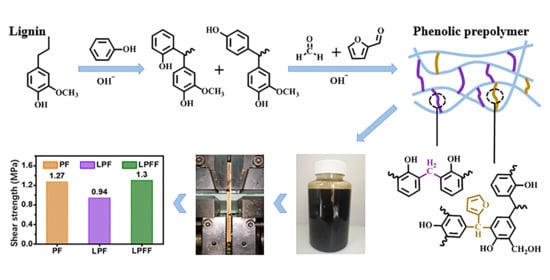
Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
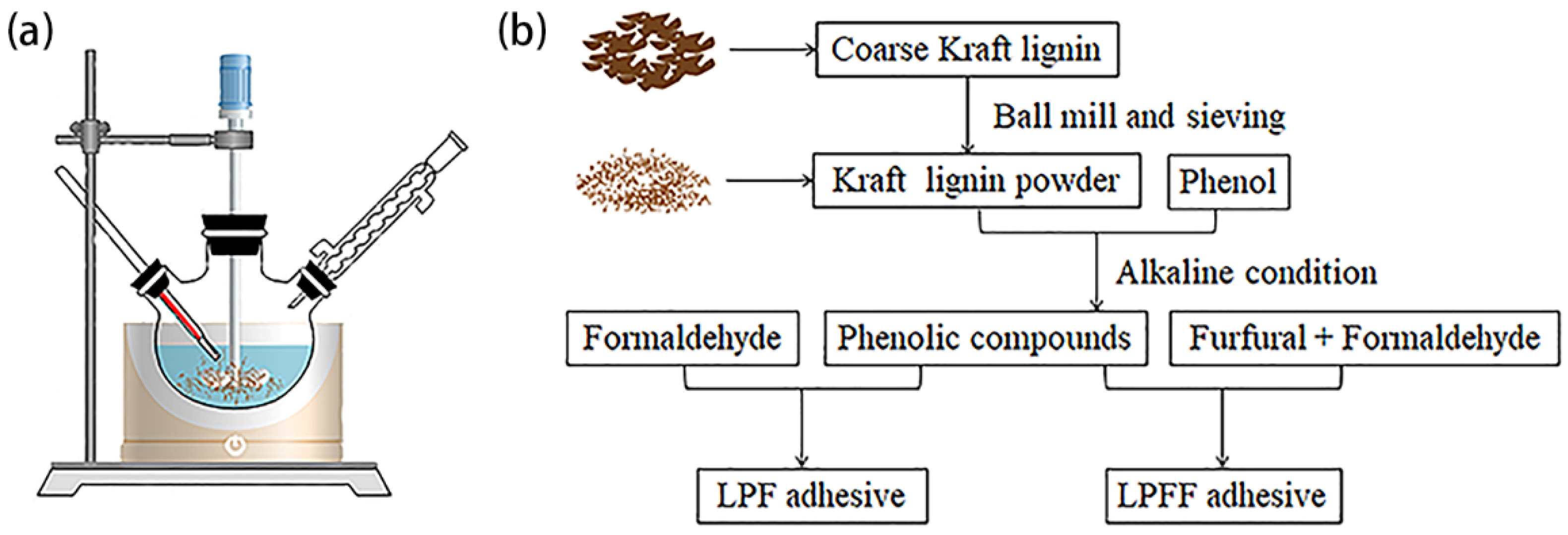
2.2. Synthesis of LPF Adhesive and LPFF Adhesive
2.3. Preparation of Three-Layer Plywood and Water-Resistance Test
2.4. Evaluation of Resin Properties
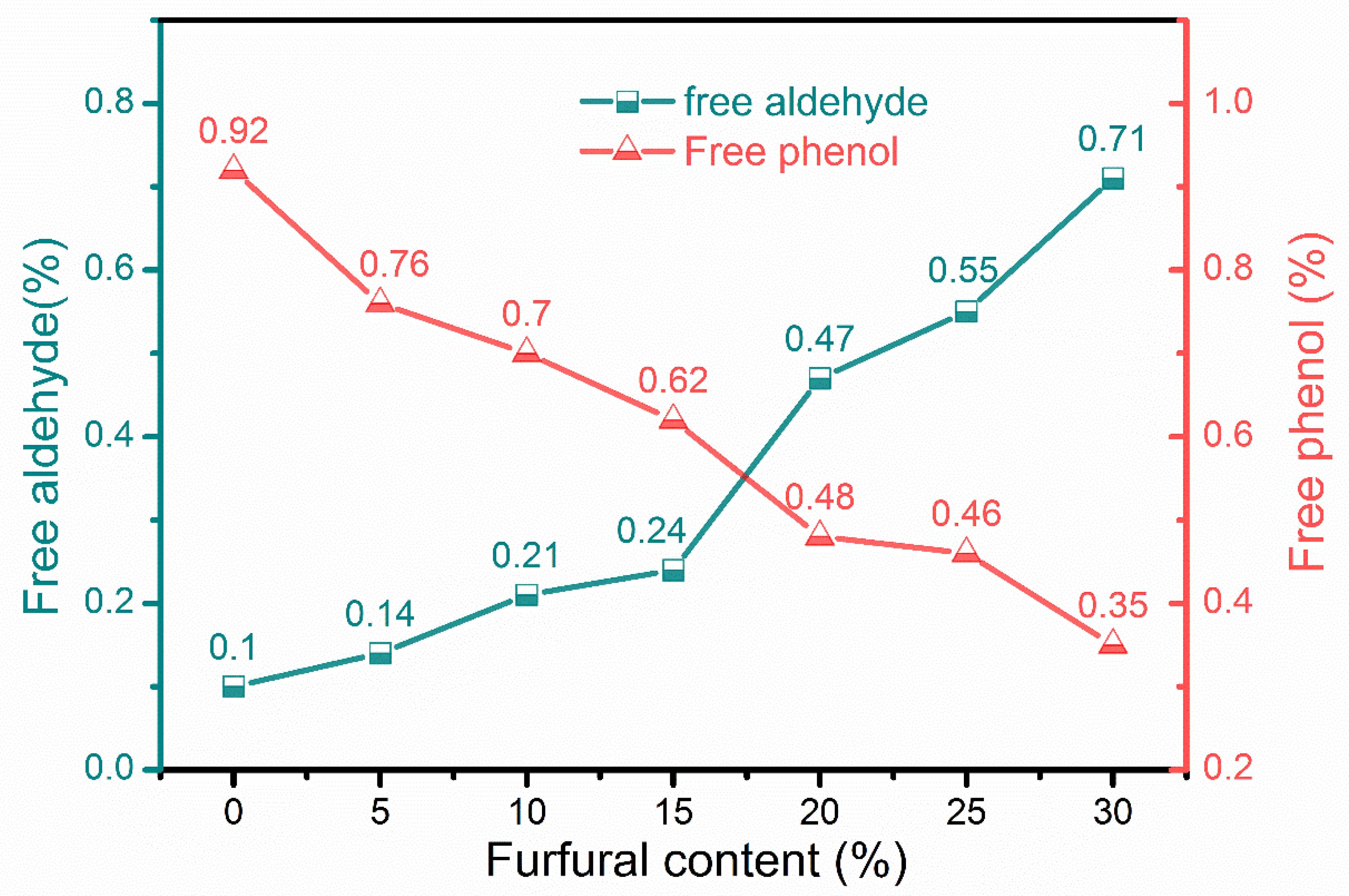
2.5. Free Aldehyde Determination by Titration
2.6. Free Phenol Determination by Titration
2.7. Characteristics
3. Results and Discussion
3.1. Water Resistance of Wood-Based Panel Adhered by Different Adhesives
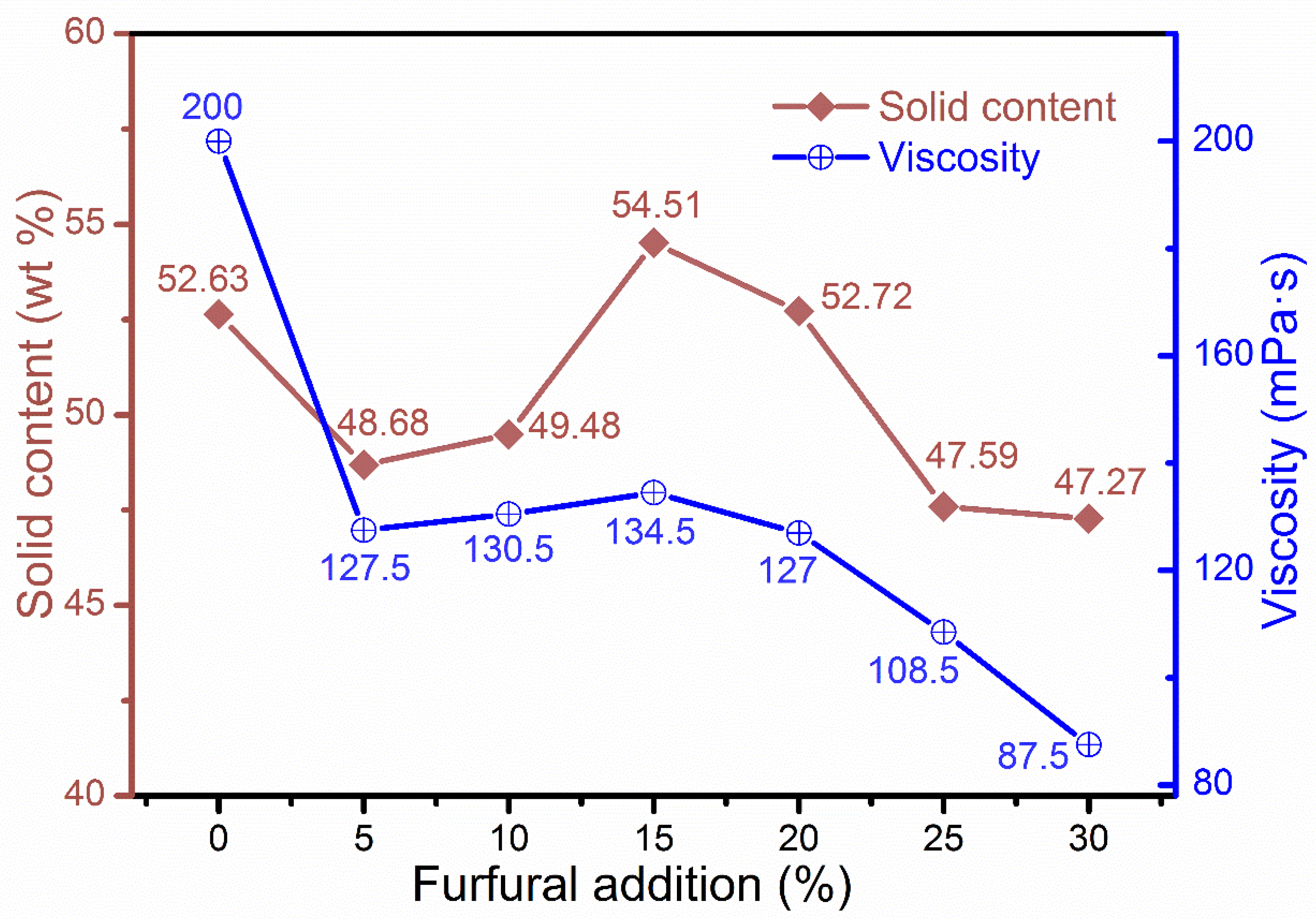
3.2. Physicochemical Properties of Different Adhesives
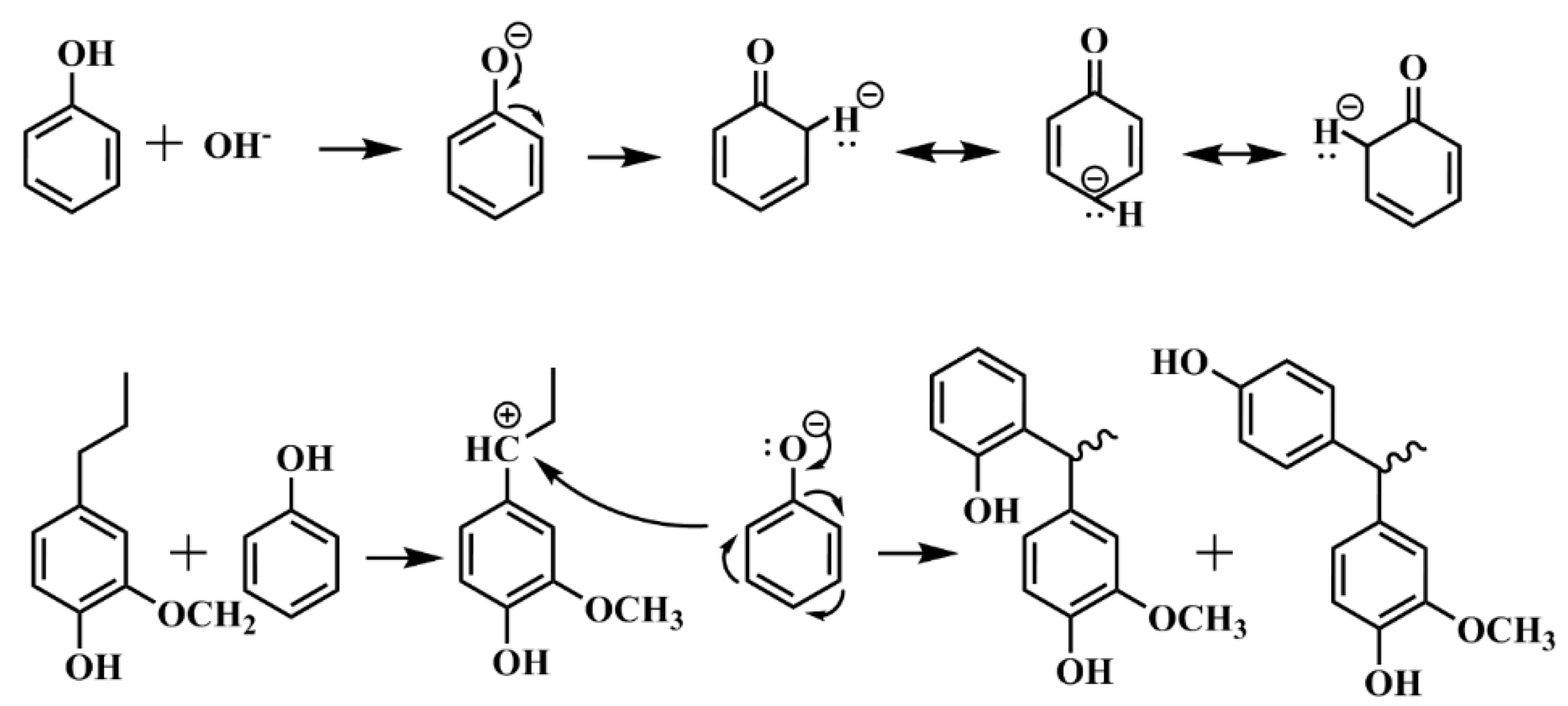
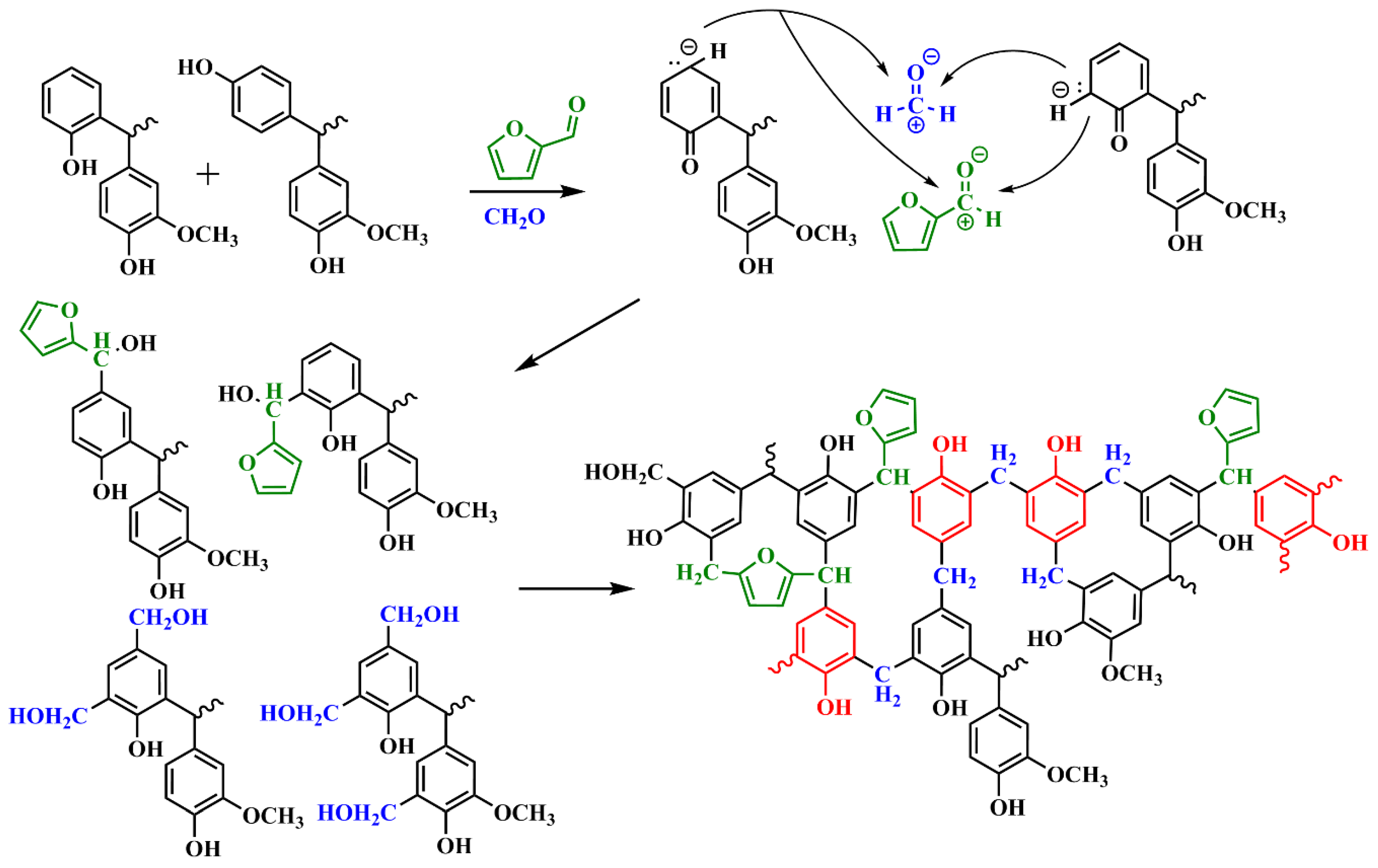
3.3. Possible Interaction between Furfural and LPF Adhesive
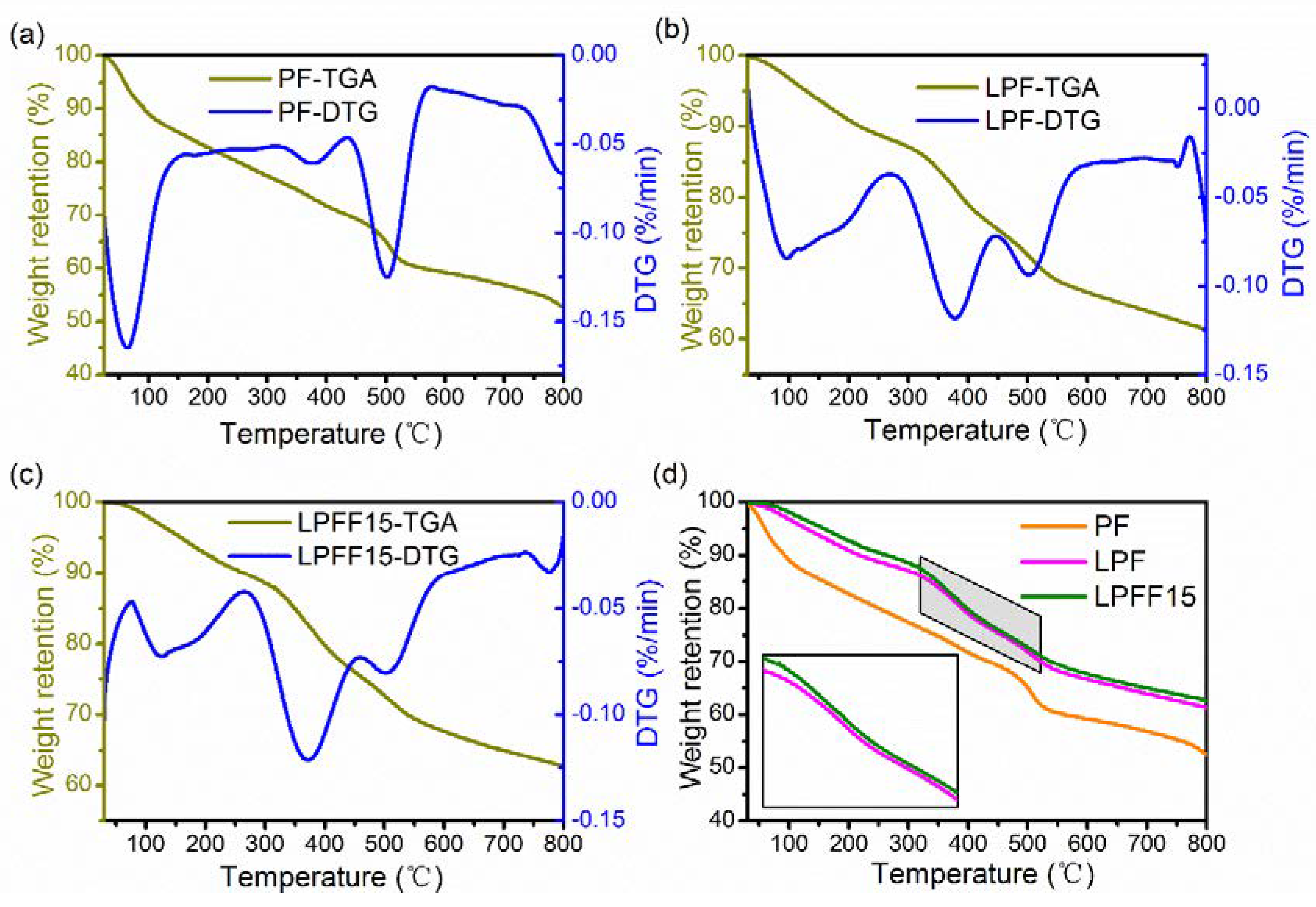
3.4. Thermal Stability Analysis
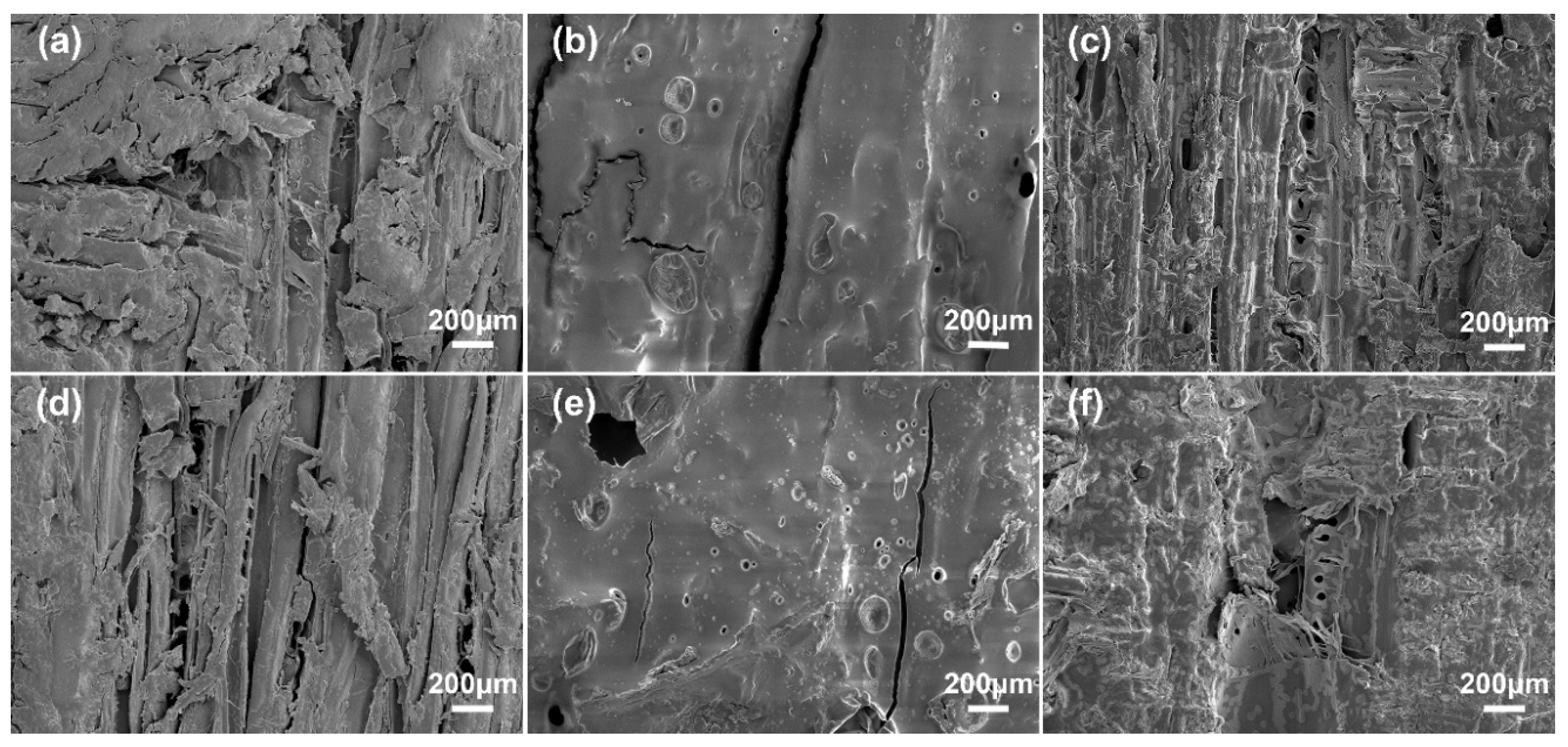
3.5. Analysis of Plywood Shear Surface Morphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Ashraf, S.M.; Malhotra, V.P. Eucalyptus bark lignin substituted phenol formaldehyde adhesives: A study on optimization of reaction parameters and characterization. J. Appl. Polym. Sci. 2004, 92, 3514–3523. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Xu, Y.; Wang, C.; Chu, F. Lignocellulosic ethanol residue-based lignin–phenol–formaldehyde resin adhesive. Int. J. Adhes. Adhes. 2013, 40, 11–18. [Google Scholar] [CrossRef]
