Synthesis, Optical, Thermal and Structural Characteristics of Novel Thermocleavable Polymers Based on Phthalate Esters
Abstract
1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Measurements
2.3. Synthesis of the Monomers
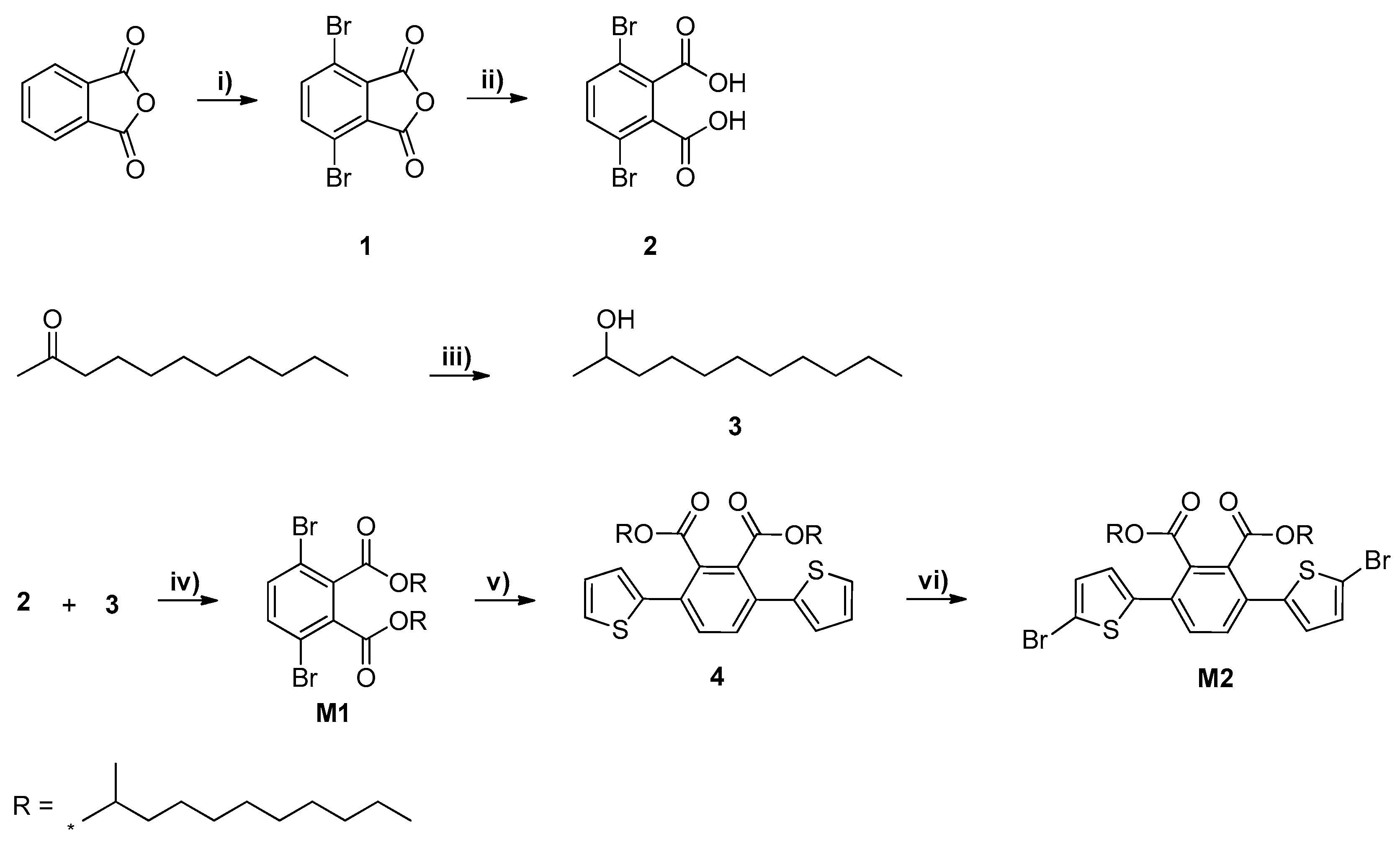
2.3.1. Synthesis of 3,6-Dibromophthalic Anhydride (1)
2.3.2. Synthesis of 3,6-Dibromophthalic Acid (2)
2.3.3. Synthesis of 2-Undecanol (3)
2.3.4. Synthesis of 3,6-Dibromo-Bis(2-Undecanyl) Phthalate (M1)
2.3.5. Synthesis of 3,6-Bis(2-Thienyl)-Bis(2-Undecanyl) Phthalate (4)
2.3.6. Synthesis of 5,5′-Dibromo-3,6-Bis(2-Thienyl)-Bis(2-Undecanyl) Phthalate (M2)
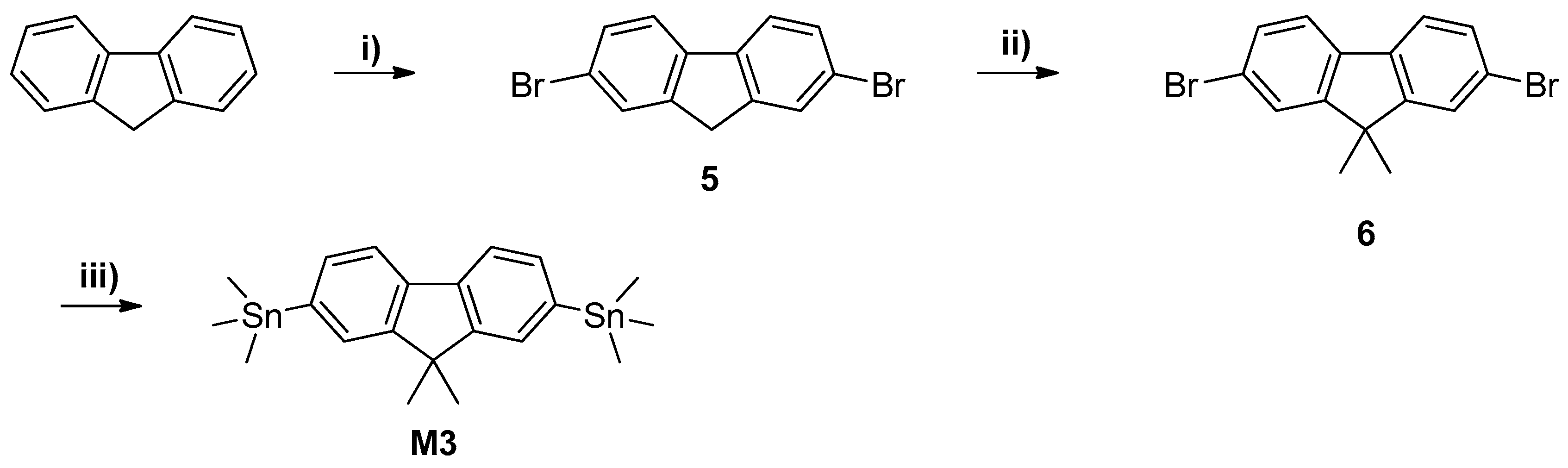
2.3.7. Synthesis of 2,7-Dibromofluorene (5)
2.3.8. Synthesis of 9,9-Dimethyl-2,7-Dibromofluorene (6)
2.3.9. Synthesis of 9,9-Dimethyl-2,7-Bis(Trimethylstannyl) Fluorine (M3)
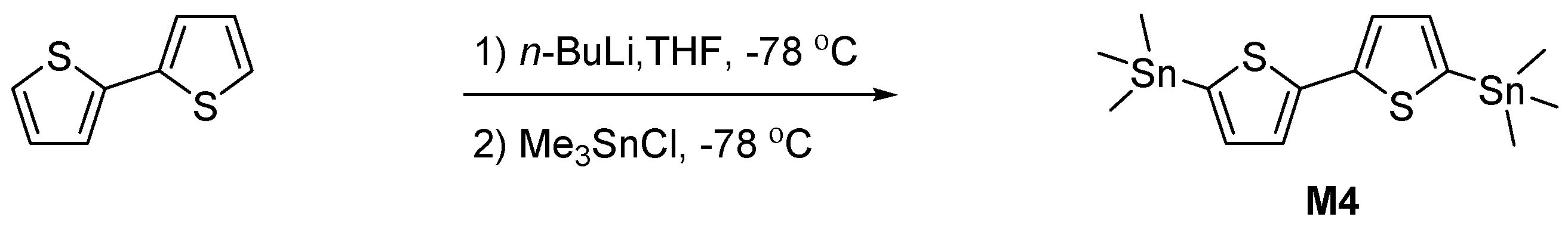
2.3.10. Synthesis of 5,5′-Bis(Trimethylstannyl)-2,2′-Bithiophene (M4)
2.4. Synthetic of the Polymers
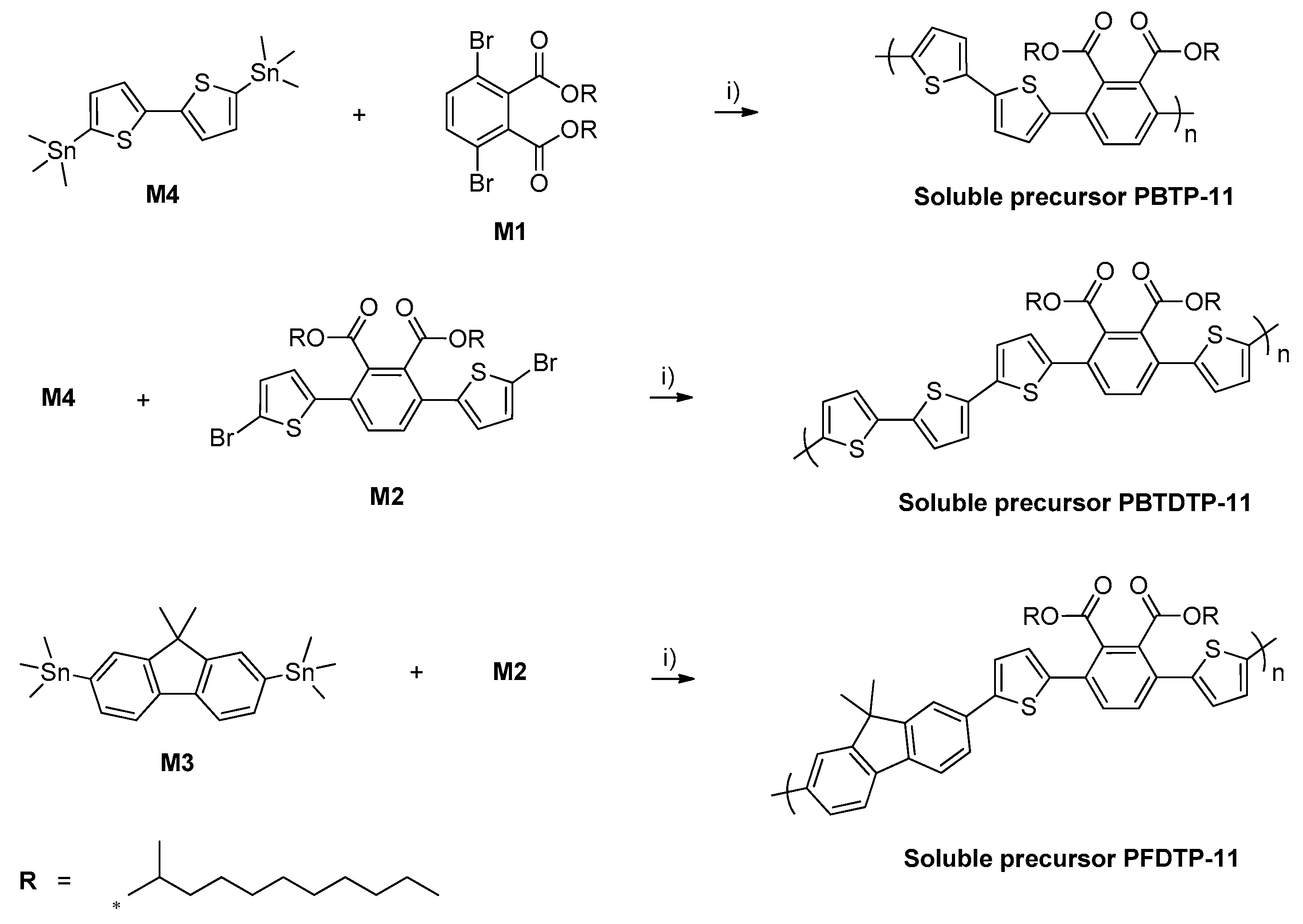
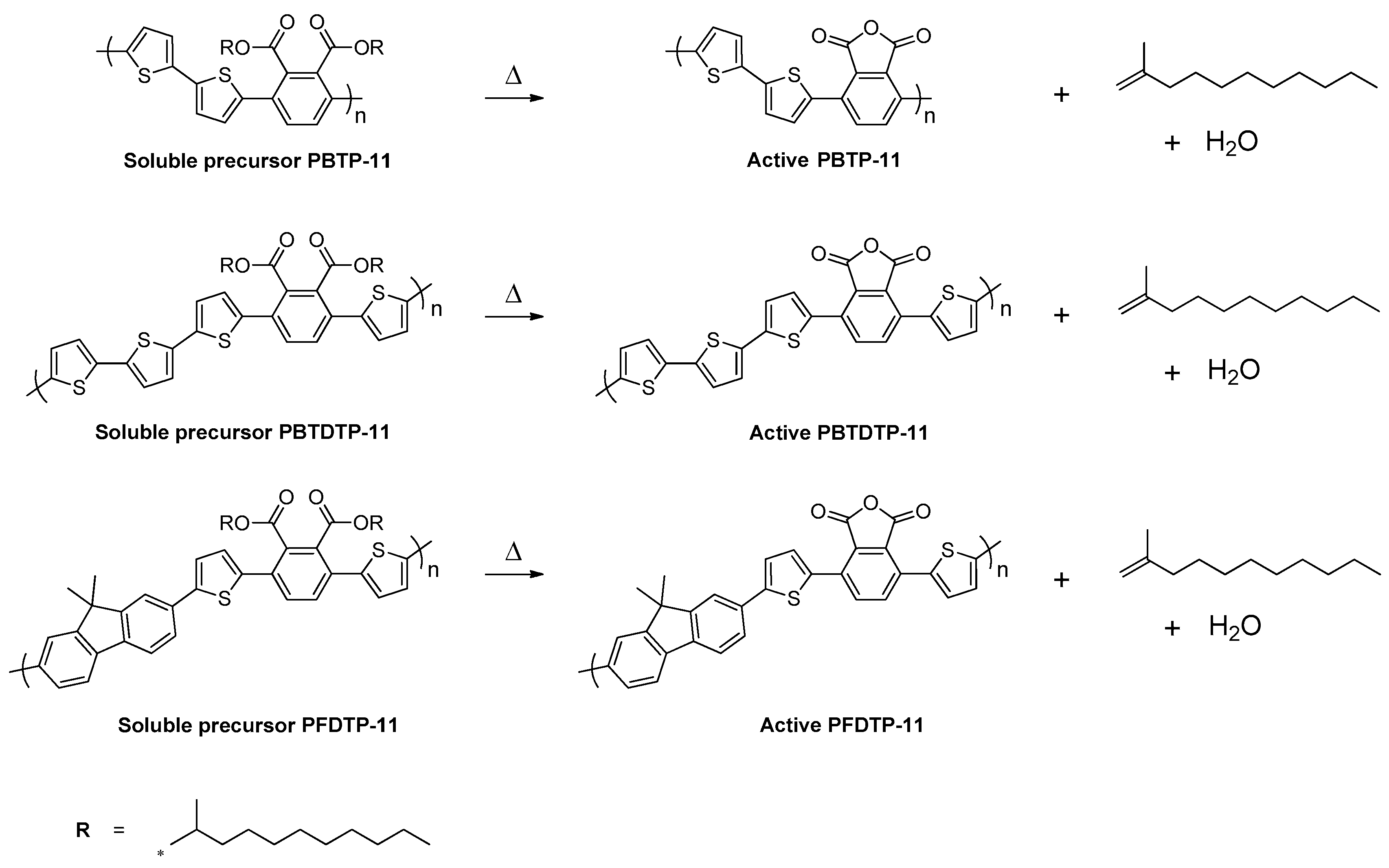
2.4.1. Synthesis of Poly[2,2′-Bithiophene-Alt-(3′,6′-bis(2-Undecanyl)Phthalate)] (PBTP-11)
2.4.2. Synthesis of Poly[2,2′-Bithiophene-Alt-5,5-(3′,6′-Bis(2-Thienyl)-Bis(2-Undecanyl)Phthalate)] (PBTDTP-11)
2.4.3. Synthesis of Poly[9,9-Dimethyl-2,7-Fluorene-Alt-5,5-(3′,6′-Bis(2-Thienyl)-Bis(2-Undecanyl)Phthalate)] (PFDTP-11)
3. Results and Discussion
3.1. Synthesis of Monomers and Polymers
3.2. Optical Properties
3.3. Thermal Properties
3.4. Powder X-Ray Diffraction (XRD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the Planet: Chemical Challenges in Solar Energy Utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible Climate Change Due to Carbon Dioxide Emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef]
