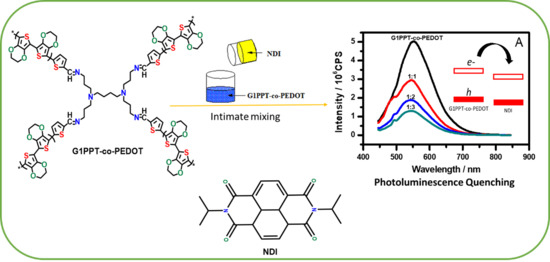
Photoluminescence Quenching of a Novel Electroconductive Poly(propylene thiophenoimine)-co-Poly(ethylenedioxy thiophene) Star Copolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthetic Routes
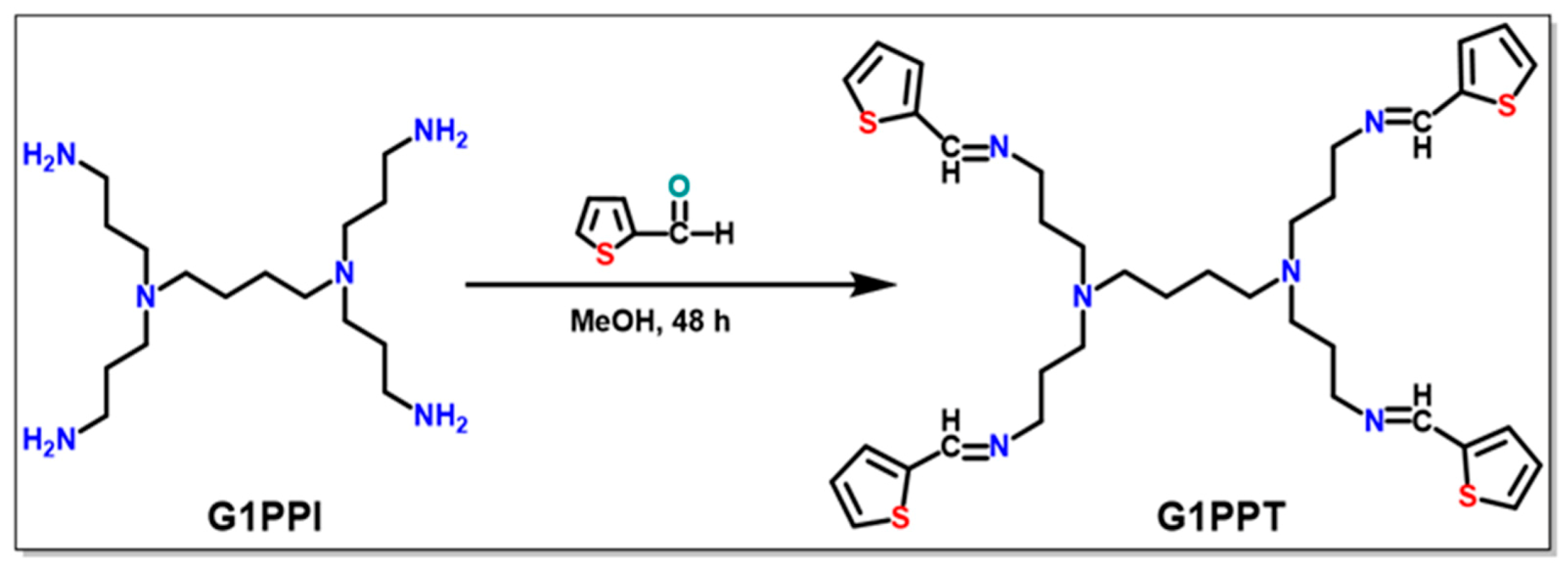
2.2.1. Generation 1 Poly(Propylene Imine) Tetramine (G1PPI) Functionalization
2.2.2. Preparation of G1PPT-co-PEDOT Star Copolymer
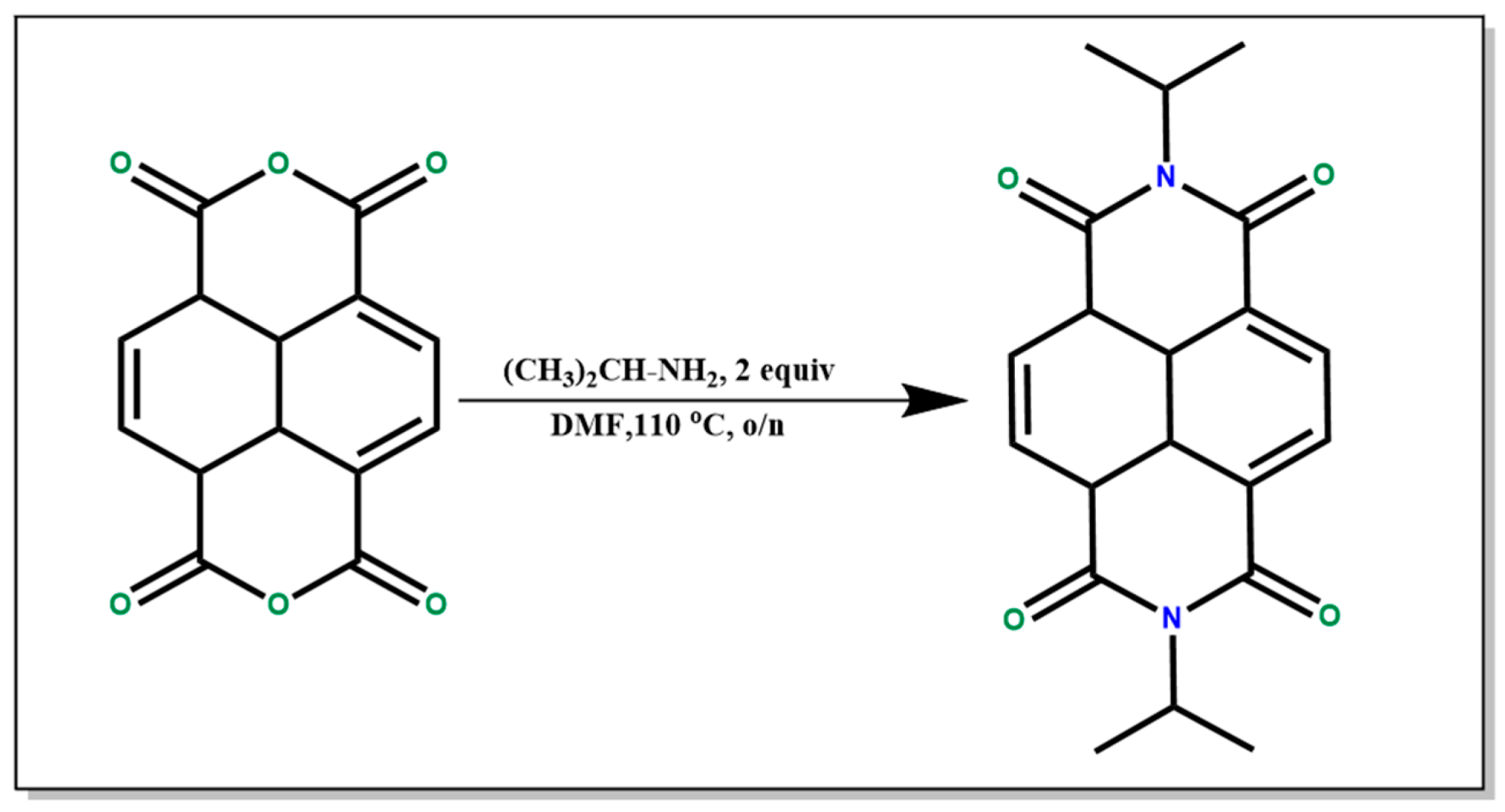
2.2.3. Synthesis of N,N′-diisopropyl naphthalene diimide (NDI)
2.3. Instrumentation
2.4. Preparation of the Solution Blends, G1PPT-co-PEDOT:NDI
3. Results and Discussion
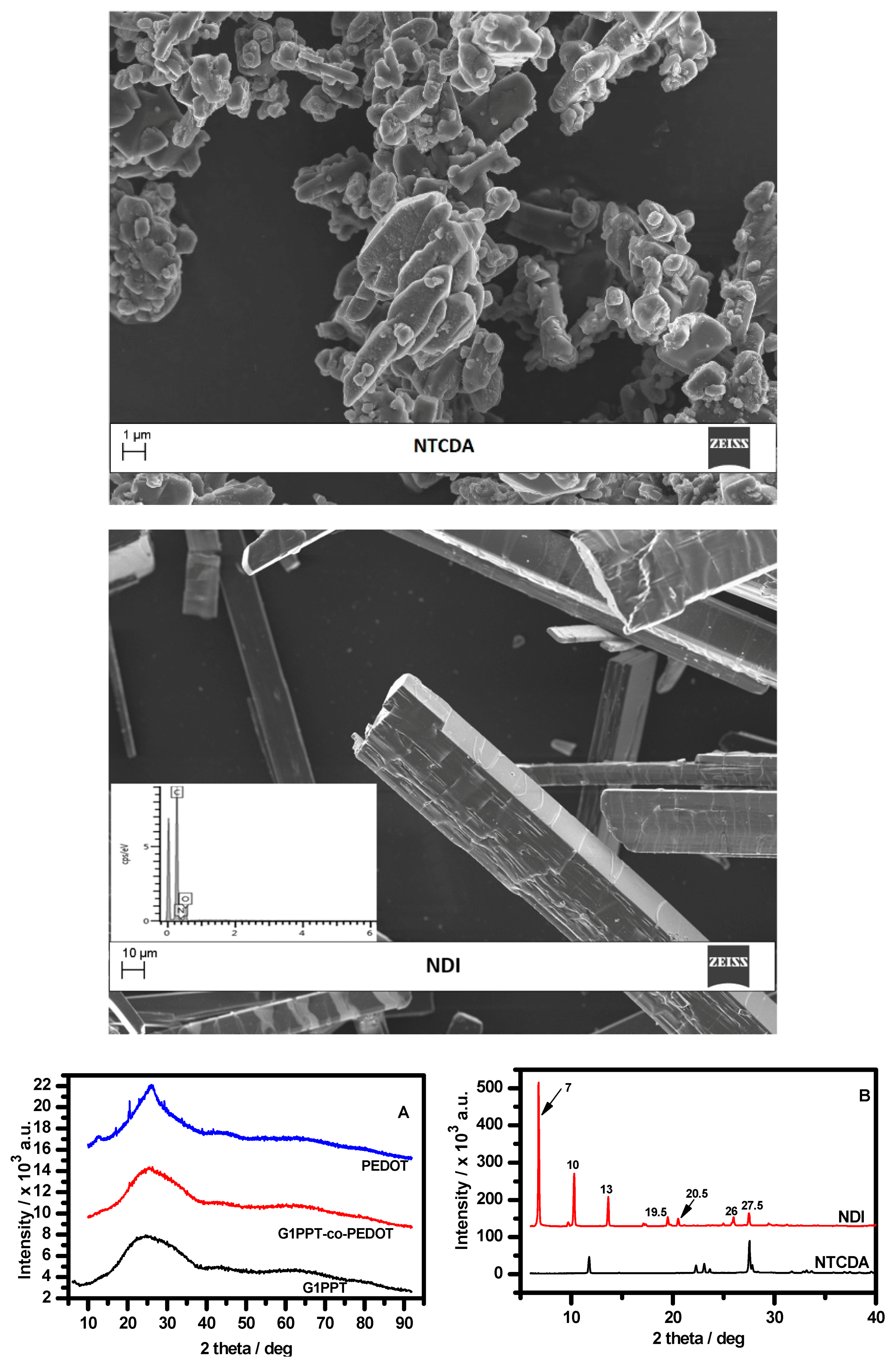
3.1. Spectroscopic and Morphological Sstudies
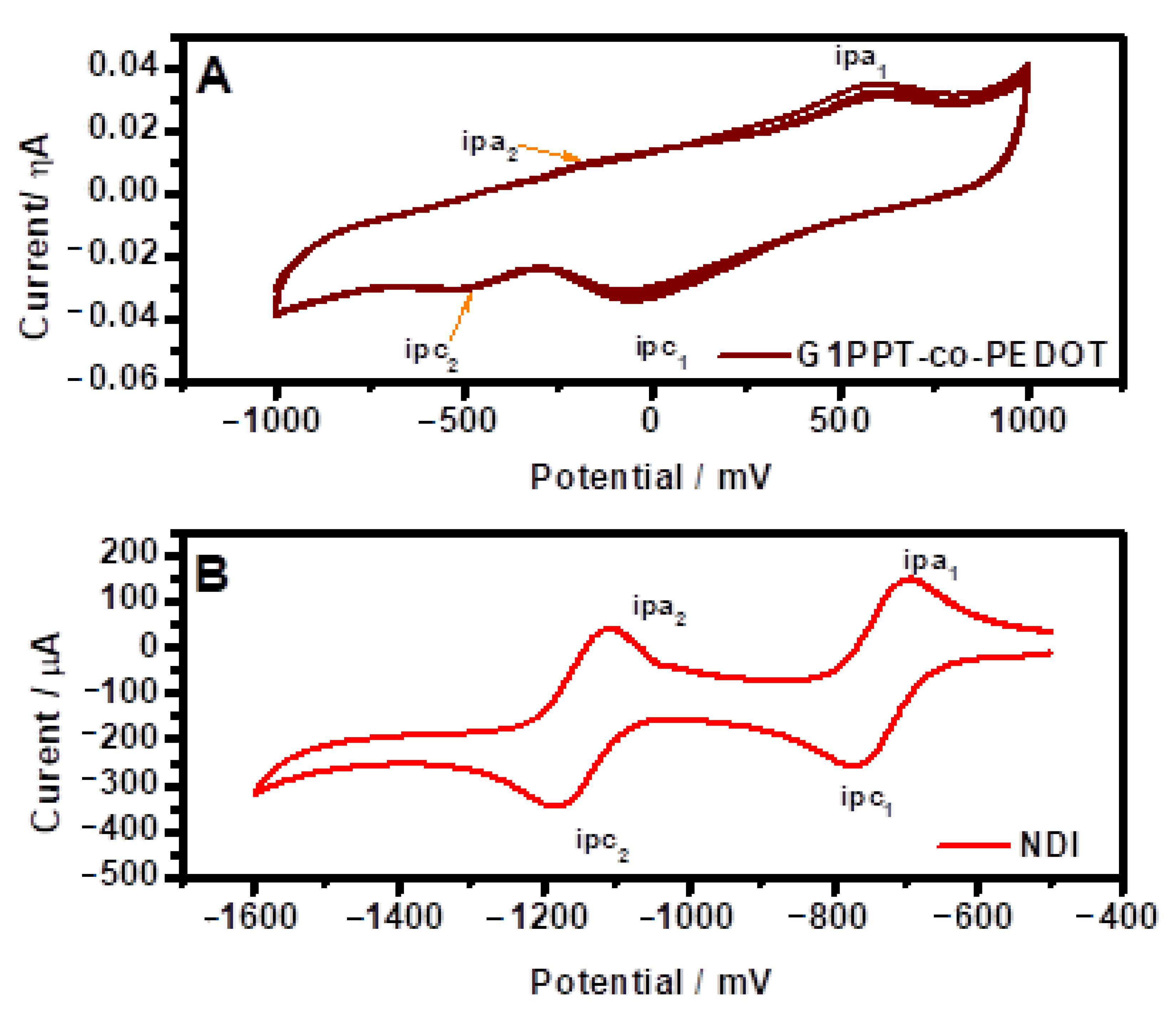
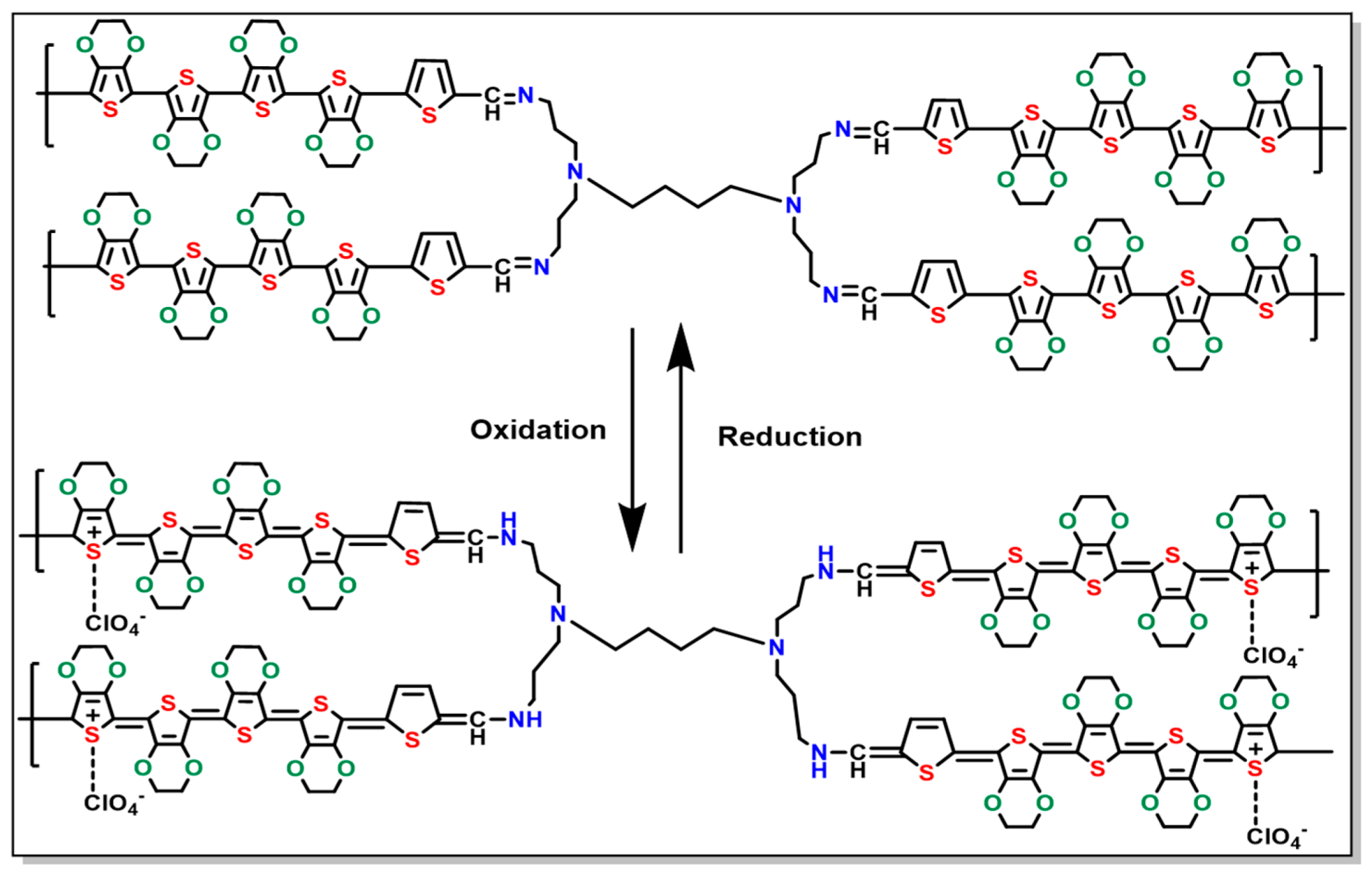
3.2. Electrochemical Studies
