FDM 3D Printed Composites for Bone Tissue Engineering Based on Plasticized Poly(3-hydroxybutyrate)/poly(d,l-lactide) Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Composites
2.2. Thermal Characterization
2.2.1. Differential Scanning Calorimetry
2.2.2. Thermogravimetric Analysis
2.3. Preparation of 3D Printing Filaments
2.4. 3D Printing and Printability Tests
2.5. Mechanical Testing
2.5.1. Tensile Test
2.5.2. Flexure Test
2.6. In Vitro Tests on Scaffolds
2.6.1. Metabolic Activity Assay
2.6.2. DNA Quantification
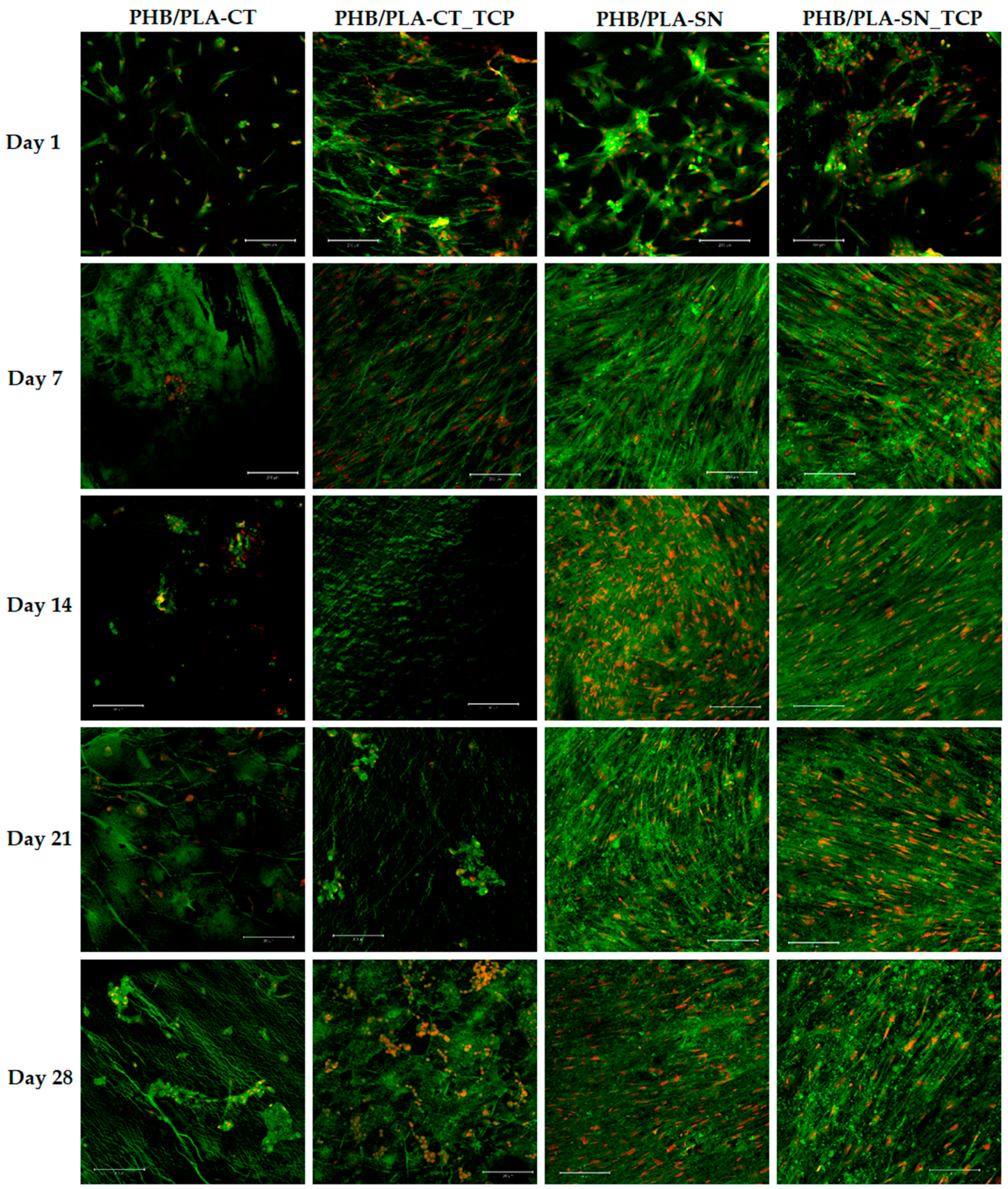
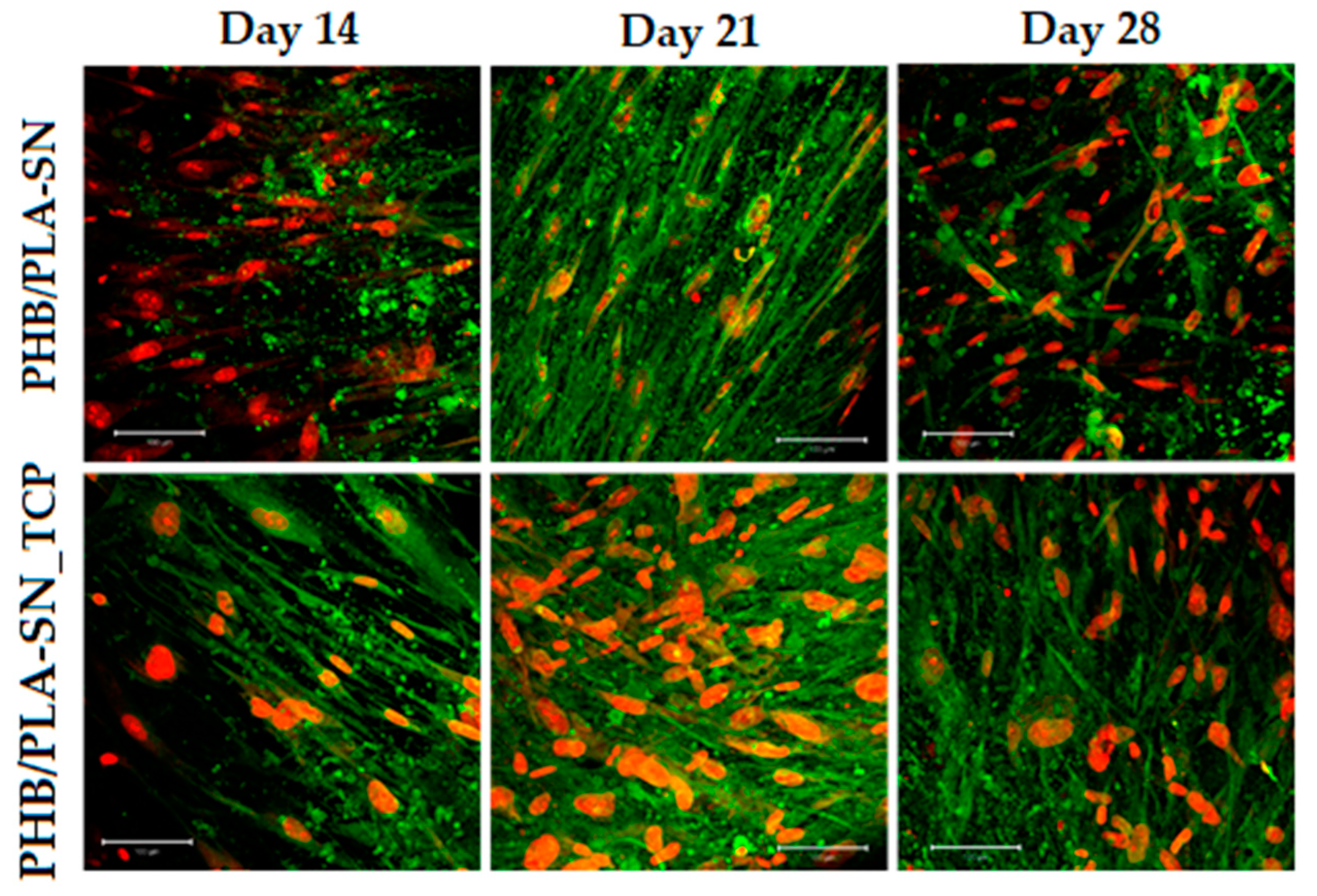
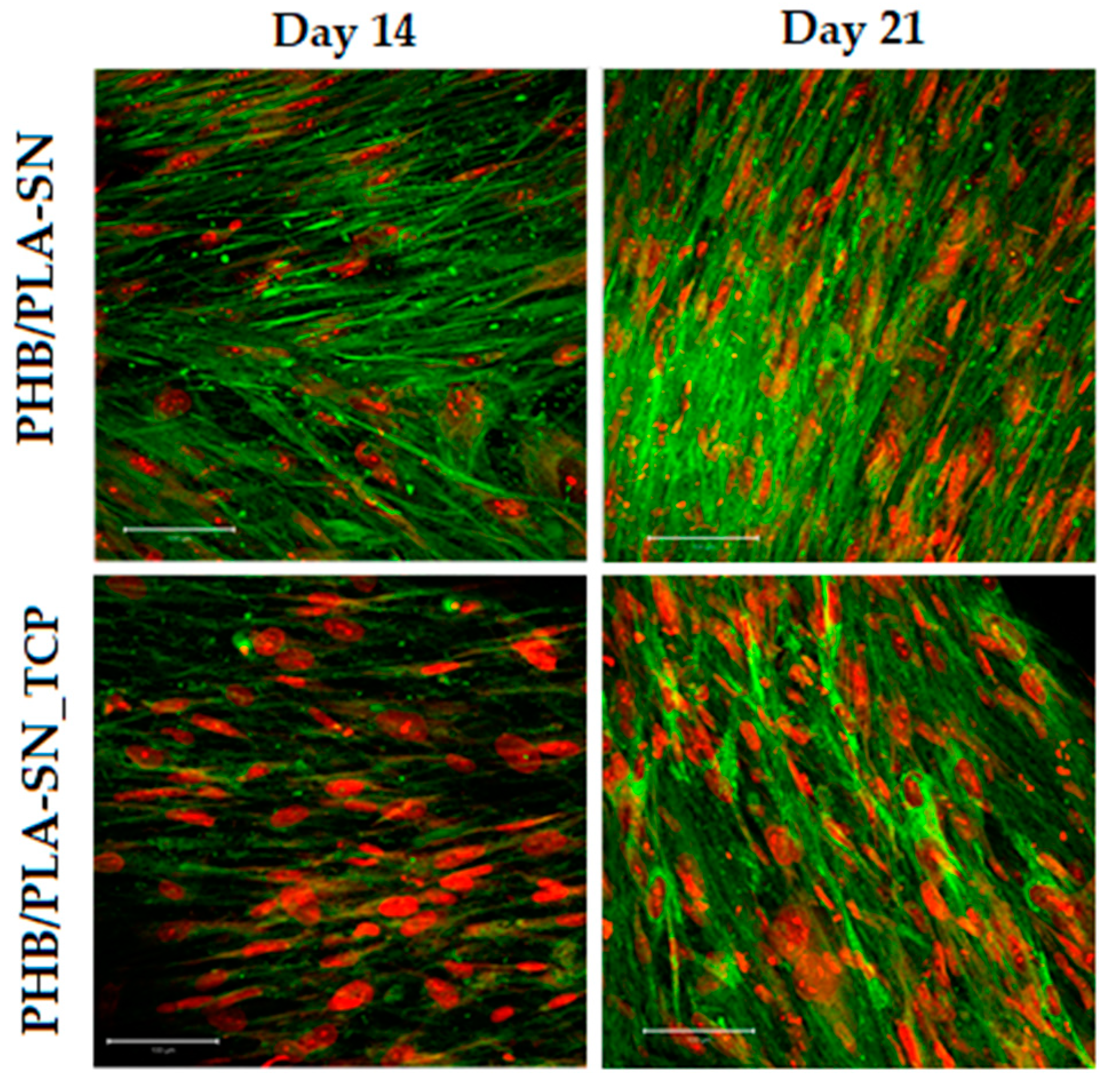
2.6.3. Cell Visualization
2.6.4. Alkaline Phosphatase Activity
2.6.5. Osteogenic Differentiation Analysis
2.6.6. Immunohistochemical Staining
2.6.7. Statistical Analysis
3. Results
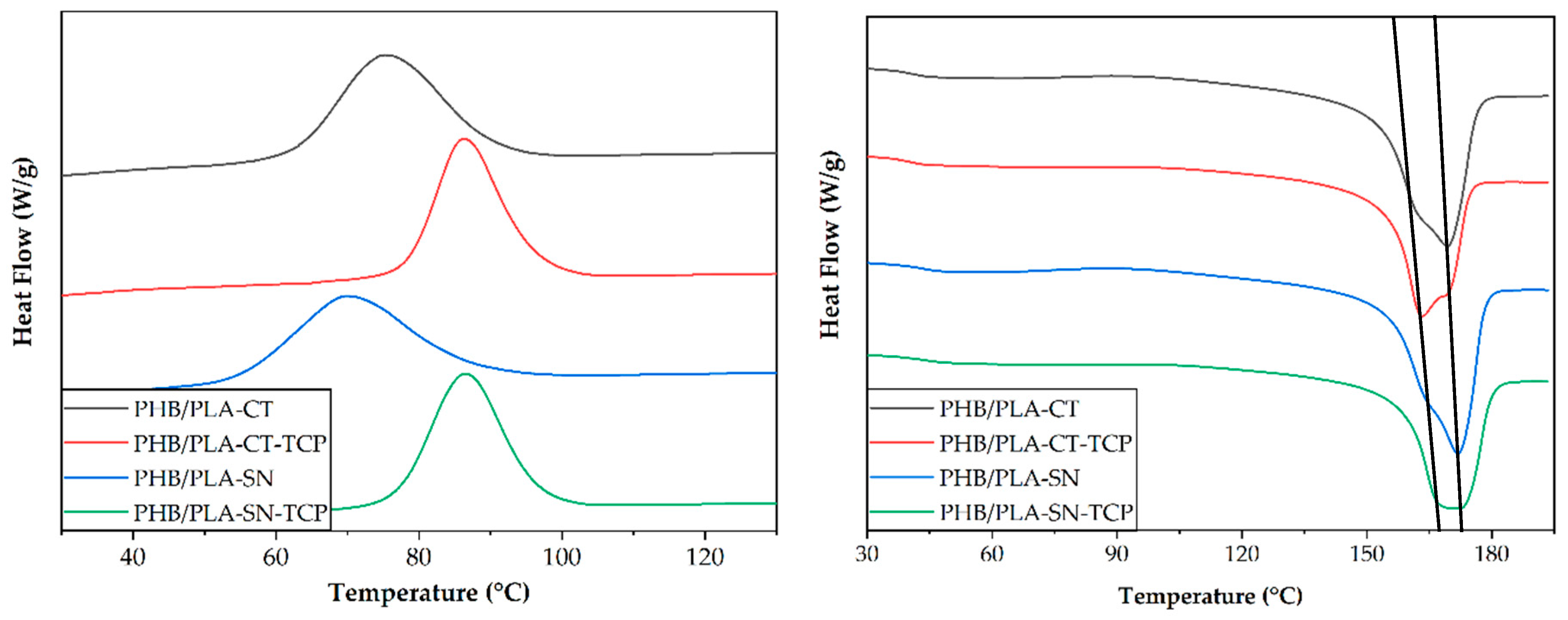
3.1. Thermal Properties of Prepared Materials
3.2. Thermal Stability of Prepared Composites
3.3. Optimal Printing Conditions and Printability
3.4. Tensile Properties of Prepared Biocomposites
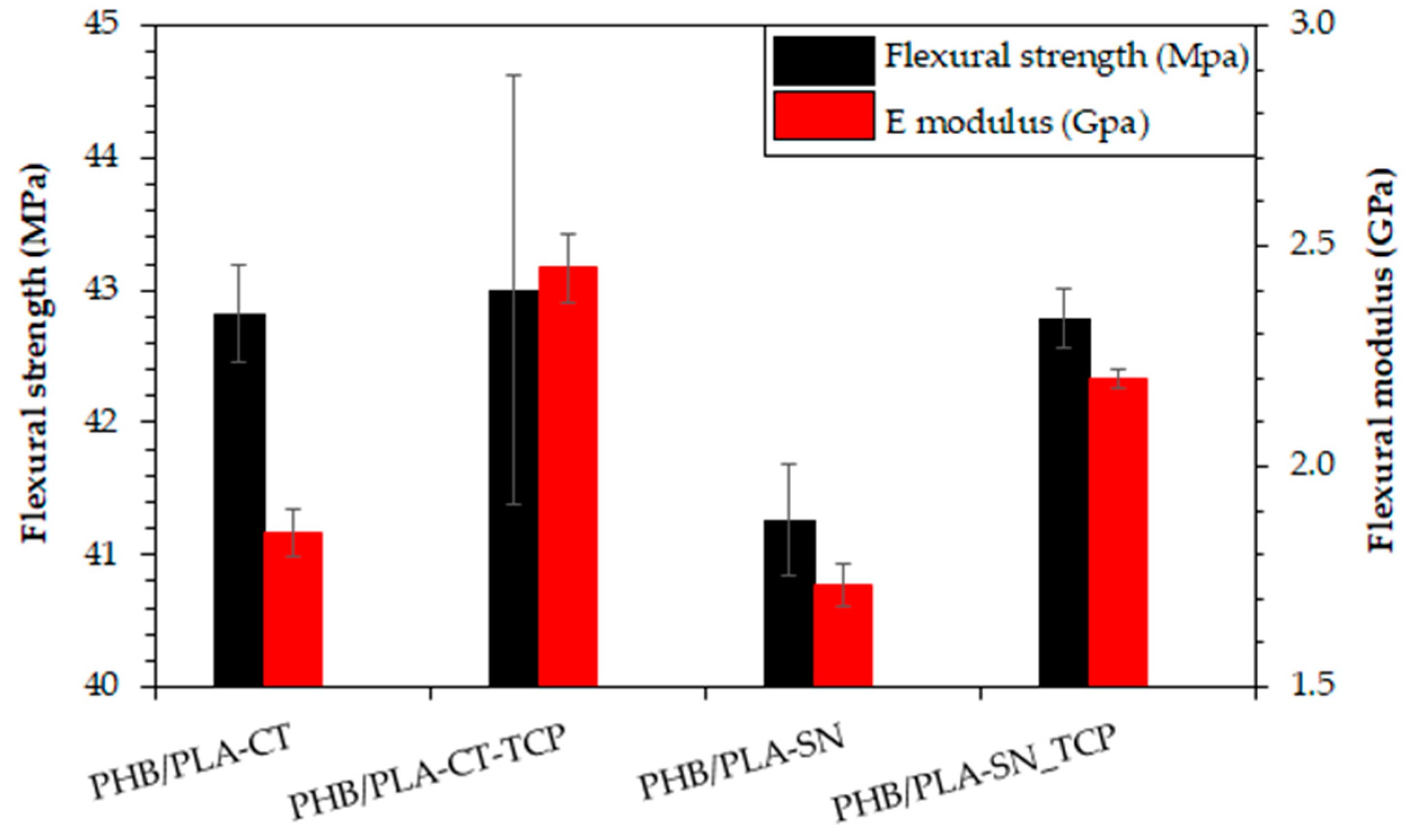
3.5. Flexural Properties of Prepared Materials
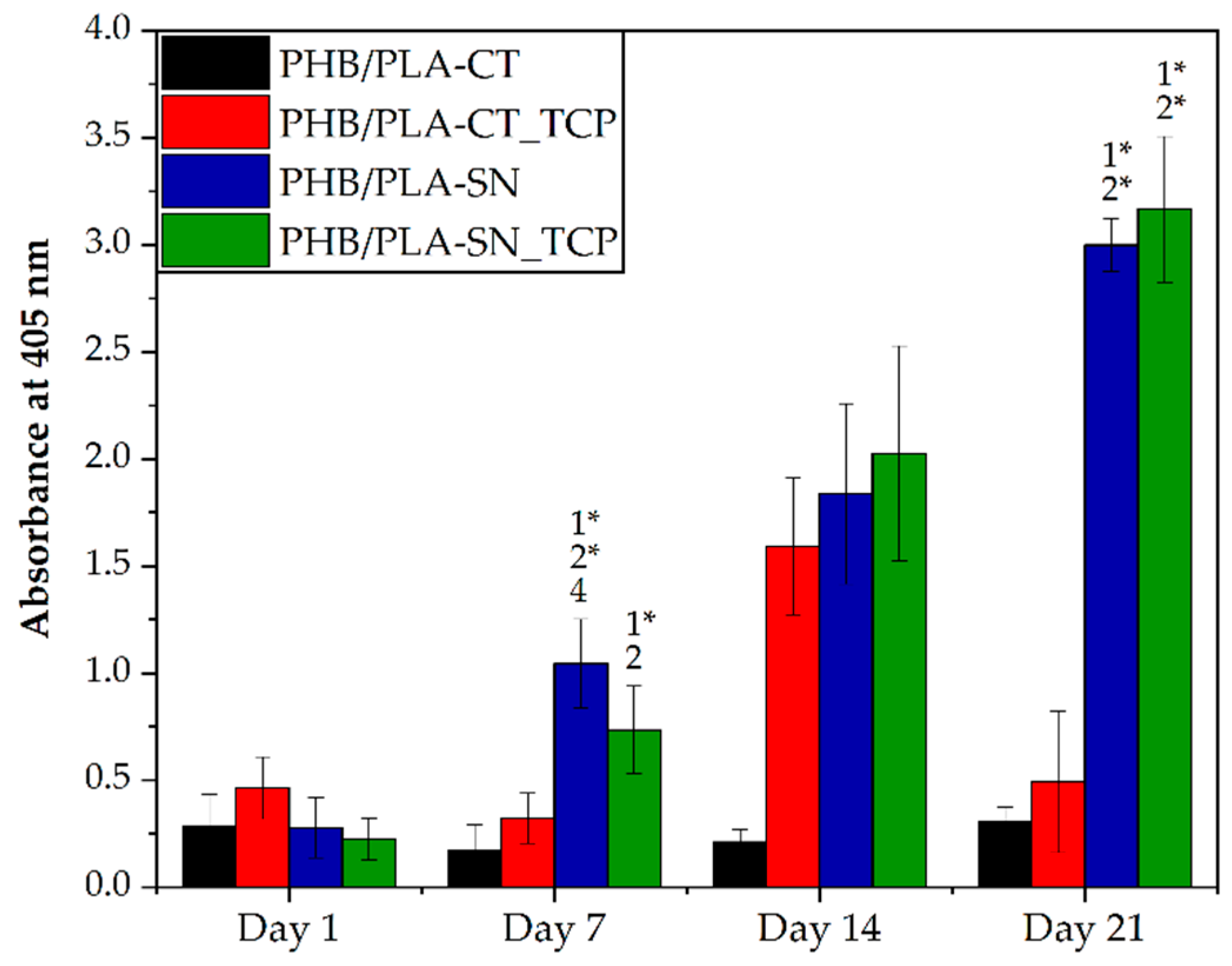
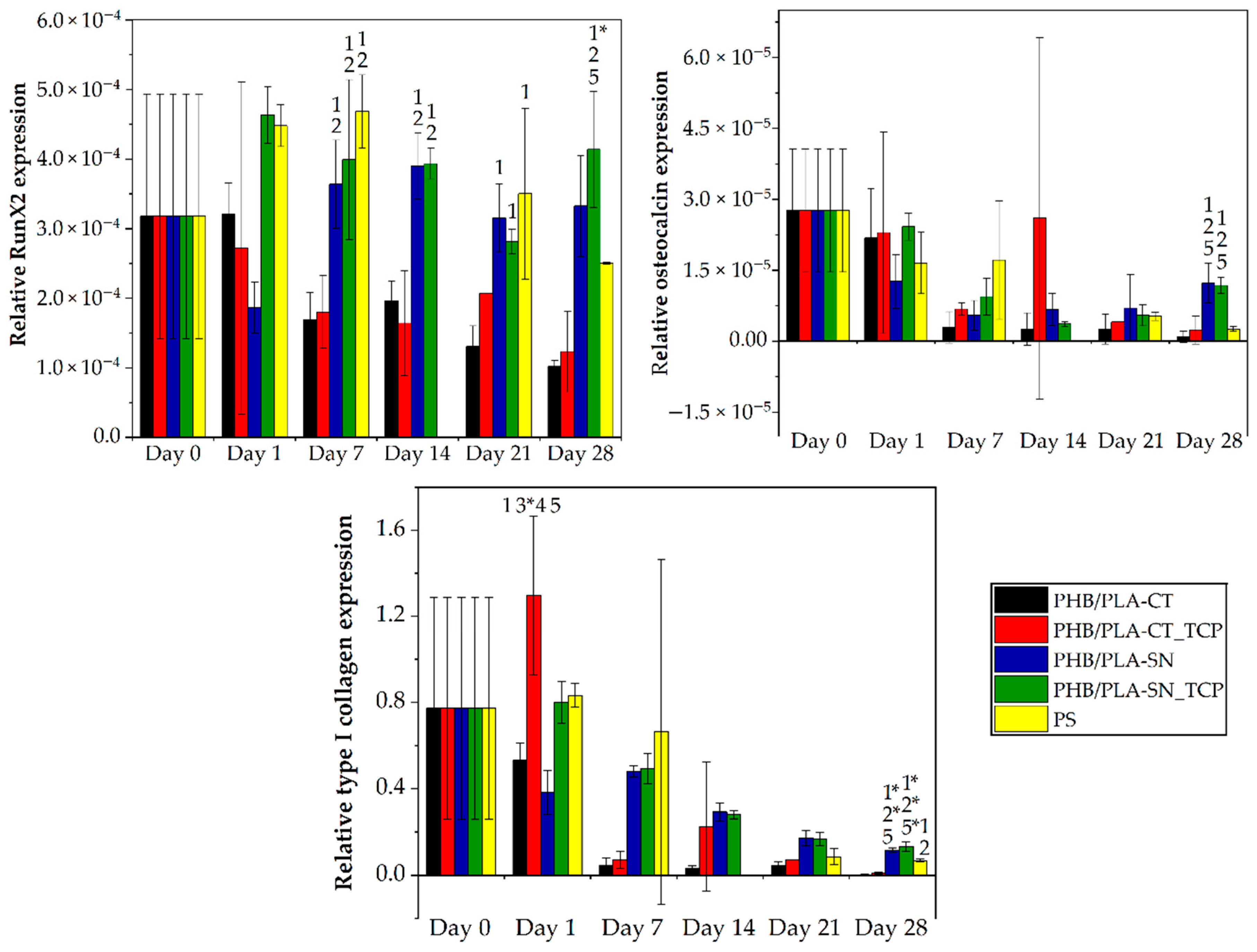
3.6. In Vitro Testing on Scaffolds
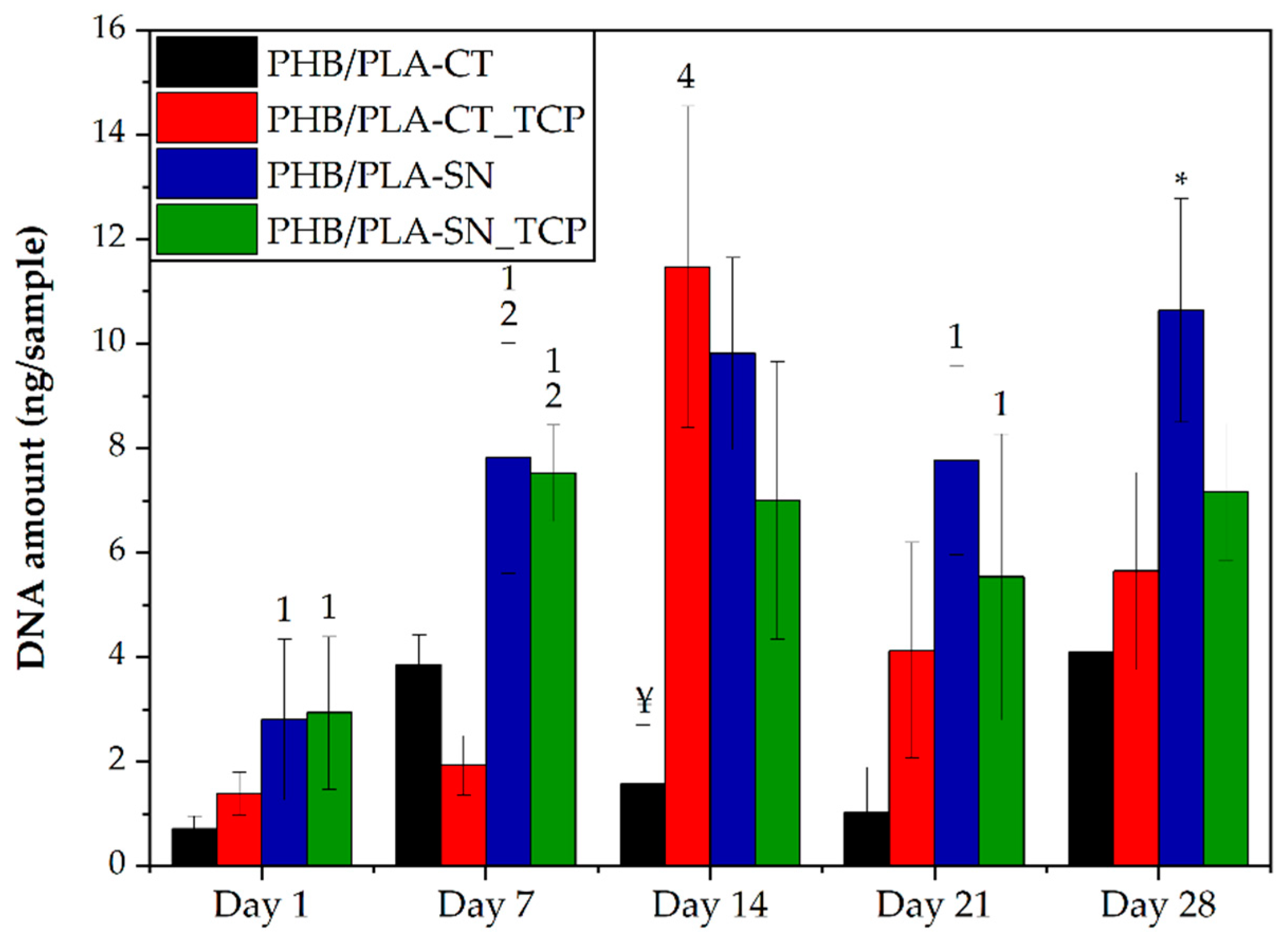
3.6.1. Cell Proliferation and Metabolic Activity