- Lewis, N.S.; Crabtree, G.; Nozik, A.J.; Wasielewski, M.R.; Alivisatos, P.; Kung, H.; Tsao, J.; Chandler, E.; Walukiewicz, W.; Spitler, M.; et al. Basic Research Needs for Solar Energy Utilization. In Report of the Basic Energy Sciences Workshop on Solar Energy Utilization; US Department of Energy: Washington, DC, USA, 2005; pp. 1–276. [Google Scholar]
- Smalley, R.E. Future Global Energy Prosperity: The Terawatt Challenge. MRS Bull. 2005, 30, 412–417. [Google Scholar] [CrossRef]
- Lewis, N.S. Toward Cost-Effective Solar Energy Use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Morton, O. Solar Energy: A New Day dawning? Silicon Valley Sunrise. Nature 2006, 443, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Chapin, D.M.; Fuller, C.S.; Pearson, G.L. A New Silicon p-n Junction Photocell for Converting Solar Radiation into Electrical Power. J. Appl. Phys. 1954, 25, 676–677. [Google Scholar] [CrossRef]
- Ameri, T.; Dennler, G.; Lungenschmied, C.; Brabec, C.J. Organic Tandem Solar Cells: A Review. Energy Environ. Sci. 2009, 2, 347–363. [Google Scholar] [CrossRef]
- Green, M.A.; Views, C. Solar Cell Efficiency Tables. Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Long, G. High Performance Photovoltaic Applications Using Solution-Processed Small Molecules. Acc. Chem. Res. 2013, 46, 2645–2655. [Google Scholar] [CrossRef]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-Processed Small-Molecule Solar Cells with 6.7% Efficiency. Nat. Mater. 2012, 11, 44–48. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Zoombelt, A.P.; Mathijssen, S.G.J.; Wienk, M.M.; Turbiez, M.; De Leeuw, D.M.; Janssen, R.A.J. Poly(diketopyrrolopyrrole−terthiophene) for Ambipolar Logic and Photovoltaics. J. Am. Chem. Soc. 2009, 131, 16616–16617. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, L.; Iraqi, A.; Zhang, Y.; Wang, T.; Lidzey, D.G. Impact of Fluorine Substitution Upon the Photovoltaic Properties of Benzothiadiazole-Fluorene Alternate Copolymers. RSC Adv. 2015, 5, 46386–46394. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cao, Y.; Zhang, J.; Wang, M.; Li, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. High-Performance Dye-Sensitized Solar Cells Based on Solvent-Free Electrolytes Produced from Eutectic Melts. Nat. Mater. 2008, 7, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C. Fabrication and Processing of Polymer Solar Cells: A Review of Printing and Coating Techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer Solar Cell Modules Prepared Using Roll-to-Roll Methods: Knife-over-Edge Coating, Slot-Die Coating and Screen Printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Krebs, F.C. Roll-to-Roll Fabrication of Monolithic Large-Area Polymer Solar Cells Free from Indium-Tin-Oxide. Sol. Energy Mater. Sol. Cells 2009, 93, 1636–1641. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design Rules for Donors in Bulk-Heterojunction Solar Cells—Towards 10% Energy-Conversion Efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Brédas, J.-L.; Norton, J.E.; Cornil, J.; Coropceanu, V. Molecular Understanding of Organic Solar Cells: The Challenges. Accounts Chem. Res. 2009, 42, 1691–1699. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltiac Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic Solar Cells: An Overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J. Structures and Properties of Conjugated Donor–Acceptor Copolymers for Solar Cell Applications. J. Mater. Chem. 2012, 22, 4178–4187. [Google Scholar] [CrossRef]
- Rispens, M.T.; Meetsma, A.; Rittberger, R.; Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Influence of the Solvent on the Crystal Structure of PCBM and the Efficiency of MDMO-PPV:PCBM ‘plastic’ Solar Cells. Chem. Commun. 2003, 13, 2116–2118. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Roy, A.; Beaupre, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk Heterojunction Solar Cells with Internal Quantum Efficiency Approaching 100 & Percent. Nat. Photonics 2009, 3, 297–302. [Google Scholar]
