Gemcitabine-Loaded Magnetically Responsive Poly(ε-caprolactone) Nanoparticles against Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
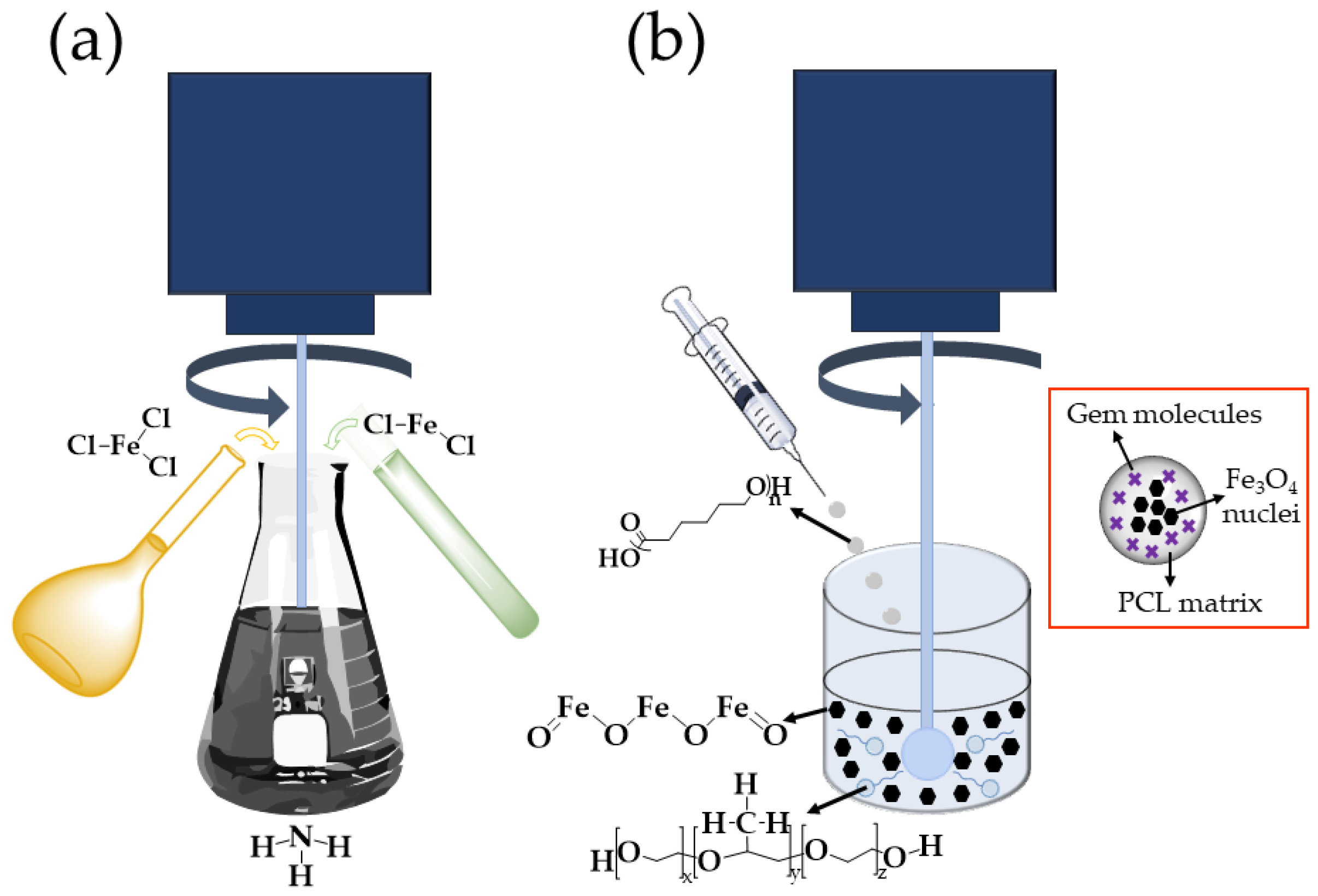
2.2.1. Preparation of Fe3O4/PCL (Core/Shell) NPs
2.2.2. Characterization
2.2.3. In Vitro Determination of Gemcitabine Loading and Release
2.2.4. In Vitro Proliferation Studies
2.2.5. Magnetic Field Responsive Behaviour
2.2.6. Statistical Analysis
3. Results and Discussion
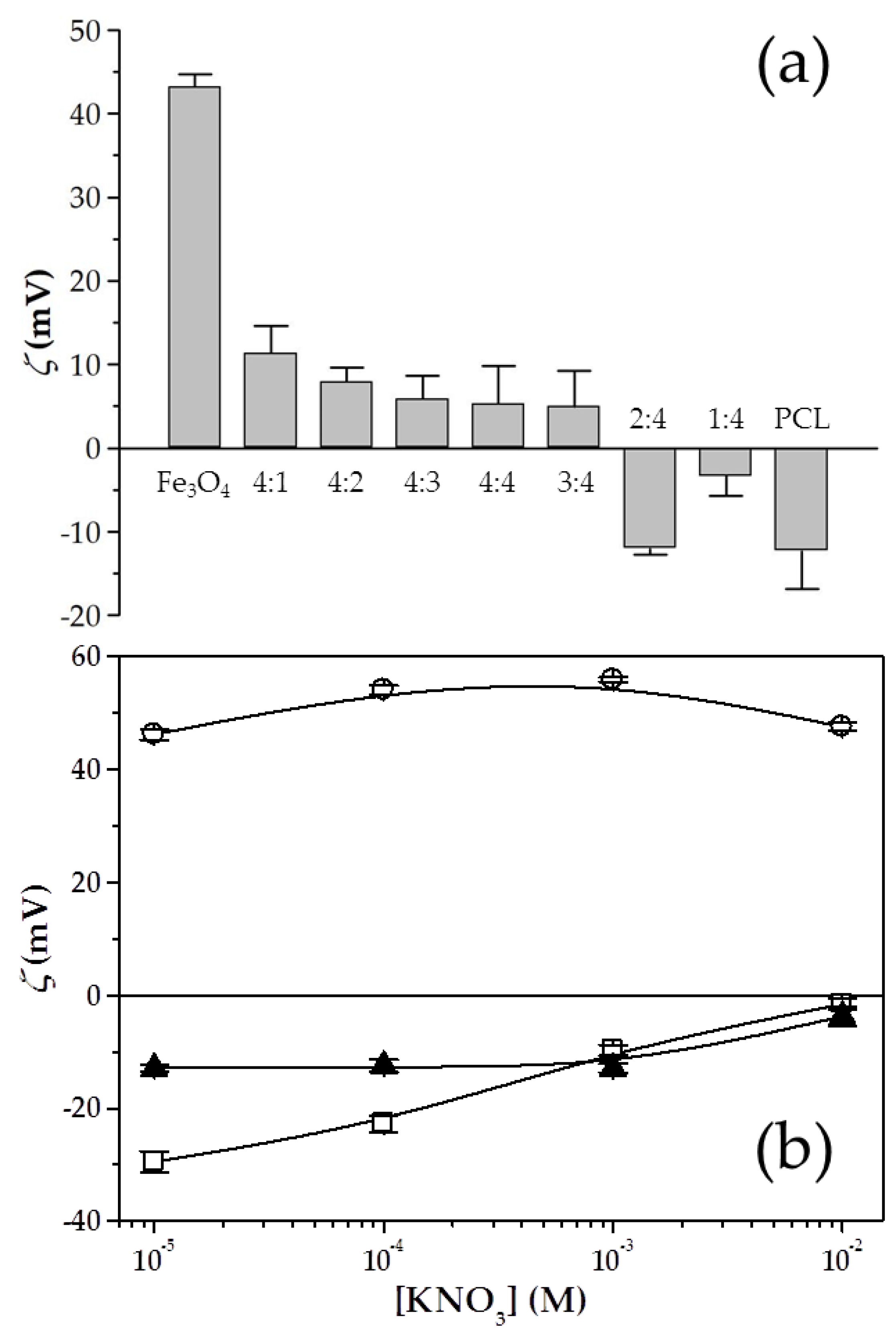
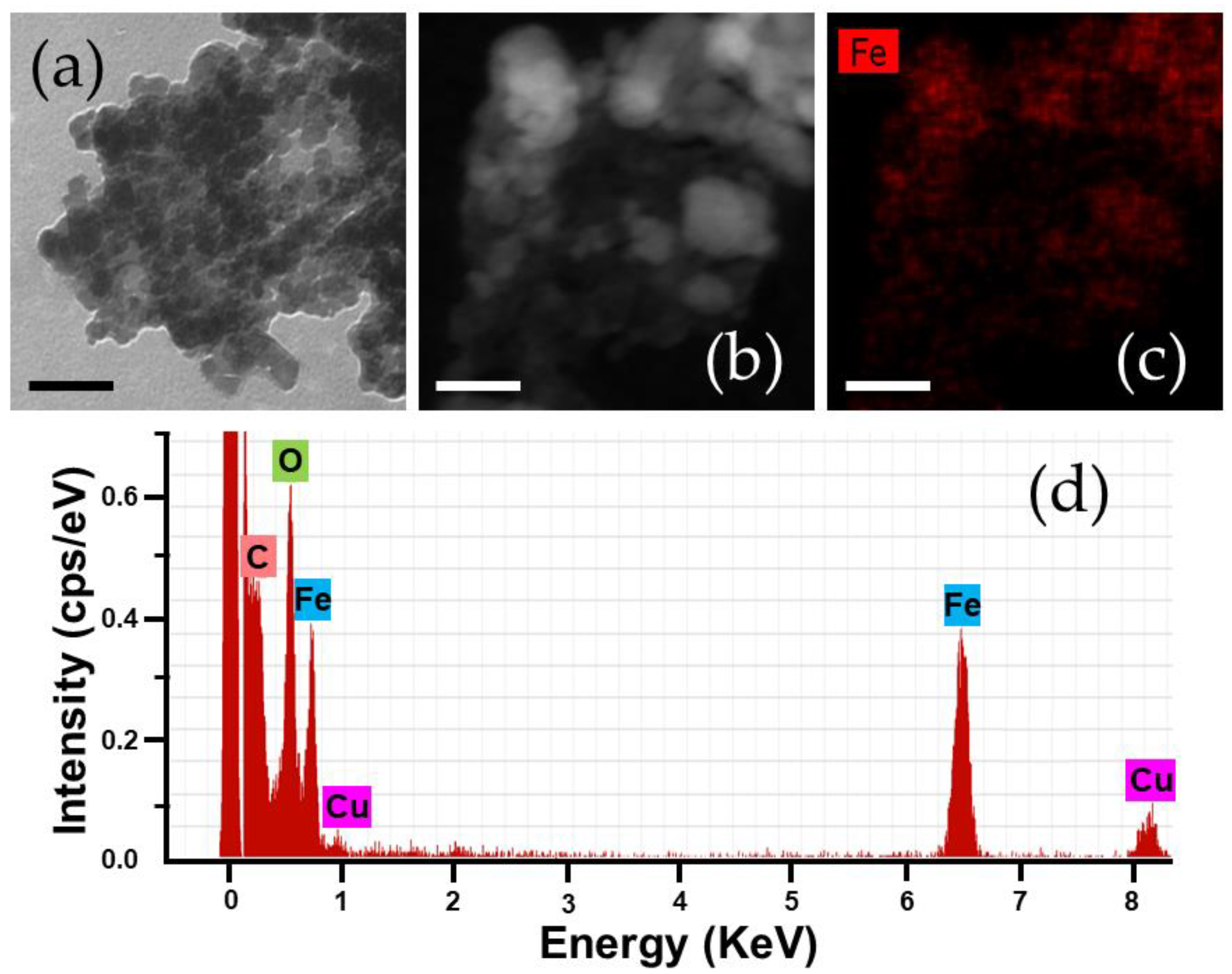
3.1. Characterization of the Fe3O4/PCL Nanocomposites
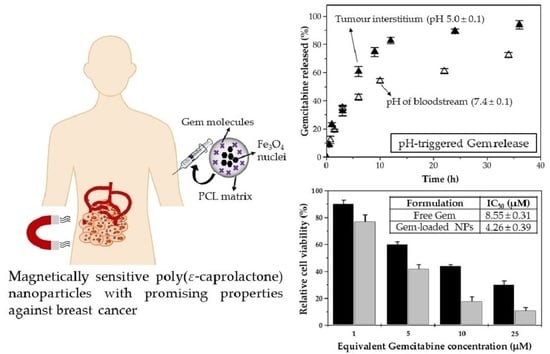
3.2. Characterization Gemcitabine Absorption and In Vitro Release
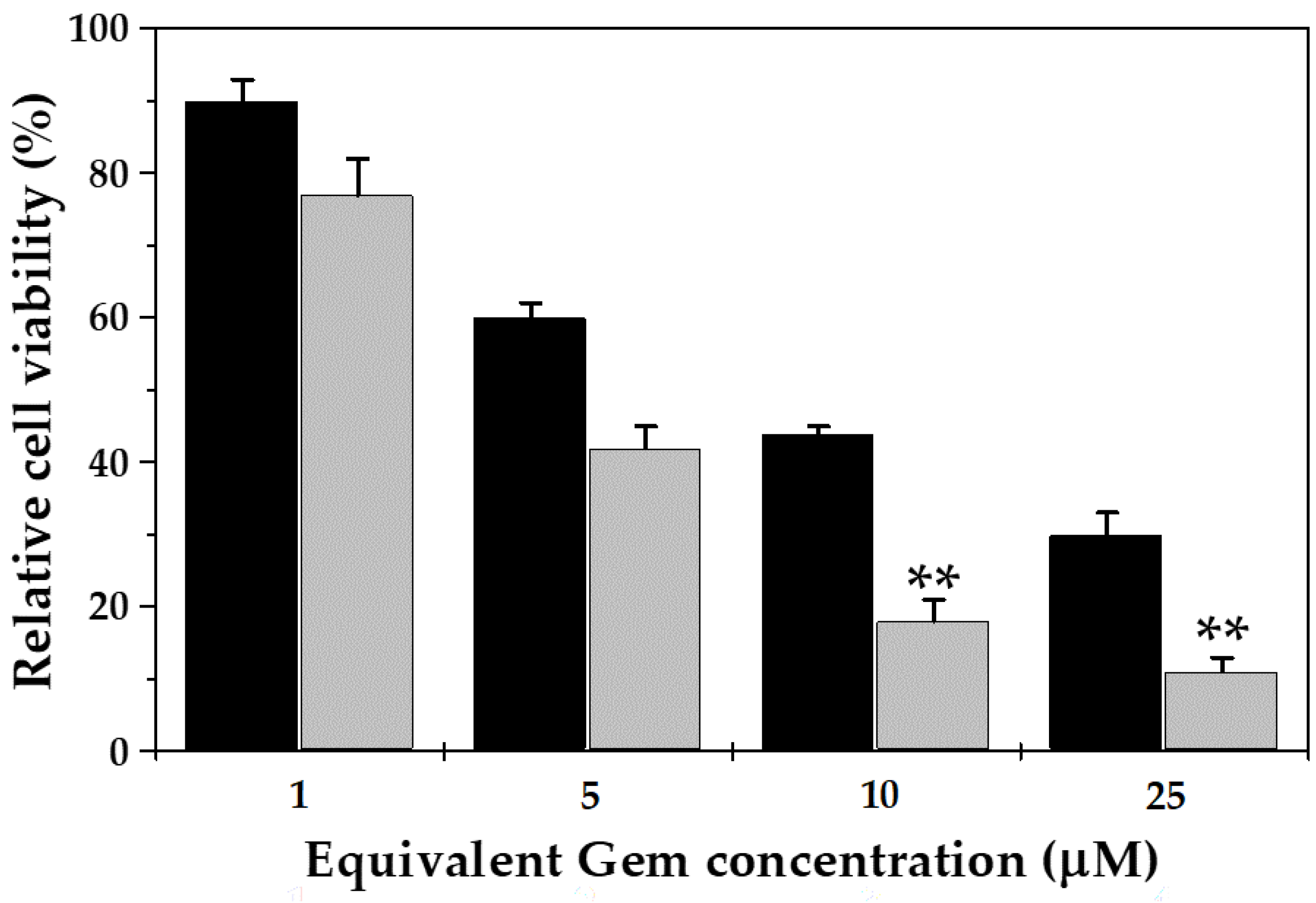
3.3. In Vitro Cytotoxicity
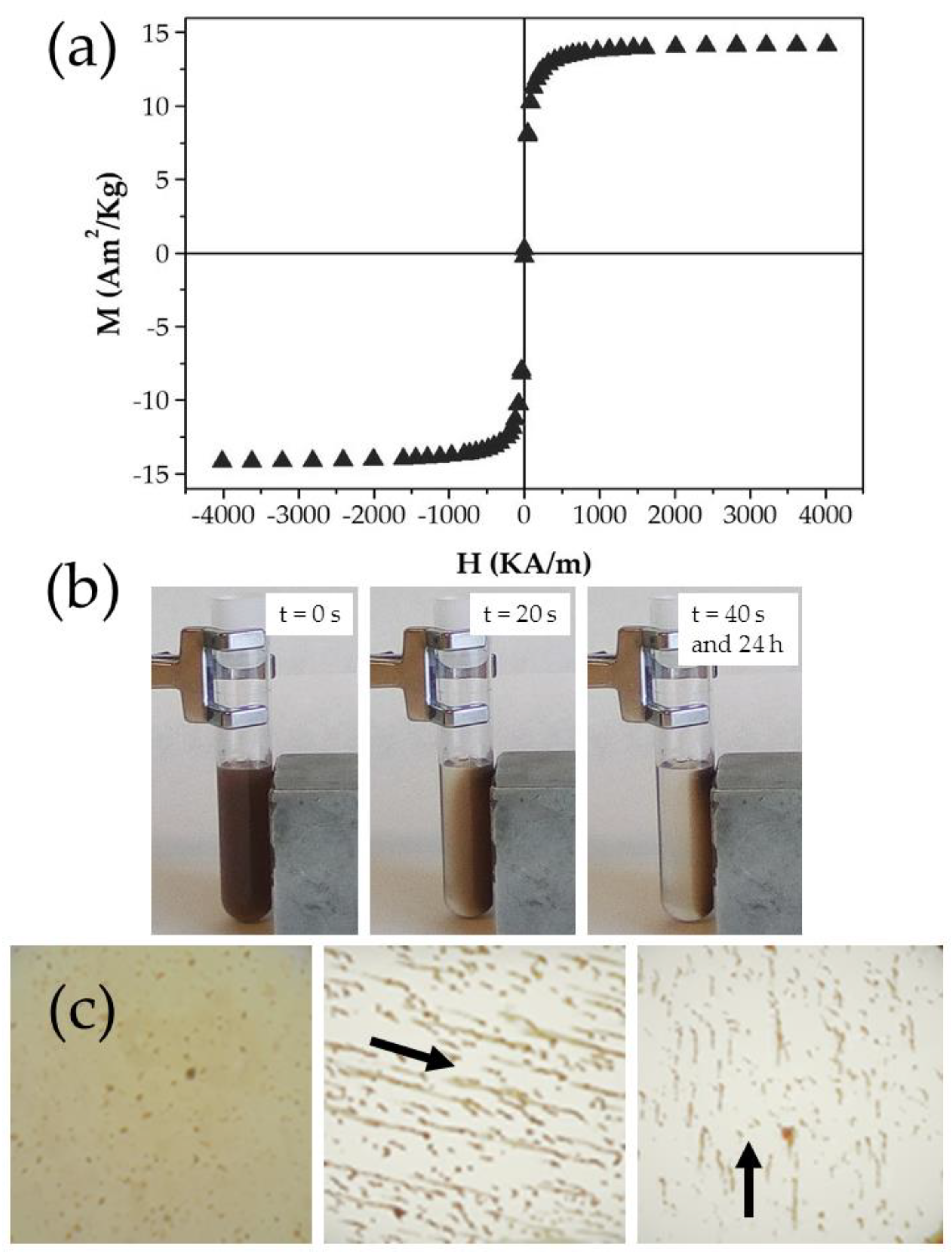
3.4. In Vitro Magnetic Responsiveness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reddy, L.; Couvreur, P. Novel approaches to deliver gemcitabine to cancers. Curr. Pharm. Des. 2008, 14, 1124–1137. [Google Scholar] [CrossRef]
- Heinemann, V. Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology 2003, 64, 191–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Mohammad, A.S.; Saralkar, P.; Sprowls, S.A.; Vickers, S.D.; John, D.; Tallman, R.M.; Lucke-Wold, B.P.; Jarrell, K.E.; Pinti, M.; et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol. Res. 2018, 132, 47–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, A.; Sasaki, T. Antitumor activity of sugar-modified cytosine nucleosides. Cancer Sci. 2004, 52, 215–229. [Google Scholar] [CrossRef] [Green Version]