- Cetin, N.S.; Özmen, N. Use of organosolv lignin in phenol-formaldehyde resins for particleboard production II Particleboard production and properties. Int. J. Adhes. Adhes. 2002, 22, 481–486. [Google Scholar] [CrossRef]
- da Silva, C.G.; Grelier, S.; Pichavant, F.; Frollini, E.; Castellan, A. Adding value to lignins isolated from sugarcane bagasse and Miscanthus. Ind. Crop. Prod. 2013, 42, 87–95. [Google Scholar] [CrossRef]
- Spiridon, I. Extraction of lignin and therapeutic applications of lignin-derived compounds. A review. Environ. Chem. Lett. 2020, 18, 771–785. [Google Scholar] [CrossRef]
- Ning, L.; Zhikang, C.; Yifu, Z. Preparation and Characterization of Phenol Formaldehyde Resin Adhesives Modified by the Phenolic Lignin. Chem. Ind. For. Prod. 2019, 39, 95–101. [Google Scholar]
- Zhang, Y.; Yuan, Z.; Mahmood, N.; Huang, S.; Xu, C. Sustainable bio-phenol-hydroxymethylfurfural resins using phenolated de-polymerized hydrolysis lignin and their application in bio-composites. Ind. Crop. Prod. 2016, 79, 84–90. [Google Scholar] [CrossRef]
- Hazwan Hussin, M.; Aziz, A.A.; Iqbal, A.; Ibrahim, M.N.M.; Latif, N.H.A. Development and characterization novel bio-adhesive for wood using kenaf core (Hibiscus cannabinus) lignin and glyoxal. Int. J. Biol. Macromol. 2019, 122, 713–722. [Google Scholar] [CrossRef]
- Solt, P.; Jaaskelainen, A.S.; Lingenfelter, P. Impact of molecular weight of kraft lignin on adhesiveperformance of lignin-bas. For. Prod. J. 2018, 68, 365–371. [Google Scholar]