- Amb, C.M.; Chen, S.; Graham, K.R.; Subbiah, J.; Small, C.E.; So, F.; Reynolds, J.R. Dithienogermole As a Fused Electron Donor in Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2011, 133, 10062–10065. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational Design of High Performance Conjugated Polymers for Organic Solar Cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Helgesen, M.; Søndergaard, R.; Krebs, F.C. Advanced Materials and Processes for Polymer Solar Cell Devices. J. Mater. Chem. 2010, 20, 36–60. [Google Scholar] [CrossRef]
- Norrman, K.; Larsen, N.B.; Krebs, F.C. Lifetimes of Organic Photovoltaics: Combining Chemical and Physical Characterisation Techniques to Study Degradation Mechanisms. Sol. Energy Mater. Sol. Cells 2006, 90, 2793–2814. [Google Scholar] [CrossRef]
- Norrman, K.; Krebs, F.C. Lifetimes of Organic Photovoltaics: Using TOF-SIMS and 18O2 Isotopic Labelling to Characterise Chemical Degradation Mechanisms. Sol. Energy Mater. Sol. Cells 2006, 90, 213–227. [Google Scholar] [CrossRef]
- Norrman, K.; Gevorgyan, S.A.; Krebs, F.C. Water-Induced Degradation of Polymer Solar Cells Studied by H218O Labeling. ACS Appl. Mater. Interfaces 2009, 1, 102–112. [Google Scholar] [CrossRef]
- Lira-Cantu, M.; Norrman, K.; Andreasen, J.W.; Krebs, F.C. Oxygen Release and Exchange in Niobium Oxide MEH-PPV Hybrid Solar Cells. Chem. Mater. 2006, 18, 5684–5690. [Google Scholar] [CrossRef]
- Manceau, M.; Rivaton, A.; Gardette, J.-L.; Guillerez, S.; Lemaître, N. The Mechanism of Photo- and Thermooxidation of poly(3-Hexylthiophene) (P3HT) Reconsidered. Polym. Degrad. Stab. 2009, 94, 898–907. [Google Scholar] [CrossRef]