- De Sousa Cavalcante, L.; Monteiro, G. Gemcitabine: Metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol. 2014, 741, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xie, J. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes Dis. 2015, 2, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papa, A.L.; Sidiqui, A.; Balasubramanian, S.U.A.; Sarangi, S.; Luchette, M.; Sengupta, S.; Harfouche, R. PEGylated liposomal gemcitabine: Insights into a potential breast cancer therapeutic. Cell. Oncol. 2013, 36, 449–457. [Google Scholar] [CrossRef]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Magnetoresponsive squalenoyl gemcitabine composite nanoparticles for cancer active targeting. Langmuir 2008, 24, 7512–7519. [Google Scholar] [CrossRef]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Huang, Y.; Wen, J. N-trimethyl chitosan nanoparticles and CSKSSDYQC peptide: N-trimethyl chitosan conjugates enhance the oral bioavailability of gemcitabine to treat breast cancer. J. Control. Release 2018, 277, 142–153. [Google Scholar] [CrossRef]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Polymeric nanoparticulate system augmented the anticancer therapeutic efficacy of gemcitabine. J. Drug Target. 2009, 17, 586–598. [Google Scholar] [CrossRef]
- Martín-Banderas, L.; Sáez-Fernández, E.; Holgado, M.Á.; Durán-Lobato, M.M.; Prados, J.C.; Melguizo, C.; Arias, J.L. Biocompatible gemcitabine-based nanomedicine engineered by Flow Focusing® for efficient antitumor activity. Int. J. Pharm. 2013, 443, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Superior preclinical efficacy of gemcitabine developed as chitosan nanoparticulate system. Biomacromolecules 2011, 12, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L. Drug targeting strategies in cancer treatment: An overview. Mini Rev. Med. Chem. 2010, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- López-Viota, M.; El-Hammadi, M.M.; Cabeza, L.; Prados, J.; Melguizo, C.; Ruiz Martinez, M.A.; Arias, J.L.; Delgado, Á.V. Development and characterization of magnetite/poly(butylcyanoacrylate) nanoparticles for magnetic targeted delivery of cancer drugs. AAPS PharmSciTech 2017, 18, 3042–3052. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Daoush, W.M. Co-precipitation and magnetic properties of magnetite nanoparticles for potential biomedical applications. J. Nanomed. Res. 2017, 5, 00118. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- El-Hammadi, M.M.; Arias, J.L. Iron oxide-based multifunctional nanoparticulate systems for biomedical applications: A patent review (2008–present). Expert Opin. Ther. Pat. 2015, 25, 691–709. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chang, X.; Chen, X.; Gu, Z.; Zhao, F.; Chai, Z.; Zhao, Y. Toxicity of inorganic nanomaterials in biomedical imaging. Biotechnol. Adv. 2014, 32, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.C.; Andronescu, E.; Vasile, B.Ș.; Truşcă, R.; Boldeiu, A.; Mogoantă, L.; Mogoșanu, G.D.; Temelie, M.; Radu, M.; Grumezescu, A.M.; et al. Fabrication and cytotoxicity of gemcitabine-functionalized magnetite nanoparticles. Molecules 2017, 22, 1080. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.R.; Xu, S.; Stains, J.P.; Bennett, C.H.; Lovering, R.M. Superparamagnetic iron oxide nanoparticles in musculoskeletal biology. Tissue Eng. Part B Rev. 2017, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; López-Viota, M.; Sáez-Fernández, E.; Ruiz, M.A. Formulation and physicochemical characterization of poly(ε-caprolactone) nanoparticles loaded with ftorafur and diclofenac sodium. Colloids Surf. B Biointerfaces 2010, 75, 204–208. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, L.; Ortiz, R.; Prados, J.; Delgado, Á.V.; Martín-Villena, M.J.; Clares, B.; Perazzoli, G.; Entrena, J.M.; Melguizo, C.; Arias, J.L. Improved antitumor activity and reduced toxicity of doxorubicin encapsulated in poly(ε-caprolactone) nanoparticles in lung and breast cancer treatment: An in vitro and in vivo study. Eur. J. Pharm. Sci. 2017, 102, 24–34. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-responsive polymer nanoparticles for drug delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [Green Version]