3.6.2. Osteogenic Differentiation of Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amin, S.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J. Trends in Fracture Incidence: A Population-Based Study Over 20 Years. J. Bone Miner. Res. 2014, 29, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Kumbar, S.G.; Laurencin, C.T. Bone tissue engineering. In Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1194–1214. [Google Scholar]
- Laurencin, C.T.; Ambrosio, A.M.A.; Borden, M.D.; Cooper, J.A. Tissue Engineering: Orthopedic Applications. Annu. Rev. Biomed. Eng. 1999, 1, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, H.; Mansouri, K.; Mansouri, N. Biomaterials, Substitutes, And Tissue Engineering in Bone Repair. Comp. Clin. Path. 2019, 28, 879–891. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Hollister, S.J. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Sloviková, A.; Vojtová, L.; Jančář, J. Preparation and modification of collagen-based scaffold for tissue engineering. Chem. Pap. 2008, 62, 417–422. [Google Scholar] [CrossRef]
- Švachová, V.; Vojtová, L.; Pavliňák, D.; Vojtek, L.; Sedláková, V.; Hyršl, P.; Alberti, M.; Jaroš, J.; Hampl, A.; Jančář, J. Novel electrospun gelatin/oxycellulose nanofibers as a suitable platform for lung disease modeling. Mater. Sci. Eng. C 2016, 67, 493–501. [Google Scholar] [CrossRef]