- Li, Y.; Li, B.; Du, F.; Wang, Y.; Pan, L.; Chen, D. Microwave-assisted hydrothermal liquefaction of lignin for the preparation of phenolic formaldehyde adhesive. J. Appl. Polym. Sci. 2017, 134, 44510. [Google Scholar] [CrossRef]
- Wang, H.; Eberhardt, T.L.; Wang, C.; Gao, S.; Pan, H. Demethylation of Alkali Lignin with Halogen Acids and Its Application to Phenolic Resins. Polymers 2019, 11, 1771. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, C.; Wu, D.; Chen, Z.; Zhu, N.; Gui, C.; Zhang, M.; Umemura, K.; Yong, Q. Utilization of enzymatic hydrolysate from corn stover as a precursor to synthesize an eco-friendly plywood adhesive. Ind. Crop. Prod. 2020, 152, 112501. [Google Scholar] [CrossRef]
- Gan, L.; Pan, X. Phenol-Enhanced Depolymerization and Activation of Kraft Lignin in Alkaline Medium. Ind. Eng. Chem. Res. 2019, 58, 7794–7800. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.-M.; Jameel, H. Phenolation to Improve Lignin Reactivity toward Thermosets Application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Luo, B.; Jia, Z.; Jiang, H.; Wang, S.; Min, D. Improving the Reactivity of Sugarcane Bagasse Kraft Lignin by a Combination of Fractionation and Phenolation for Phenol-Formaldehyde Adhesive Applications. Polymers 2020, 12, 1825. [Google Scholar] [CrossRef]
- Dong, L.; Hu, H.; Cheng, F.; Yang, S. The water resistance of corrugated paper improved by lipophilic extractives and lignin in APMP effluent. J. Wood Sci. 2015, 61, 412–419. [Google Scholar] [CrossRef]