- Asadi, N.; Annabi, N.; Mostafavi, E.; Anzabi, M.; Khalilov, R.; Saghfi, S.; Mehrizadeh, M.; Akbarzadeh, A. Synthesis, characterization and in vitro evaluation of magnetic nanoparticles modified with PCL–PEG–PCL for controlled delivery of 5FU. Artif. Cells Nanomed. B 2018, 46, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Gang, J.; Park, S.B.; Hyung, W.; Choi, E.H.; Wen, J.; Kim, H.S.; Shul, Y.G.; Haam, S.; Song, S.Y. Magnetic poly epsilon-caprolactone nanoparticles containing Fe3O4 and gemcitabine enhance anti-tumor effect in pancreatic cancer xenograft mouse model. J. Drug Target. 2007, 15, 445–453. [Google Scholar] [CrossRef]
- Yang, J.; Park, S.B.; Yoon, H.G.; Huh, Y.M.; Haam, S. Preparation of poly epsilon-caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int. J. Pharm. 2006, 324, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Lyon, R.J.P. Infrared absorption spectroscopy. In Physical Methods in Determinative Mineralogy; Zussman, J., Ed.; Academic Press: London, UK, 1967; pp. 371–399. [Google Scholar]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds, 6th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Van Oss, C.J. Interfacial Forces in Aqueous Media, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Fe3O4/chitosan nanocomposite for magnetic drug targeting to cancer. J. Mater. Chem. 2012, 22, 7622–7632. [Google Scholar] [CrossRef]
- Arias, J.L.; Gómez-Gallo, A.; Delgado, Á.V.; Gallardo, V. Study of the stability of Kollidon® SR suspensions for pharmaceutical applications. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 107–113. [Google Scholar] [CrossRef]