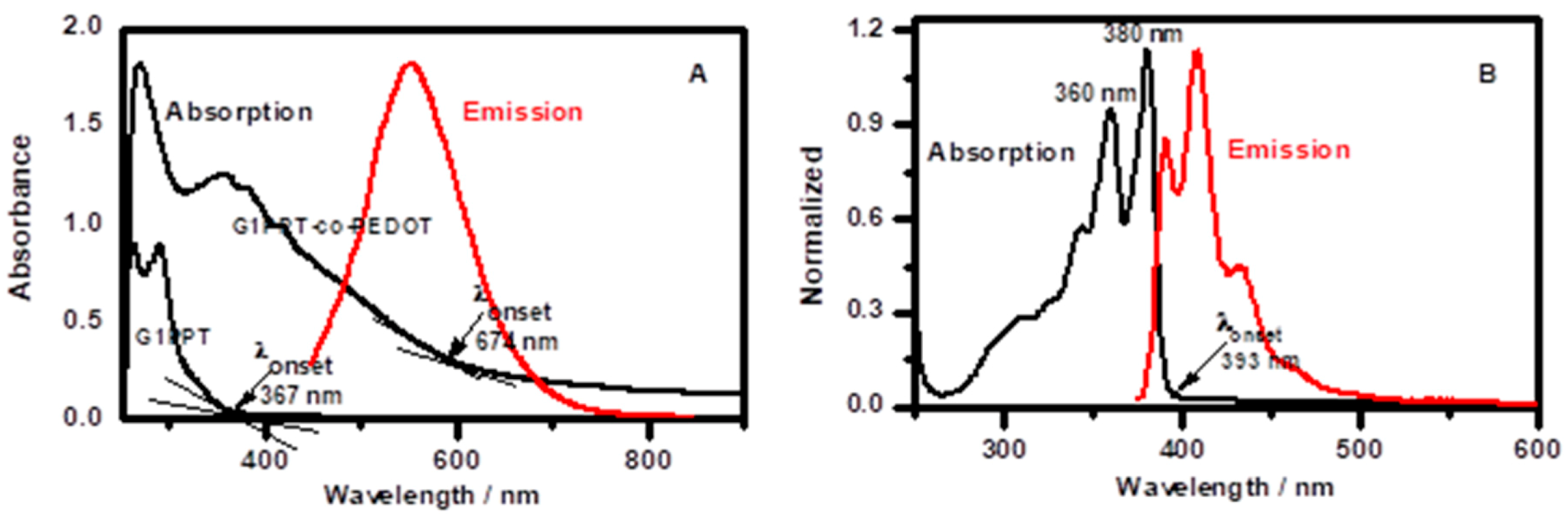
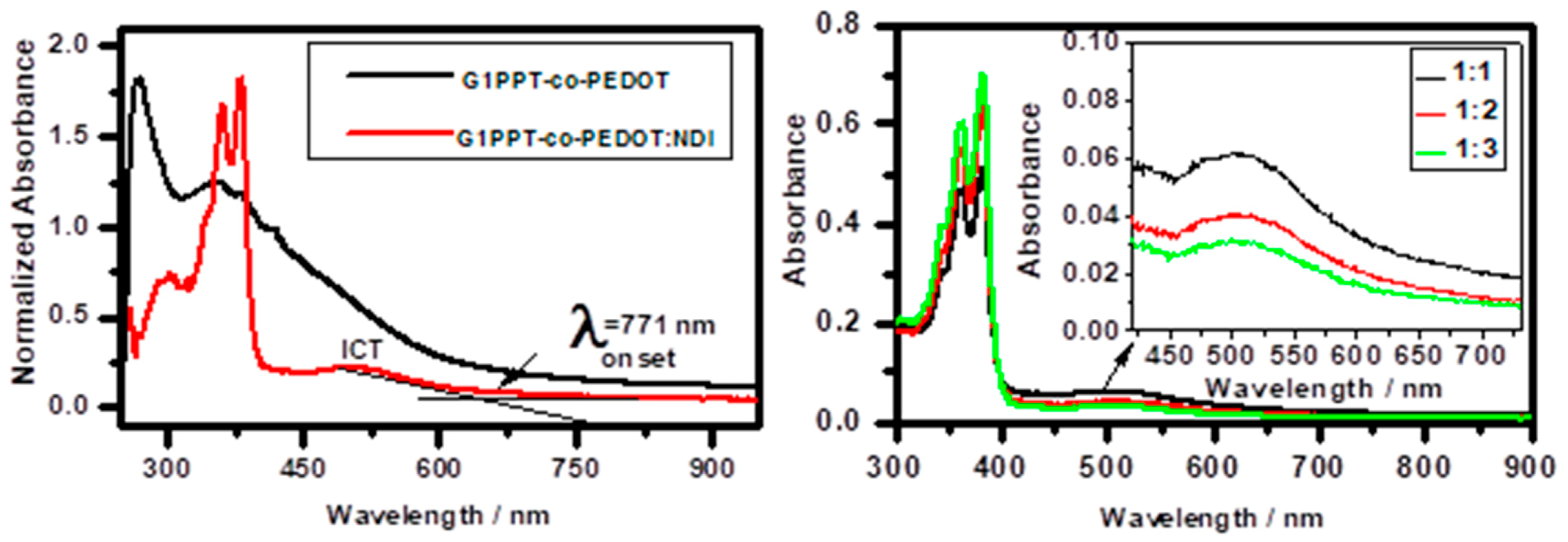
3.3. Optical and Photophysical Analyses of G1PPT-co-PEDOT, NDI, and Their Blends
3.4. Photoluminescence Quenching
4. Conclusios
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Terao, J. Synthesis of conjugated polyrotaxanes and its application to molecular wires. In Molecular Architectonics; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 487–512. [Google Scholar]
- Liao, K.S.; Yambem, S.D.; Haldar, A.; Alley, N.J.; Curran, S.A. Designs and Architectures for the Next Generation of Organic Solar Cells. Energies 2010, 3, 1212–1250. [Google Scholar] [CrossRef]
- Gómez, R.; Segura, J.L. Handbook of Organic Electronics and Photonics; American Scientific Pub: New Orleans, LA, USA, 2007; Volume 2, pp. 109–147. [Google Scholar]