- Gu, Y.; Cheng, L.; Gu, Z.B.; Hong, Y.; Li, Z.F.; Li, C.M. Preparation, characterization and properties of starch-based adhesive for wood-based panels. Int. J. Biol. Macromol. 2019, 134, 247–254. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Zhang, C.; Tang, J.; Zeng, X.; Wan, X. Versatile value-added application of hyperbranched lignin derivatives: Water-resistance adhesive, UV protection coating, self-healing and skin-adhesive sensing. Chem. Eng. J. 2021, 404, 126358. [Google Scholar] [CrossRef]
- Lépine, E.; Riedl, B.; Wang, X.-M.; Pizzi, A.; Delmotte, L.; Hardy, J.-M.; Da Cruz, M.J.R. Synthesis of bio-adhesives from soybean flour and furfural: Relationship between furfural level and sodium hydroxide concentration. Int. J. Adhes. Adhes. 2015, 63, 74–78. [Google Scholar] [CrossRef]
- Sakostschikoff, A.P.; Iwanowa, W.T.; Kurennowa, A.M.; Moscow, U.S.S.R. Determination of Pentosans in Vegetable MaterialsContaining Tannins. Ind. Eng. Chem. 1934, 6, 205–208. [Google Scholar]
- Koley, R.; Kasilingam, R.; Sahoo, S.; Chattopadhyay, S.; Bhowmick, A.K. Synthesis and Characterization of Phenol Furfural Resin from Moringa Oleifera Gum and Biophenol and Its Application in Styrene Butadiene Rubber. Ind. Eng. Chem. Res. 2019, 58, 18519–18532. [Google Scholar] [CrossRef]
- Liu, J.; Xuan, D.; Chai, J.; Guo, D.; Huang, Y.; Liu, S.; Chew, Y.T.; Li, S.; Zheng, Z. Synthesis and Thermal Properties of Resorcinol-Furfural Thermosetting Resin. ACS Omega 2020, 5, 10011–10020. [Google Scholar] [CrossRef]