- Martínez, N.A.; Fernández-Álvarez, F.; Delgado, Á.V.; Badillo-García, M.L.; Raba, J.; Cerutti, S.E.; Arias, J.L. First steps in the formulation of praziquantel nanosuspensions for pharmaceutical applications. Pharm. Dev. Technol. 2020, 25, 892–898. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Delgado, Á.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int. J. Pharm. 2017, 516, 61–70. [Google Scholar] [CrossRef]
- Hamoudeh, M.; Fessi, H. Preparation, characterization and surface study of poly-epsilon caprolactone magnetic microparticles. J. Colloid Interface Sci. 2006, 300, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Lee, S.B.; Lee, Y.M. Preparation and characterization of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric nanospheres for tumor-specific folate-mediated targeting of anticancer drugs. Biomaterials 2005, 26, 1053–1061. [Google Scholar] [CrossRef]
- Nakatuka, Y.; Yoshida, H.; Fukui, K.; Matuzawa, M. The effect of particle size distribution on effective zeta-potential by use of the sedimentation method. Adv. Powder Technol. 2015, 26, 650–656. [Google Scholar] [CrossRef]
- Plaza, R.C.; Arias, J.L.; Espín, M.; Jiménez, M.L.; Delgado, A.V. Aging effects in the electrokinetics of colloidal iron oxides. J. Colloid Interface Sci. 2002, 245, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Cózar-Bernal, M.J.; Holgado, M.A.; Arias, J.L.; Muñoz-Rubio, I.; Martín-Banderas, L.; Alvarez-Fuentes, J.; Fernández-Arévalo, M. Insulin-loaded PLGA microparticles: Flow focusing versus double emulsion/solvent evaporation. J. Microencapsul. 2011, 28, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Hoshiar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Lyklema, J. The role of surface conduction in the development of electrokinetics. In Interfacial Electrokinetics and Electrophoresis; Delgado, A.V., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 87–97. [Google Scholar]