- Balletti, C.; Ballarin, M.; Guerra, F. 3D printing: State of the art and future perspectives. J. Cult. Herit. 2017, 26, 172–182. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Pereira, T.F.; Oliveira, M.; Maia, I.A.; Da Silva, J.V.L.; Costa, M.; Thiré, R.M.D.S.M. 3D Printing of Poly(3-Hydroxybutyrate) Porous Structures Using Selective Laser Sintering. Macromol. Symp. 2012, 319, 64–73. [Google Scholar] [CrossRef]
- Lee, S.; Zhou, W.Y.; Wang, M.; Cheung, W.L.; Ip, W.Y. Selective Laser Sintering of Poly(L-Lactide) Porous Scaffolds for Bone Tissue Engineering. J. Biomim. Biomater. Tissue Eng. 2008, 1, 81–89. [Google Scholar] [CrossRef]
- Saska, S.; Pires, L.C.; Cominotte, M.A.; Mendes, L.S.; de Oliveira, M.F.; Maia, I.A.; da Silva JV, L.; Ribeiro SJ, L.; Cirelli, J.A. Three-Dimensional Printing and in Vitro Evaluation of Poly(3-Hydroxybutyrate) Scaffolds Functionalized with Osteogenic Growth Peptide for Tissue Engineering. Mater. Sci. Eng. C 2018, 89, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boparai, K.S.; Singh, R.; Singh, H. Development of rapid tooling using fused deposition modeling: A review. Rapid Prototyp. J. 2016, 22, 281–299. [Google Scholar] [CrossRef]