- Edder, C.; Armstrong, P.B.; Prado, K.B.; Fréchet, J.M.J. Benzothiadiazole- and Pyrrole-Based Polymers Bearing Thermally Cleavable Solubilizing Groups as Precursors for Low Bandgap Polymers. Chem. Commun. 2006, 18, 1965–1967. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, O.; Bjerring, M.; Nielsen, N.C.; Krebs, F.C. All Solution Processed Tandem Polymer Solar Cells Based on Thermocleavable materials. Sol. Energy Mater. Sol. Cells 2008, 92, 1327–1335. [Google Scholar] [CrossRef]
- Bjerring, M.; Nielsen, J.S.; Siu, A.; Nielsen, N.C.; Krebs, F.C. An Explanation for the High Stability of Polycarboxythiophenes in Photovoltaic devices—A Solid-State NMR Dipolar Recoupling Study. Sol. Energy Mater. Sol. Cells 2008, 92, 772–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Hau, S.K.; Yip, H.-L.; Sun, Y.; Acton, O.; Jen, A.K.-Y. Efficient Polymer Solar Cells Based on the Copolymers of Benzodithiophene and Thienopyrroledione. Chem. Mater. 2010, 22, 2696–2698. [Google Scholar] [CrossRef]
- Piliego, C.; Holcombe, T.W.; Douglas, J.D.; Woo, C.H.; Beaujuge, P.M.; Fréchet, J.M.J. Synthetic Control of Structural Order in N-Alkylthieno[3,4-c]pyrrole-4,6-Dione-Based Polymers for Efficient Solar Cells. J. Am. Chem. Soc. 2010, 132, 7595–7597. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, Y.; Zhang, Q.; Xie, Z. Benzo[1,2-b:4,5-b′]dithiophene-Dioxopyrrolothiophen Copolymers for High Performance Solar Cells. Chem. Commun. 2010, 46, 4997. [Google Scholar] [CrossRef]
- Cabanetos, C.; El Labban, A.; Bartelt, J.A.; Douglas, J.D.; Mateker, W.R.; Fréchet, J.M.J.; McGehee, M.D.; Beaujuge, P.M. Linear Side Chains in Benzo[1,2-b:4,5-b′]dithiophene–Thieno[3,4-c]pyrrole-4,6-Dione Polymers Direct Self-Assembly and Solar Cell Performance. J. Am. Chem. Soc. 2013, 135, 4656–4659. [Google Scholar] [CrossRef]