- Muñoz de Escalona, M.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Arias, J.L. Magnetic solid lipid nanoparticles in hyperthermia against colon cancer. Int. J. Pharm. 2016, 504, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Cabeza, L.; Arias, J.L.; Melguizo, C.; Álvarez, P.J.; Vélez, C.; Clares, B.; Áranega, A.; Prados, J. Poly(butylcyanoacrylate) and poly(ε-caprolactone) nanoparticles loaded with 5-fluorouracil increase the cytotoxic effect of the drug in experimental colon cancer. AAPS J. 2015, 17, 918–929. [Google Scholar] [CrossRef] [Green Version]
- Arias, J.L.; Gallardo, V.; Gómez-Lopera, S.A.; Plaza, R.C.; Delgado, A.V. Synthesis and characterization of poly(ethyl-2-cyanoacrylate) nanoparticles with a magnetic core. J. Control. Release 2001, 77, 309–321. [Google Scholar] [CrossRef]
- Arias, J.L.; Ruiz, M.A.; Gallardo, V.; Delgado, A.V. Tegafur loading and release properties of magnetite/poly(alkylcyanoacrylate) (core/shell) nanoparticles. J. Control. Release 2008, 125, 50–58. [Google Scholar] [CrossRef]
- Arias, J.L.; Gallardo, V.; Ruiz, M.A.; Delgado, A.V. Magnetite/poly(alkylcyanoacrylate) (core/shell) nanoparticles as 5-Fluorouracil delivery systems for active targeting. Eur. J. Pharm. Biopharm. 2008, 69, 54–63. [Google Scholar] [CrossRef]
- Santos, D.P.; Ruiz, M.A.; Gallardo, V.; Zanoni, M.V.B.; Arias, J.L. Multifunctional antitumor magnetite/chitosan-L-glutamic acid (core/shell) nanocomposites. J. Nanopart. Res. 2011, 13, 4311–4323. [Google Scholar] [CrossRef]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef]