- Carrell, J.; Gruss, G.; Gomez, E. Four-dimensional printing using fused-deposition modeling: A review. Rapid Prototyp. J. 2020, 26, 855–869. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Kumar, K.M.; Ram, P.B.; Ravichandran, M.; Vinayagamoorthy, M. A review on recent advancements in fused deposition modeling. Mater. Today Proc. 2020, 27, 752–756. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Mohan, N.; Senthil, P.; Vinodh, S.; Jayanth, N. A review on composite materials and process parameters optimisation for the fused deposition modelling process. Virtual Phys. Prototyp. 2017, 12, 47–59. [Google Scholar] [CrossRef]
- Chiulan, I.; Frone, A.N.; Brandabur, C.; Panaitescu, D.M. Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Shunmugasundaram, M.; Maughal, A.A.B.; Ajay Kumar, M. A review of bio-degradable materials for fused deposition modeling machine. Mater. Today Proc. 2020, 27, 1596–1600. [Google Scholar] [CrossRef]
- Wurm, M.C.; Möst, T.; Bergauer, B.; Rietzel, D.; Neukam, F.W.; Cifuentes, S.C.; Von Wilmowsky, C. In-vitro evaluation of Polylactic acid (PLA) manufactured by fused deposition modeling. J. Biol. Eng. 2017, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C 2017, 79, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Butt, F.I.; Muhammad, N.; Hamid, A.; Moniruzzaman, M.; Sharif, F. Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications—Review. Int. J. Biol. Macromol. 