.; Lagcher, R.; Pennings, A.J. The effect of diameter on the mechanical properties of amorphous carbon fibres from linear low density polyethylene. Polym. Bull. 1991, 25, 405–412. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Q. Structure and properties development during the conversion of polyethylene precursors to carbon fibers. J. Appl. Polym. Sci. 1996, 62, 367–373. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M. Mechanical properties of activated carbon fibers. In Activated Carbon Fiber and Textiles; Chen, J.Y., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2017; pp. 167–179. [Google Scholar]
- Karacan, I.; Benli, H. Use of sulfonation procedure for the development of thermally stabilized isotactic polypropylene fibers prior to carbonization. J. Appl. Polym. Sci. 2012, 123, 234–245. [Google Scholar] [CrossRef]









| Temperature (°C) | 120, 130, 140, 150 |
|---|---|
| Time (Min) | 60, 90, 120, 150 |
| Loading (MPa) | 0.25 |
| Crosslinking Time (min) | 0 | 60 | 90 | 120 | 150 | |
|---|---|---|---|---|---|---|
| Crosslink percentage (%) | HDPE | 0 | 43.9 | 67.95 | 100 | 100 |
| LDPE | 0 | 91.88 | 100 | 100 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, J.S.; Lee, H.R.; Lee, M.J.; Jeon, M.H.; Lee, S.G.; Joo, Y.L. Fabrication of Activated Carbon Fibers with Sheath-Core, Hollow, or Porous Structures via Conjugated Melt Spinning of Polyethylene Precursor. Polymers 2020, 12, 2895. https://doi.org/10.3390/polym12122895
Won JS, Lee HR, Lee MJ, Jeon MH, Lee SG, Joo YL. Fabrication of Activated Carbon Fibers with Sheath-Core, Hollow, or Porous Structures via Conjugated Melt Spinning of Polyethylene Precursor. Polymers. 2020; 12(12):2895. https://doi.org/10.3390/polym12122895
Chicago/Turabian StyleWon, Jong Sung, Ha Ram Lee, Min Jun Lee, Min Hong Jeon, Seung Goo Lee, and Yong Lak Joo. 2020. "Fabrication of Activated Carbon Fibers with Sheath-Core, Hollow, or Porous Structures via Conjugated Melt Spinning of Polyethylene Precursor" Polymers 12, no. 12: 2895. https://doi.org/10.3390/polym12122895
APA StyleWon, J. S., Lee, H. R., Lee, M. J., Jeon, M. H., Lee, S. G., & Joo, Y. L. (2020). Fabrication of Activated Carbon Fibers with Sheath-Core, Hollow, or Porous Structures via Conjugated Melt Spinning of Polyethylene Precursor. Polymers, 12(12), 2895. https://doi.org/10.3390/polym12122895