- Dong, A.; Lan, S.; Huang, J.; Wang, T.; Zhao, T.; Xiao, L.; Wang, W.; Zheng, X.; Liu, F.; Gao, G.; et al. Modifying Fe3O4-functionalized nanoparticles with N-halamine and their magnetic/antibacterial properties. ACS Appl. Mater. Interfaces 2011, 3, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.V.; Rudzinski, W.E. Biodegradable polymeric microparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Blanco, M.D.; Bernardo, M.V.; Sastre, R.L.; Olmo, R.; Muñiz, E.; Teijón, J.M. Preparation of bupivacaine-loaded poly(ε-caprolactone) microspheres by spray drying: Drug release studies and biocompatibility. Eur. J. Pharm. Biopharm. 2003, 55, 229–236. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Ghoroghchian, P.P.; Li, G.; Levine, D.H.; Davis, K.P.; Bates, F.S.; Hammer, D.A.; Therien, M.J. Bioresorbable vesicles formed through spontaneous self-assembly of amphiphilic poly(ethylene oxide)-block-polycaprolactone. Macromolecules 2006, 39, 1673–1675. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.H.; Che, J.H.; Seo, J.M.; Jeong, J.; Kim, E.T.; Lee, S.W.; Koo, K.I.; Suaning, G.J.; Lovell, N.H.; Cho, D.I.; et al. In vitro biocompatibility of various polymer-based microelectrode arrays for retinal prosthesis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2653–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varan, C.; Bilensoy, E. Caitonic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017, 8, 1446–1456. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.A.; Nguyen, V.C. Formation of nanoparticles in aqueous solution from poly(ε-caprolactone)–poly(ethylene glycol)–poly(ε-caprolactone). Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 025012. [Google Scholar] [CrossRef] [Green Version]
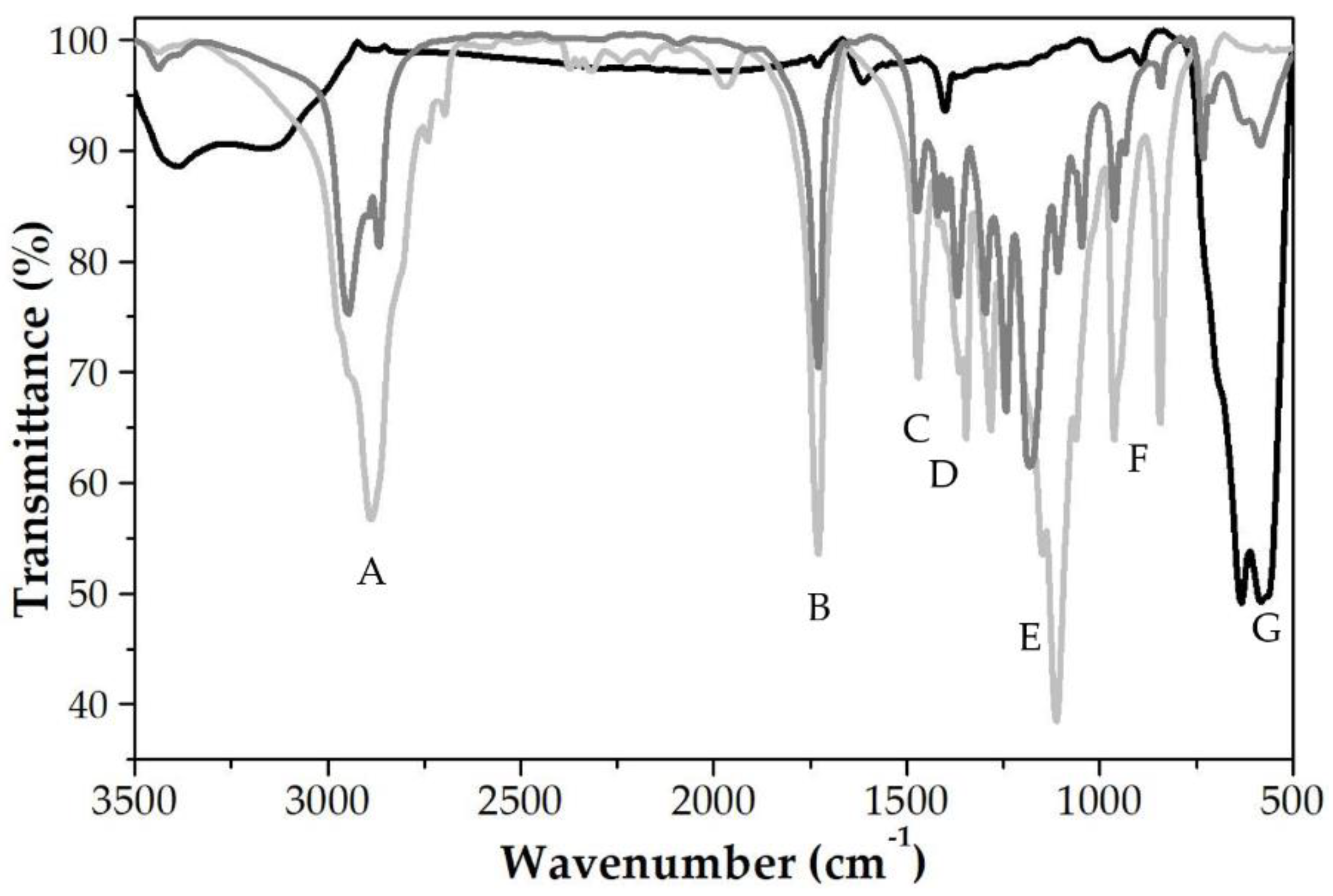
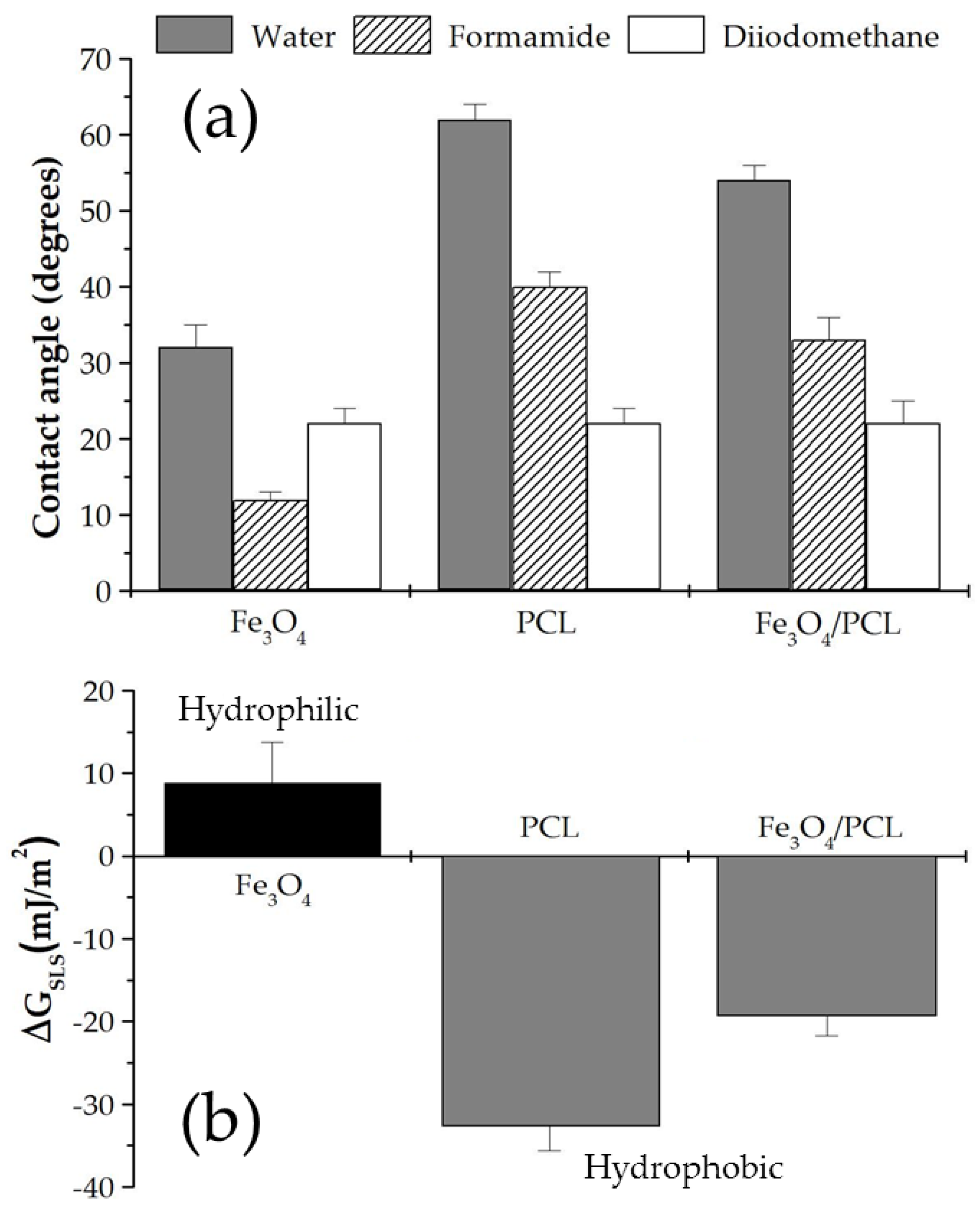
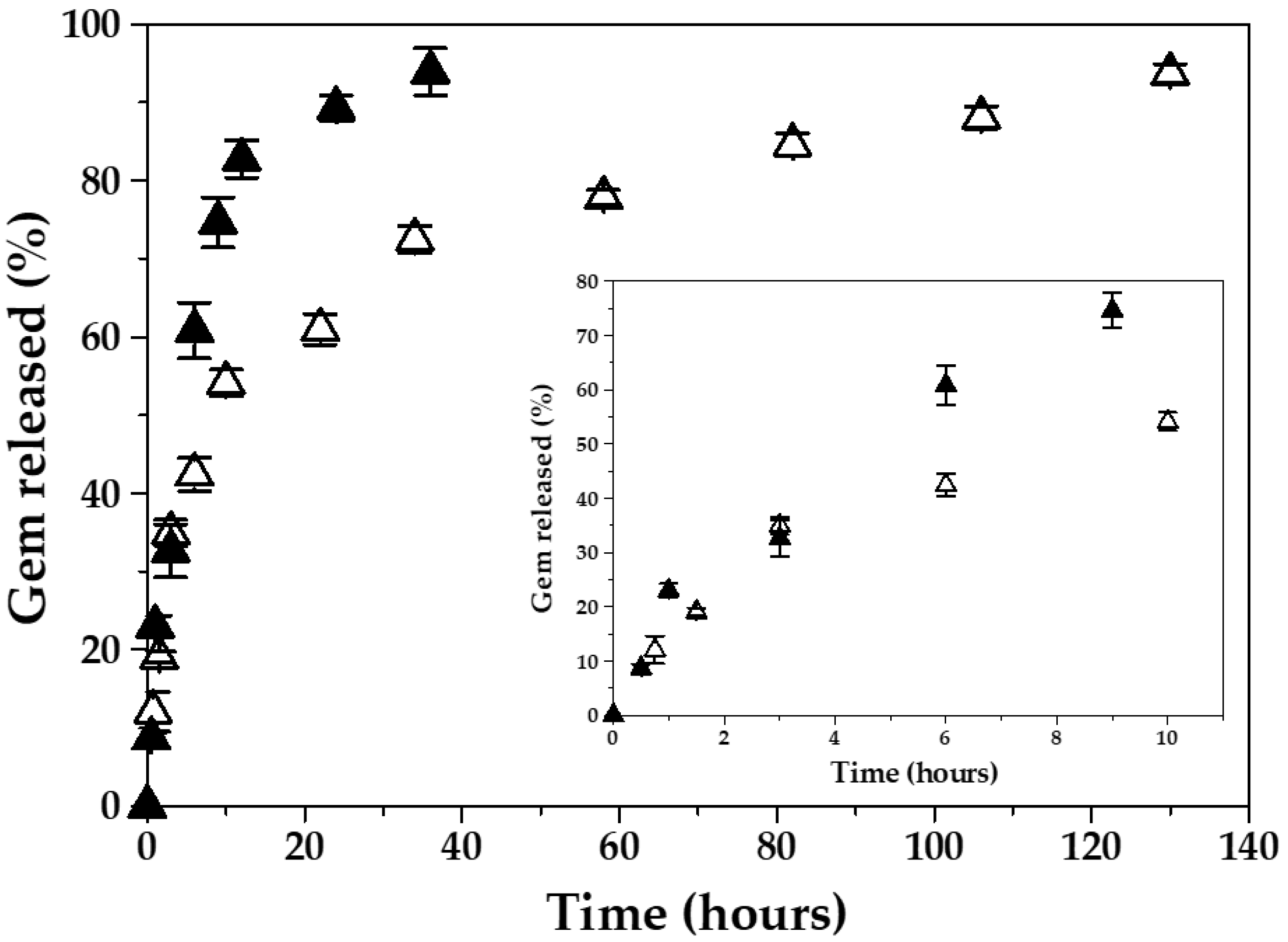
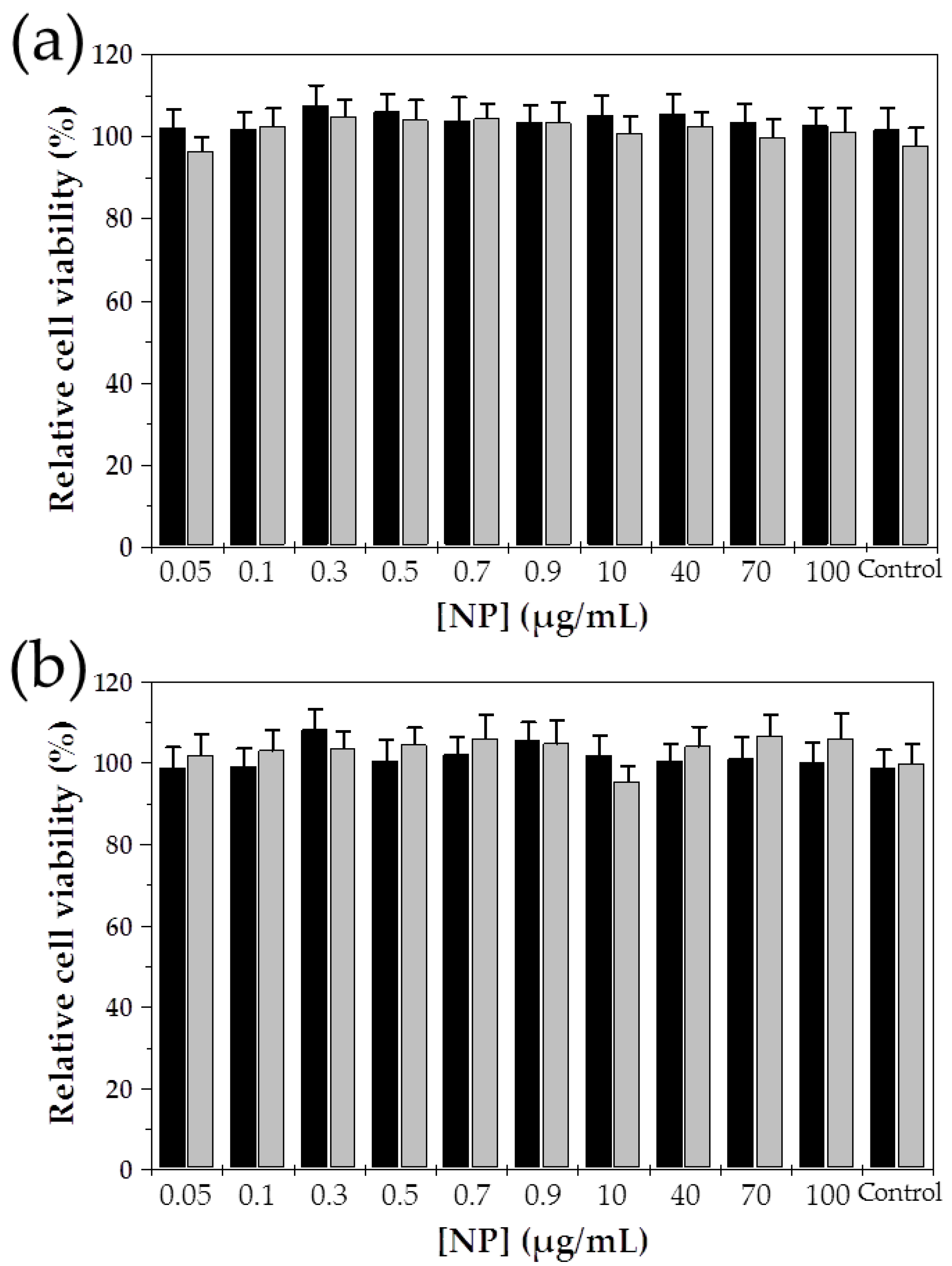
| Fe3O4:PCL Weight Ratio | 4:1 | 4:2 | 4:3 | 4:4 | 3:4 | 2:4 | 1:4 |
|---|---|---|---|---|---|---|---|
| Yield (%) | ≈15 | ≈20 | ≈30 | 80.1 ± 7.6 | 69.1 ± 12.2 | 91.5 ± 8.1 | 57.9 ± 6.3 |