2018, 120, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Kovalcik, A.; Sangroniz, L.; Kalina, M.; Skopalova, K.; Humpolíček, P.; Omastova, M.; Mundigler, N.; Müller, A.J. Properties of scaffolds prepared by fused deposition modeling of poly(hydroxyalkanoates). Int. J. Biol. Macromol. 2020, 161, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Ausejo, J.; Rydz, J.; Musioł, M.; Sikorska, W.; Sobota, M.; Włodarczyk, J.; Adamus, G.; Janeczek, H.; Kwiecień, I.; Hercog, A.; et al. A comparative study of three-dimensional printing directions: The degradation and toxicological profile of a PLA/PHA blend. Polym. Degrad. Stab. 2018, 152, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Esposito Corcione, C.; Scalera, F.; Gervaso, F.; Montagna, F.; Sannino, A.; Maffezzoli, A. One-step solvent-free process for the fabrication of high loaded PLA/HA composite filament for 3D printing. J. Therm. Anal. Calorim. 2018, 134, 575–582. [Google Scholar] [CrossRef]
- Xuan, Y.; Tang, H.; Wu, B.; Ding, X.; Lu, Z.; Li, W.; Xu, Z. A specific groove design for individualized healing in a canine partial sternal defect model by a polycaprolactone/hydroxyapatite scaffold coated with bone marrow stromal cells. J. Biomed. Mater. Res. Part A 2014, 102, 3401–3408. [Google Scholar] [CrossRef]