- Liu, J.; Kadnikova, E.N.; Liu, Y.; McGehee, M.D.; Fréchet, J.M.J. Polythiophene Containing Thermally Removable Solubilizing Groups Enhances the Interface and the Performance of Polymer−Titania Hybrid Solar Cells. J. Am. Chem. Soc. 2004, 126, 9486–9487. [Google Scholar] [CrossRef]
- Helgesen, M.; Gevorgyan, S.A.; Krebs, F.C.; Janssen, R.A.J. Substituted 2,1,3-Benzothiadiazole- And Thiophene-Based Polymers for Solar Cells—Introducing a New Thermocleavable Precursor. Chem. Mater. 2009, 21, 4669–4675. [Google Scholar] [CrossRef]
- Helgesen, M.; Krebs, F.C. Photovoltaic Performance of Polymers Based on Dithienylthienopyrazines Bearing Thermocleavable Benzoate Esters. Macromolecules 2010, 43, 1253–1260. [Google Scholar] [CrossRef]
- Petersen, M.H.; A Gevorgyan, S.; Krebs, F.C. Thermocleavable Low Band Gap Polymers and Solar Cells Therefrom with Remarkable Stability Toward Oxygen. Macromolecules 2008, 41, 8986–8994. [Google Scholar] [CrossRef]
- Helgesen, M.; Bjerring, M.; Nielsen, N.C.; Krebs, F.C. Influence of the Annealing Temperature on the Photovoltaic Performance and Film Morphology Applying Novel Thermocleavable Materials. Chem. Mater. 2010, 22, 5617–5624. [Google Scholar] [CrossRef]
- Guo, X.; Kim, F.S.; Jenekhe, S.A.; Watson, M.D. Phthalimide-Based Polymers for High Performance Organic Thin-Film Transistors. J. Am. Chem. Soc. 2009, 131, 7206–7207. [Google Scholar] [CrossRef]
- Eleya, N.; Patonay, T.; Villinger, A.; Langer, P. Synthesis of Dimethyl Tetraarylphthalates by Suzuki-Miyaura Reactions of Dimethyl Tetrabromophthalate. Helvetica Chim. Acta 2013, 96, 408–413. [Google Scholar] [CrossRef]
- Albanese, D.C.M.; Benaglia, M.; Landini, D.; Maia, A.; Lupi, A.V.; Penso, M. Use of a Quaternary Ammonium Salt Supported on a Liposoluble Poly(ethylene Glycol) Matrix for Laboratory and Industrial Synthetic Applications of Phase-Transfer Catalysis. Ind. Eng. Chem. Res. 2002, 41, 4928–4935. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.-M.; Song, K.W.; Moon, D.K. Synthesis and Photovoltaic Property of Polymer Semiconductor with Phthalimide Derivative as a Promising Electron Withdrawing Material. Eur. Polym. J. 2012, 48, 532–540. [Google Scholar] [CrossRef]