- Zhou, J.B.; Li, P.L.; Zhou, J.F.; Liao, X.P.; Shi, B. Preparation of Formaldehyde-free Melamine Resin using Furfural as Condensation Agent and its Retanning Performances Investigation. J. Am. Leather Chem. Assoc. 2018, 113, 198–206. [Google Scholar]
- Dongre, P.; Driscoll, M.; Amidon, T.; Bujanovic, B. Lignin-Furfural Based Adhesives. Energies 2015, 8, 7897–7914. [Google Scholar] [CrossRef]
- Chang, L.; Guo, W.; Tang, Q. Assessing the Tensile Shear Strength and InterfacialBonding Mechanism of Poplar. Bioresources 2017, 12, 571–585. [Google Scholar]
- Gao, Z.-H.; Zhang, Y.-H.; Fang, B.; Zhang, L.-P.; Shi, J. The effects of thermal-acid treatment and crosslinking on the water resistance of soybean protein. Ind. Crop. Prod. 2015, 74, 122–131. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast Curing Bio-Based Phenolic Resins via Lignin Demethylated under Mild Reaction Condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Fu, Y.; Chang, J. Synthesis and characterization of phenol–furfural resins using lignin modified by a low transition temperature mixture. RSC Adv. 2016, 6, 94588–94594. [Google Scholar] [CrossRef]
- Ugryumov, S.A. A study of the viscosity of phenol–formaldehyde resin modified with furfural–acetone monomer FA. Polym. Sci. Ser. D 2017, 10, 99–102. [Google Scholar] [CrossRef]