- Shinar, R.; Shinar, J. Organic Electronics in Sensors and Biotechnology; McGraw-Hill: New York, NY, USA, 2009; pp. 419–425. [Google Scholar]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Pal, S.; Roy, D.; Mondal, M.K.; Chowdhury, P. Synthesis of water-soluble conjugated polymer, poly(N-3-sulfopropylaniline) and the study of its glucose sensing property. J. Polym. Res. 2019, 26, 1–9. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Vanlaeke, P.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; Manca, J.; Heremans, P.; Poortmans, J. Polythiophene based bulk heterojunction solar cells: Morphology and its implications. Thin Solid Films 2006, 511–512, 358–361. [Google Scholar] [CrossRef]
- Deckers, S.; Steverlynck, J.; Willot, P.; Vandendriessche, S.; Koeckelberghs, G.; Asselberghs, I.; Verbiest, T.; Van der Veen, M.A. Regioregularity Increases Second-Order Nonlinear Optical Response of Polythiophenes in Solution. J. Phys. Chem. C 2015, 119, 18513–18517. [Google Scholar]
- Iovu, M.C.; Sheina, E.E.; Gil, R.R.; McCullough, R.D. Experimental evidence for the quasi-“living” nature of the grignard metathesis method for the synthesis of regioregular poly(S-alkylthiophenes). Macromolecules 2005, 38, 8649–8656. [Google Scholar] [CrossRef]
- Espinosa-Roa, A.; De Jesús Cruz-Carrillo, M.; Ledesma-Juárez, A.; Del Angel, A.M.; Romero-Borja, D.; Güizado-Rodríguez, M.; Rodríguez, M.; Galindo, R.; Maldonado, J.L.; Barba, V. Synthesis of polyfluorenes by oxidative polymerization, their characterization and implementation in organic solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 2716–2725. [Google Scholar] [CrossRef]
- Ando, D.; Ijichi, J.; Uno, T.; Itoh, T.; Kubo, M. Preparation of donor–acceptor polyfluorenes with pendant carboxyl or amine functionalities and their photoluminescence properties. Polym. Bull. 2019, 76, 6137–6151. [Google Scholar] [CrossRef]
- Akcelrud, L. Electroluminescent polymers. Prog. Polym. Sci. 2003, 28, 875–962. [Google Scholar]
- Moliton, A.; Hiorns, R.C. Review of electronic and optical properties of semiconducting π-conjugated polymers: Applications in optoelectronics. Polym. Int. 2004, 53, 1397–1412. [Google Scholar] [CrossRef]
- Kopidakis, N.; Mitchell, W.J.; Bozell, J.J.; Piris, J.; Ginley, D.S.; Rumbles, G.; Shaheen, S.E. Bulk Heterojunction Organic Photovoltaic Devices Using Dendrimers. Sol. Energy Technol. 2005, 1–2. Available online: http://www.nrel.gov/docs/fy06osti/39051.pdf (accessed on 4 August 2020).