- Lee, J.Y.; Song, K.W.; Ku, J.R.; Sung, T.H.; Moon, D.K. Development of DA-Type Polymers with Phthalimide Derivatives As Electron Withdrawing Units and a Promising Strategy for the Enhancement of Photovoltaic Properties. Sol. Energy Mater. Sol. Cells 2011, 95, 3377–3384. [Google Scholar] [CrossRef]
- Li, Q.; Guo, H.; Ma, L.; Wu, W.; Liu, Y.; Zhao, J. Tuning the Photophysical Properties of N^N Pt(ii) Bisacetylide Complexes with Fluorene Moiety and Its Applications for triplet–triplet-Annihilation Based Upconversion. J. Mater. Chem. 2012, 22, 5319–5329. [Google Scholar] [CrossRef]
- Singh, V.; Wang, S.; Kool, E.T.; Chan, K.M.; A Clark, S. Genetically Encoded Multispectral Labeling of Proteins with Polyfluorophores on a DNA Backbone. J. Am. Chem. Soc. 2013, 135, 6184–6191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, P.; Lalancette, R.A.; Jäkle, F. Applying the Oligomer Approach to Luminescent Conjugated Organoboranes. J. Am. Chem. Soc. 2011, 133, 8802–8805. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.S.; Park, J.W.; Kim, S.-O.; Heo, K.; Park, C.E.; Ree, M.; Kim, Y.-H.; Kwon, S.-K. Alternating Copolymers Containing Bithiophene and Dialkoxynaphthalene for the Applications to Field Effect Transistor and Photovoltaic Cell: Performance and Stability. Chem. Mater. 2009, 21, 5499–5507. [Google Scholar] [CrossRef]
- Zhu, Z.; Waller, D.; Gaudiana, R.; Morana, M.; Mühlbacher, D.; Scharber, A.M.; Brabec, C. Panchromatic Conjugated Polymers Containing Alternating Donor/Acceptor Units for Photovoltaic Applications. Macromolecules 2007, 40, 1981–1986. [Google Scholar] [CrossRef]