- Yang, S.; Wu, J.-Q.; Zhang, Y.; Yuan, T.-Q.; Sun, R.-C. Preparation of Lignin-Phenol-Formaldehyde Resin AdhesiveBased on Active Sites of Technical Lignin. J. Biobased Mater. Bioenergy 2015, 9, 266–272. [Google Scholar] [CrossRef]
- Yan, L.; Cui, Y.; Gou, G.; Wang, Q.; Jiang, M.; Zhang, S.; Hui, D.; Gou, J.; Zhou, Z. Liquefaction of lignin in hot-compressed water to phenolic feedstock for the synthesis of phenol-formaldehyde resins. Compos. Part B Eng. 2017, 112, 8–14. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Y.; Xu, C. Synthesis and thermomechanical property study of Novolac phenol-hydroxymethyl furfural (PHMF) resin. RSC Adv. 2014, 4, 31829–31835. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Z.; Xiao, Y.; Pan, Z.; Jia, P.; Feng, G.; Bao, C.; Zhou, Y.; Hua, L. Lignin-based phenolic resin modified with whisker silicon and its application. J. Bioresour. Bioprod. 2020, 5, 67–77. [Google Scholar] [CrossRef]
- Aziz, N.A.; Latip, A.F.A.; Peng, L.C.; Latif, N.H.A.; Brosse, N.; Hashim, R.; Hussin, M.H. Reinforced lignin-phenol-glyoxal (LPG) wood adhesives from coconut husk. Int. J. Biol. Macromol. 2019, 141, 185–196. [Google Scholar] [CrossRef]
- Sui, G.; Cheng, Y.; Yang, X.; Wang, X.; Wang, Z. Use of sustainable glucose and furfural in the synthesis of formaldehyde-free phenolic resole resins. J. Appl. Polym. Sci. 2019, 136, 47732. [Google Scholar] [CrossRef]
- Pizzi, A.; Pasch, H.; Simon, C.; Rode, K. Structure of resorcinol, phenol, and furan resins by MALDI-TOF mass spectrometry and 13C NMR. J. Appl. Polym. Sci. 2004, 92, 2665–2674. [Google Scholar] [CrossRef]