- Lo, S.C.; Burn, P.L. Development of dendrimers: Macromolecules for use in organic light-emitting diodes and solar cells. Chem. Rev. 2007, 107, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.L.; Mastalerz, M.; Ma, C.Q.; Wienk, M.; Janssen, R.; Bäuerle, P. Synthesis and photovoltaic performance of pyrazinoquinoxaline containing conjugated thiophene-based dendrimers and polymers. Macromolecules 2013, 46, 2141–2151. [Google Scholar] [CrossRef]
- Roncali, J. Molecular engineering of the band gap of π-conjugated systems: Facing technological applications. Macromol. Rapid Commun. 2007, 28, 1761–1775. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Forrest, S.R.; Thompson, M.E. Introduction: Organic electronics and optoelectronics. Chem. Rev. 2007, 107, 923–925. [Google Scholar] [CrossRef]
- Dayyani, N.; Ramazani, A.; Khoee, S.; Shafiee, A. Synthesis and Characterization of the First Generation of Polyamino-Ester Dendrimer-Grafted Magnetite Nanoparticles from 3-Aminopropyltriethoxysilane (APTES) via the Convergent Approach. Silicon 2018, 10, 595–601. [Google Scholar] [CrossRef]
- Mu, B.; Liu, T.; Tian, W. Long-Chain Hyperbranched Polymers: Synthesis, Properties, and Applications. Macromol. Rapid Commun. 2019, 40, 1970042. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Markham, J.P.; Namdas, E.B.; Samuel, I.D.; Lo, S.C.; Burn, P.L. Highly efficient single-layer dendrimer light-emitting diodes with balanced charge transport. Appl. Phys. Lett. 2003, 82, 4824–4826. [Google Scholar] [CrossRef]
- Kopidakis, N.; Mitchell, W.J.; Van De Lagemaat, J.; Ginley, D.S.; Rumbles, G.; Shaheen, S.E.; Rance, W.L. Bulk heterojunction organic photovoltaic devices based on phenyl-cored thiophene dendrimers. Appl. Phys. Lett. 2006, 89, 1–4. [Google Scholar] [CrossRef]
- Zeng, W.; Qi, Q.; Wu, J. Toward Long Rylene Ribbons and Quinoidal Rylene Diradicaloids. Eur. J. Org. Chem. 2018, 2018, 7–17. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Jani, C.H.; Langford, S.J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Jiang, W.; Hou, J.; Wang, Z. New developments in non-fullerene small molecule acceptors for polymer solar cells. Mater. Chem. Front. 2017, 1, 1291–1303. [Google Scholar] [CrossRef]
- Guo, X.; Kim, F.S.; Seger, M.J.; Jenekhe, S.A.; Watson, M.D. Naphthalene Diimide-Based Polymer Semiconductors: Synthesis, Structure—Property Correlations, and n-Channel and Ambipolar Field-Effect Transistors. Chem. Mater. 2012, 24, 1434–1442. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Tan, H.S.; Guo, Y.; Di, C.A.; Yu, G.; Liu, Y.; Lin, M.; Lim, S.H.; Zhou, Y.; et al. Stable solution-processed polymer semiconductor with record high-mobility for printed transistors. Sci. Rep. 2012, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shejul, D.A.; Wagalgave, S.M.; Jadhav, R.W.; Al Kobaisi, M.; La, D.D.; Jones, L.A.; Bhosale, R.S.; Bhosale, S.V.; Bhosale, S.V. Aggregation-induced emission characteristics and solvent triggered hierarchical self-assembled chiral superstructures of naphthalenediimide amphiphiles. New J. Chem. 2020, 44, 1615–1623. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Iwashita, Y.; Shirakawa, M.; Kawano, S.I.; Fujita, N.; Shinkai, S. Spontaneous Colorimetric Sensing of the Positional Isomers of Dihydroxynaphthalene in a 1D Organogel Matrix. Angew. Chem. Int. Ed. 2006, 45, 1592–1595. [Google Scholar] [CrossRef]
- Schubert, M.; Dolfen, D.; Frisch, J.; Roland, S.; Steyrleuthner, R.; Stiller, B.; Chen, Z.; Scherf, U.; Koch, N.; Facchetti, A.; et al. Influence of Aggregation on the Performance of All-Polymer Solar Cells Containing Low-Bandgap Naphthalenediimide Copolymers. Adv. Energy Mater. 2012, 2, 369–380. [Google Scholar] [CrossRef]
- Stewart, W.W. Lucifer dyes—highly fluorescent dyes for biological tracing. Nature 1981, 292, 17–21. [Google Scholar] [CrossRef]
- Erten, Ş.; Posokhov, Y.; Alp, S.; İçli, S. The study of the solubility of naphthalene diimides with various bulky flanking substituents in different solvents by UV-vis spectroscopy. Dye Pigment. 2005, 64, 171–178. [Google Scholar] [CrossRef]
- Fallon, G.D.; Langford, S.J.; Lee, M.P. N,N’-Bis(2-carboxyethyl)-1,4,5,8-naphthalenetetra- carboxylic diimine dimethylformamide disolvate. Acta Crystallogr. Sect. E Struct. 2004, E60, o542–o543. [Google Scholar] [CrossRef]