| Size (nm) | 136 ± 2 | 142 ± 3 | 138 ± 3 | 196 ± 16 | 278 ± 7 | 126 ± 1 | 455 ± 107 |
| PdI | 0.21 ± 0.02 | 0.23 ± 0.01 | 0.23 ± 0.02 | 0.41 ± 0.01 | 0.31 ± 0.01 | 0.26 ± 0.03 | 0.93 ± 0.06 |
| Solid | (mJ/m2) | (mJ/m2) | (mJ/m2) |
|---|---|---|---|
| Fe3O4 | 47.17 ± 0.64 | 0.68 ± 0.15 | 37.75 ± 3.01 |
| PCL | 47.17 ± 0.64 | 0.16 ± 0.01 | 14.75 ± 1.23 |
| Fe3O4/PCL | 47.17 ± 0.96 | 0.33 ± 0.04 | 20.45 ± 0.91 |
| [Gem] (M) | EE (%) | DL (%) |
|---|---|---|
| 10−5 | 12.232 ± 1.483 | 0.016 ± 0.012 |
| 5 × 10−5 | 31.286 ± 1.014 | 0.163 ± 0.013 |
| 10−4 | 56.007 ± 0.264 | 1.094 ± 0.185 |
| 5 × 10−4 | 71.013 ± 0.994 | 6.433 ± 1.141 |
| 10−3 | 87.468 ± 0.332 | 11.174 ± 3.222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, G.; Fernández-Álvarez, F.; Cabeza, L.; Delgado, Á.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Gemcitabine-Loaded Magnetically Responsive Poly(ε-caprolactone) Nanoparticles against Breast Cancer. Polymers 2020, 12, 2790. https://doi.org/10.3390/polym12122790
García-García G, Fernández-Álvarez F, Cabeza L, Delgado ÁV, Melguizo C, Prados JC, Arias JL. Gemcitabine-Loaded Magnetically Responsive Poly(ε-caprolactone) Nanoparticles against Breast Cancer. Polymers. 2020; 12(12):2790. https://doi.org/10.3390/polym12122790
Chicago/Turabian StyleGarcía-García, Gracia, Fátima Fernández-Álvarez, Laura Cabeza, Ángel V. Delgado, Consolación Melguizo, José C. Prados, and José L. Arias. 2020. "Gemcitabine-Loaded Magnetically Responsive Poly(ε-caprolactone) Nanoparticles against Breast Cancer" Polymers 12, no. 12: 2790. https://doi.org/10.3390/polym12122790
APA StyleGarcía-García, G., Fernández-Álvarez, F., Cabeza, L., Delgado, Á. V., Melguizo, C., Prados, J. C., & Arias, J. L. (2020). Gemcitabine-Loaded Magnetically Responsive Poly(ε-caprolactone) Nanoparticles against Breast Cancer. Polymers, 12(12), 2790. https://doi.org/10.3390/polym12122790