- Shahid, S.A.; Ali, M.; Zafar, Z.I. Cure Kinetics, Bonding Performance, Thermal Degradation, and Biocidal Studies of Phenol-Formaldehyde Resins Modified with Crude Bio-oil Prepared from Ziziphus mauritiana Endocarps. Bioresources 2015, 10, 105–122. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, W.; Zhang, W.; Li, J. Preparation of tannin–formaldehyde–furfural resin with pretreatment of depolymerization of condensed tannin and ring opening of furfural. J. Adhes. Sci. Technol. 2016, 30, 947–959. [Google Scholar] [CrossRef]
- Ji, X.; Guo, M. Preparation and properties of a chitosan-lignin wood adhesive. Int. J. Adhes. Adhes. 2018, 82, 8–13. [Google Scholar] [CrossRef]
- Vega-Aguilar, C.A.; Lutz, G.; Mata-Segreda, J.F. Phenolic resin derived from Jatropha curcas seed-husk lignin as phenol substitute. Res. J. Costa Rican Distance Educ. Univ. 2015, 7, 217–223. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Zhang, J.; Bai, Y.; Gao, Q.; Li, J.; Li, L. A New Flexible Soy-Based Adhesive Enhanced with Neopentyl Glycol Diglycidyl Ether: Properties and Application. Polymers 2016, 8, 346. [Google Scholar] [CrossRef] [PubMed]

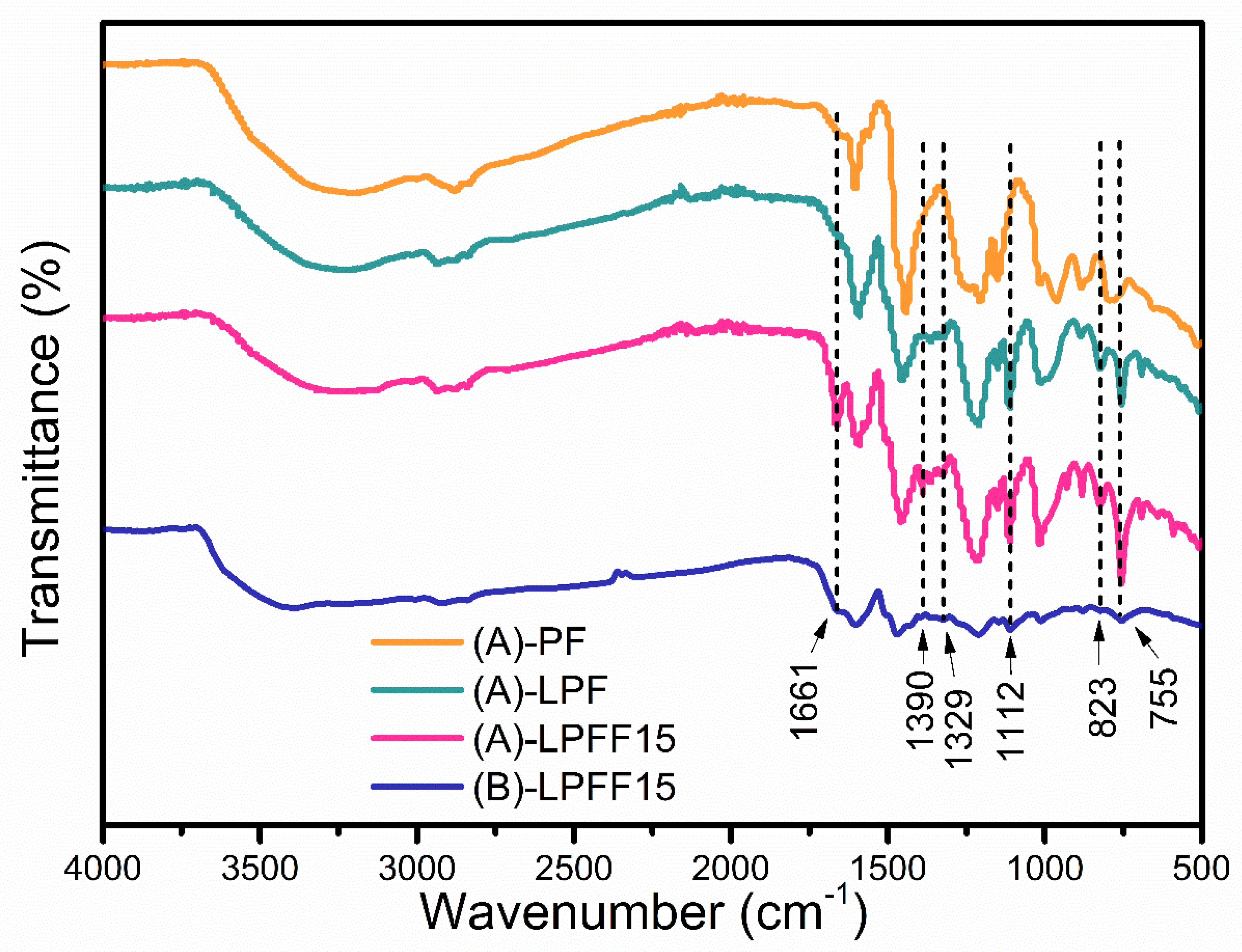

| Assignments | (A)-PF | (A)-LPF | (A)-LPFF15 | (B)-LPFF15 |
|---|---|---|---|---|
| Wavenumber (cm−1) | ||||
| –OH groups stretching | 3203 | 3216 | 3234 | 3387 |