- Hansen, J.G.; Feeder, N.; Hamilton, D.G.; Gunter, M.J.; Becher, J.; Sanders, J.K.M. Macrocyclization and Molecular Interlocking via Mitsunobu Alkylation: Highlighting the Role of C−H···O Interactions in Templating. Org. Lett. 2000, 2, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, J.; Xiao, Y.; Li, Z.; Qian, X. Core-Perfluoroalkylated Perylene Diimides and Naphthalene Diimides: Versatile Synthesis, Solubility, Electrochemistry, and Optical Properties. J. Org. Chem. 2010, 75, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, B.; Tajima, K.; Nakano, M.; Takimiya, K.; Tang, A.; Zhou, E. Comparison among Perylene Diimide (PDI), Naphthalene Diimide (NDI), and Naphthodithiophene Diimide (NDTI) Based n-Type Polymers for All-Polymer Solar Cells Application. Macromolecules 2017, 50, 3179–3185. [Google Scholar] [CrossRef]
- Ahmed, E.; Ren, G.; Kim, F.S.; Hollenbeck, E.C.; Jenekhe, S.A. Design of New Electron Acceptor Materials for Organic Photovoltaics: Synthesis, Electron Transport, Photophysics, and Photovoltaic Properties of Oligothiophene-Functionalized Naphthalene Diimides. Chem. Mater. 2011, 23, 4563–4577. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.H.; Yu, H.; Lee, C.; Kang, H.; Song, I.; Kim, Y.; Oh, J.H.; Kim, B.J. Importance of Electron Transport Ability in Naphthalene Diimide-Based Polymer Acceptors for High-Performance, Additive-Free, All-Polymer Solar Cells. Chem. Mater. 2015, 27, 5230–5237. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Earmme, T.; Courtright, B.A.; Eberle, F.N.; Jenekhe, S.A. n-Type Semiconducting Naphthalene Diimide-Perylene Diimide Copolymers: Controlling Crystallinity, Blend Morphology, and Compatibility Toward High-Performance All-Polymer Solar Cells. J. Am. Chem. Soc. 2015, 137, 4424–4434. [Google Scholar] [CrossRef]
- Olowu, R.A.; Williams, A.; Ndangili, P.M.; Ngece, R.F.; Mailu, S.N.; Baker, P.; Iwuoha, E. Impedimetry and microscopy of electrosynthetic poly (propylene thiophenoimine)-co-poly(3,4 ethylene dioxythiophene) dendritic star copolymer. J. Electrochem. Sci. 2011, 6, 1855–1870. [Google Scholar]
- Ali, M.A.; Kim, H.H.; Lee, C.Y.; Soh, H.S.; Lee, J.G. Effects of the FeCl3 concentration on the polymerization of conductive poly(3,4-ethylenedioxythiophene) thin films on (3-aminopropyl) trimethoxysilane monolayer-coated SiO2 surfaces. Met. Mater. Int. 2009, 15, 977–981. [Google Scholar] [CrossRef]
- Makelane, H.R.; Tovide, O.; Sunday, C.E.; Waryo, T.; Iwuoha, E.I. Electrochemical interrogation of G3-poly(propylene thiophenoimine) dendritic star polymer in phenanthrene sensing. Sensors 2015, 15, 22343–22363. [Google Scholar] [CrossRef]
- Ganesamoorthy, R.; Sathiyan, G.; Thangamuthu, R.; Sakthivel, P. Synthesis and characterization of bay substituted perylene diimide small molecule for organic solar cell application. In Recent Trends in Materials Science and Applications; Springer: Cham, Switzerland, 2017; Volume 189, pp. 401–415. [Google Scholar]
- Abd Almonam, A.B.; Jahed, N.M.; Arotiba, O.A.; Mailu, S.N.; Hendricks, N.R.; Baker, P.G.; Iwuoha, E.I. Synthesis and characterization of poly(propylene imine) dendrimer—Polypyrrole conducting star copolymer. J. Electroanal. Chem. 2011, 652, 18–25. [Google Scholar]
- Olowu, R.A.; Ndangili, P.M.; Baleg, A.A.; Ikpo, C.O.; Njomo, N.; Baker, P.; Iwuoha, E. Spectroelectrochemical dynamics of dendritic poly (propylene imine)-polythiophene star copolymer aptameric 17β-estradiol biosensor. Int. J. Electrochem. Sci. 2011, 6, 1686–1708. [Google Scholar]
- Ndipingwi, M.M.; Ikpo, C.O.; Hlongwa, N.W.; Myalo, Z.; Ross, N.; Masikini, M.; John, S.V.; Baker, P.G.; Roos, W.D.; Iwuoha, E.I. Orthorhombic Nanostructured Li2MnSiO4 /Al2O3 Supercapattery Electrode with Efficient Lithium-Ion Migratory Pathway. Batter. Supercaps 2018, 1, 223–235. [Google Scholar] [CrossRef]
- Katz, H.E.; Lovinger, A.J.; Johnson, J.; Kloc, C.; Siegrist, T.; Li, W.; Lin, Y.Y.; Dodabalapur, A. A soluble and air-stable organic semiconductor with high electron mobility. Nature 2000, 404, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Şahin, Z.; Meunier-Prest, R.; Dumoulin, F.; Işci, U.; Bouvet, M. Alkylthio-tetrasubstituted μ-Nitrido Diiron Phthalocyanines: Spectroelectrochemistry, Electrical Properties, and Heterojunctions for Ammonia Sensing. Inorg. Chem. 2020, 59, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.J.; Lee, K.; Sun, J.; Andreou, A.G.; Katz, H.E. Air-operable, high-mobility organic transistors with semifluorinated side chains and unsubstituted naphthalenetetracarboxylic diimide cores: High mobility and environmental and bias stress stability from the perfluorooctylpropyl side chain. Adv. Funct. Mater. 2010, 20, 2930–2944. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Stoneking, S.; You, W. A weak donor-strong acceptor strategy to design ideal polymers for organic solar cells. ACS Appl. Mater. Interfaces 2010, 2, 1377–1383. [Google Scholar] [CrossRef]
- Hohnholz, D.; MacDiarmid, A.G.; Sarno, D.M.; Jones, W.E. Uniform thin films of poly-3,4-ethylenedioxythiophene (PEDOT) prepared by in-situ deposition. Chem. Commun. 2001, 23, 2444–2445. [Google Scholar] [CrossRef]