- Konopnicki, S.; Sharaf, B.; Resnick, C.; Patenaude, A.; Pogal-Sussman, T.; Hwang, K.-G.; Abukawa, H.; Troulis, M.J. Tissue-Engineered Bone With 3-Dimensionally Printed β-Tricalcium Phosphate and Polycaprolactone Scaffolds and Early Implantation: An In Vivo Pilot Study in a Porcine Mandible Model. J. Oral Maxillofac. Surg. 2015, 73, 1016.e1–1016.e11. [Google Scholar] [CrossRef]
- Dávila, J.L.; Freitas, M.S.; Inforçatti Neto, P.; Silveira, Z.C.; Silva JV, L.; d’Ávila, M.A. Fabrication of PCL/Β-TCP Scaffolds By 3D Mini-Screw Extrusion Printing. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Kontárová, S.; Přikryl, R.; Melčová, V.; Menčík, P.; Horálek, M.; Figalla, S.; Plavec, R.; Feranc, J.; Sadílek, J.; Pospíšilová, A. Printability, Mechanical and Thermal Properties of Poly(3-Hydroxybutyrate)-Poly(Lactic Acid)-Plasticizer Blends for Three-Dimensional (3D) Printing. Materials 2020, 13, 4736. [Google Scholar] [CrossRef]
- Menčík, P.; Přikryl, R.; Stehnová, I.; Melčová, V.; Kontárová, S.; Figalla, S.; Alexy, P.; Bočkaj, J. Effect of Selected Commercial Plasticizers on Mechanical, Thermal, and Morphological Properties of Poly(3-hydroxybutyrate)/Poly(lactic acid)/Plasticizer Biodegradable Blends for Three-Dimensional (3D) Print. Materials 2018, 11, 1893. [Google Scholar] [CrossRef] [Green Version]


| Amount (wt.%) | |||||
|---|---|---|---|---|---|
| Sample | PHB | PLA | Citroflex | Syncroflex | TCP |
| PHB/PLA-CT | 62 | 26 | 12 | 0 | 0 |
| PHB/PLA-CT_TCP | 54 | 23 | 10 | 0 | 13 |