| C–H | 2880 | 2934 | 2934 | 2927 |
| Carbonyl C=O group of furfural | - | - | 1661 | 1661 |
| Aromatic ring structure stretching | 1605 | 1594 | 1594 | 1604 |
| –CH2 bridge | 1443 | 1455 | 1455 | 1467 |
| Furfural double bond | - | - | 1391 | - |
| Syringyl C–O stretching | - | 1326 | 1328 | 1325 |
| Conjugate C–O groups stretching | 1206 | 1212 | 1212 | 1212 |
| Syringyl aromatic ring C–H in-plane deformation | - | 1112 | 1112 | 1112 |
| Unconjugated C–O groups stretching | 1014 | 1013 | 1016 | 1012 |
| C–H out of plane, para-substituted | - | 823 | 824 | 823 |
| C–H out of plane, ortho-substituted | - | 755 | 755 | 755 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, N.; Chen, Z.; Ding, C.; Zheng, Q.; Xu, J.; Meng, Q. Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent. Polymers 2020, 12, 2805. https://doi.org/10.3390/polym12122805
Zhang Y, Li N, Chen Z, Ding C, Zheng Q, Xu J, Meng Q. Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent. Polymers. 2020; 12(12):2805. https://doi.org/10.3390/polym12122805
Chicago/Turabian StyleZhang, Yufei, Ning Li, Zhikang Chen, Chen Ding, Qi Zheng, Jindi Xu, and Qiulu Meng. 2020. "Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent" Polymers 12, no. 12: 2805. https://doi.org/10.3390/polym12122805
APA StyleZhang, Y., Li, N., Chen, Z., Ding, C., Zheng, Q., Xu, J., & Meng, Q. (2020). Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent. Polymers, 12(12), 2805. https://doi.org/10.3390/polym12122805