- Boudreault, P.L.T.; Najari, A.; Leclerc, M. Processable low-bandgap polymers for photovoltaic applications. Chem. Mater. 2011, 23, 456–469. [Google Scholar] [CrossRef]
- Bundgaard, E.; Krebs, F.C. Low band gap polymers for organic photovoltaics. Sol. Energy Mater. Sol. Cells 2007, 91, 954–985. [Google Scholar] [CrossRef]
- Jones, A.; Facchetti, A.; Marks, T.J.; Wasielewski, M.R. Tuning orbital energetics in arylene diimide semiconductors. Materials design for ambient stability of n-type charge transport. Chem. Mater. 2007, 19, 2703–2705. [Google Scholar] [CrossRef]
- Kminek, I.; Vyprachticky, D.; Kriz, J.; Dybal, J.; Cimrova, V. Low-band gap Copolymers Containing Thienothiadazole Units: Synthesis, Optical, and Electrochemical properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2743–2756. [Google Scholar]
- Cimrová, V.; Výprachtický, D.; Pokorná, V.; Babičová, P. Donor-acceptor copolymers with 1,7-regioisomers of: N, N ’-dialkylperylene-3,4,9,10-tetracarboxydiimide as materials for photonics. J. Mater. Chem. C 2019, 7, 14678–14692. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hammed, W.A.; Yahyah, R.; Mahmud, H.N.M.E. Optoelectrical and photoluminescence quenching properties of poly(N-vinyl carbazole)-polypyrrole/reduced graphene oxide nanocomposites. Synth. Met. 2017, 226, 188–194. [Google Scholar] [CrossRef]
- Kabongo, G.L.; Mbule, P.S.; Mhlongo, G.H.; Mothudi, B.M.; Hillie, K.T.; Dhlamini, M.S. Photoluminescence Quenching and Enhanced Optical Conductivity of P3HT-Derived Ho3+-Doped ZnO Nanostructures. Nanoscale Res. Lett. 2016, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Sharma, S.K.; Roy, M.S.; Sharma, G.D. Photocurrent mechanism and photovoltaic properties of photo-electrochemical device based on PPAT and PPAT:TY blend. Synth. Met. 2009, 159, 52–61. [Google Scholar] [CrossRef]
- Zapunidy, S.A.; Martyanov, D.S.; Nechvolodova, E.M.; Tsikalova, M.V.; Novikov, Y.N.; Paraschuk, D.Y. Approaches to low-bandgap polymer solar cells: Using polymer charge-transfer complexes and fullerene metallocomplexes. Pure Appl. Chem. 2008, 80, 2151–2161. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, T.; Bai, H.; Lu, G.; Li, C.; Shi, G. Layer-by-layer deposited multilayer films of oligo(pyrenebutyric acid) and a perylene diimide derivative: Structure and photovoltaic. Langmuir 2008, 24, 4380–4387. [Google Scholar] [CrossRef]
- Dou, C.; Ding, Z.; Zhang, Z.; Xie, Z.; Liu, J.; Wang, L. Developing conjugated polymers with high electron affinity by replacing a c-c unit with a b←n unit. Angew. Chem. Int. Ed. 2015, 54, 3648–3652. [Google Scholar] [CrossRef]
- Hussain, S.A. An Introduction to Fluorescence Resonance Energy Transfer (FRET). Energy 2009, 132, 1–4. [Google Scholar]




| Materials | Wavenumber (cm−1) | Vibrational Mode |
|---|---|---|
| G1PPT-co-PEDOT | 687 | α-position C–H bending |
| 1212 | C–O stretching | |
| 1310, 1357 | C–C stretching | |
| 1495 | C=C stretching | |
| 1620 | C=N stretching | |
| 2842 | C–H stretching | |
| NDI | 1325 | C–N stretching |
| 1653 | C=C stretching | |
| 1698 | C=O stretching | |
| 2932, 2978 3084 | C–H stretching (isopropyl group) C–H stretching (aromatic) |
| Materials | (V) | (V) | E(HOMO) (eV) | E(LUMO) (eV) | (eV) |
|---|---|---|---|---|---|
| G1PPT-co-PEDOT | 0.87 | −0.69 | 5.3 | 3.7 | 1.6 |
| NDI | −0.50 | −0.62 | - | 3.78 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djoumessi Yonkeu, A.L.; Ndipingwi, M.M.; Ikpo, C.; Nwambaekwe, K.; Yussuf, S.; Tesfay, H.; Iwuoha, E. Photoluminescence Quenching of a Novel Electroconductive Poly(propylene thiophenoimine)-co-Poly(ethylenedioxy thiophene) Star Copolymer. Polymers 2020, 12, 2894. https://doi.org/10.3390/polym12122894
Djoumessi Yonkeu AL, Ndipingwi MM, Ikpo C, Nwambaekwe K, Yussuf S, Tesfay H, Iwuoha E. Photoluminescence Quenching of a Novel Electroconductive Poly(propylene thiophenoimine)-co-Poly(ethylenedioxy thiophene) Star Copolymer. Polymers. 2020; 12(12):2894. https://doi.org/10.3390/polym12122894
Chicago/Turabian StyleDjoumessi Yonkeu, Anne Lutgarde, Miranda Mengwi Ndipingwi, Chinwe Ikpo, Kelechi Nwambaekwe, Sodiq Yussuf, Hayelom Tesfay, and Emmanuel Iwuoha. 2020. "Photoluminescence Quenching of a Novel Electroconductive Poly(propylene thiophenoimine)-co-Poly(ethylenedioxy thiophene) Star Copolymer" Polymers 12, no. 12: 2894. https://doi.org/10.3390/polym12122894
APA StyleDjoumessi Yonkeu, A. L., Ndipingwi, M. M., Ikpo, C., Nwambaekwe, K., Yussuf, S., Tesfay, H., & Iwuoha, E. (2020). Photoluminescence Quenching of a Novel Electroconductive Poly(propylene thiophenoimine)-co-Poly(ethylenedioxy thiophene) Star Copolymer. Polymers, 12(12), 2894. https://doi.org/10.3390/polym12122894