| PHB/PLA-SN | 62 | 26 | 0 | 12 | 0 |
| PHB/PLA-SN_TCP | 54 | 23 | 0 | 10 | 13 |
| Filament diameter | 1.75 mm |
| Nozzle diameter | 0.4 mm |
| Layer height | 0.2 mm |
| Width of printed layer | 0.45 mm |
| Perimeter printing speed | 45 mm∙s−1 |
| Fill print speed | 200 mm∙s−1 |
| Bed temperature | 20 °C |
| Cooling fan power | 100% |
| Glass Transition Temperature PLA Tg (°C) | Crystallization Temperature PHB Tc1 (°C) | Melting Temperature PHB Tm2 (°C) | Crystallinity PHB Xc1 | |
|---|---|---|---|---|
| PHB/PLA-CT | 42.8 | 75.6 | 169.3 | 59.9 |
| PHB/PLA-CT_TCP | 41.1 | 86.3 | 163.3 | 56.7 |
| PHB/PLA-SN | 40.8 | 69.8 | 171.9 | 61.1 |
| PHB/PLA-SN_TCP | 42.3 | 86.6 | 169.9 | 59.1 |
| Maximum Rate of Degradation | Residue Amount w% at 600 °C | ||
|---|---|---|---|
| PHB Td (°C) | PLA Td (°C) | ||
| PHB/PLA-CT | 281 | 337 | - |
| PHB/PLA-CT_TCP | 283 | 348 | 11.9 |
| PHB/PLA-SN | 281 | 346 | - |
| PHB/PLA-SN_TCP | 284 | 348 | 12.9 |
| Optimal Nozzle Temperature (°C) | Optimal Flow (%) | Warping Coefficient (-) | |||
|---|---|---|---|---|---|
| 185 °C | 190 °C | 195 °C | |||
| PHB/PLA-CT | 185–190 | 100 | 2.2 ± 0.1 | 2.0 ± 0.1 | |
| PHB/PLA-CT_TCP | 190–195 | 90 | 3.0 ± 0.2 | 3.2 ± 0.3 | |
| PHB/PLA-SN | 190–195 | 100 | 2.1 ± 0.1 | 2.0 ± 0.1 | |
| PHB/PLA-SN_TCP | 190–195 | 100 | 2.2 ± 0.2 | 2.5 ± 0.1 | |
| Young’s Modulus ET (GPa) | Tensile Strength σmax (MPa) | Elongation at Break εmax (%) | |
|---|---|---|---|
| PHB/PLA-CT | 2.3 ± 0.1 | 32.2 ± 1.3 | 22.6 ± 13.1 |
| PHB/PLA-CT_TCP | 2.6 ± 0.1 | 28.3 ± 0.8 | 3.4 ± 2.0 |
| PHB/PLA-SN | 2.4 ± 0.2 | 37.6 ± 3.4 | 16.9 ± 3.0 |
| PHB/PLA-SN_TCP | 2.5 ± 0.0 | 32.1 ± 0.7 | 6.4 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melčová, V.; Svoradová, K.; Menčík, P.; Kontárová, S.; Rampichová, M.; Hedvičáková, V.; Sovková, V.; Přikryl, R.; Vojtová, L. FDM 3D Printed Composites for Bone Tissue Engineering Based on Plasticized Poly(3-hydroxybutyrate)/poly(d,l-lactide) Blends. Polymers 2020, 12, 2806. https://doi.org/10.3390/polym12122806
Melčová V, Svoradová K, Menčík P, Kontárová S, Rampichová M, Hedvičáková V, Sovková V, Přikryl R, Vojtová L. FDM 3D Printed Composites for Bone Tissue Engineering Based on Plasticized Poly(3-hydroxybutyrate)/poly(d,l-lactide) Blends. Polymers. 2020; 12(12):2806. https://doi.org/10.3390/polym12122806
Chicago/Turabian StyleMelčová, Veronika, Kateřina Svoradová, Přemysl Menčík, Soňa Kontárová, Michala Rampichová, Věra Hedvičáková, Věra Sovková, Radek Přikryl, and Lucy Vojtová. 2020. "FDM 3D Printed Composites for Bone Tissue Engineering Based on Plasticized Poly(3-hydroxybutyrate)/poly(d,l-lactide) Blends" Polymers 12, no. 12: 2806. https://doi.org/10.3390/polym12122806