- Aziz, S.B. Modifying Poly(Vinyl Alcohol) (PVA) from Insulator to Small-Bandgap Polymer: A Novel Approach for Organic Solar Cells and Optoelectronic Devices. J. Electron. Mater. 2016, 45, 736–745. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hassan, A.Q.; Mohammed, S.J.; Karim, W.O.; Kadir, M.F.Z.; Tajuddin, H.A.; Chan, N.N.M.Y. Structural and Optical Characteristics of PVA:C-Dot Composites: Tuning the Absorption of Ultra Violet (UV) Region. Nanomaterials 2019, 9, 216. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Ahang, M.H.; Hameed, M.A. Fabrication of Polymer Blend Composites Based on [PVA-PVP](1−x):(Ag2S) × (0.01 ≤ × ≤ 0.03) With Small Optical Band Gaps: Structural and Optical Properties. Mater. Sci. Semicond. Process. 2017, 71, 197–203. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Ahmed, H.M. Synthesis of Polymer Nanocomposites Based on [Methyl Cellulose] (1−x):(CuS) × (0.02 M ≤ × ≤ 0.08 M) With Desired Optical Band Gaps. Polymers 2017, 9, 194. [Google Scholar] [CrossRef]
- Brza, M.A.; Aziz, S.B.; Anuar, H.; Al Hazza, M.H.F. From Green Remediation to Polymer Hybrid Fabrication with Improved Optical Band Gaps. Int. J. Mol. Sci. 2019, 20, 3910. [Google Scholar] [CrossRef]
- Abdullah, R.M.; Aziz, S.B.; Mamand, S.M.; Hassan, A.; Hussen, S.A.; Kadir, M.F.Z. Reducing the Crystallite Size of Spherulites in PEO-Based Polymer Nanocomposites Mediated by Carbon Nanodots and Ag Nanoparticles. Nanomaterials 2019, 9, 874. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Abdulwahid, R.T.; Rsaul, H.A.; Ahmed, H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci. Mater. Electron. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]




| Polymer | Hexane Fraction | Toluene Fraction | ||||
|---|---|---|---|---|---|---|
| Mn (g mol−1) | Mw (g mol−1) | PDI | Mn (g mol−1) | Mw (g mol−1) | PDI | |
| PBTP-11 | 9600 | 13,500 | 1.3 | |||
| PBTDTP-11 | 9500 | 14,300 | 1.5 | |||
| PFDTP-11 | 16,400 | 30,300 | 1.8 | |||
| Polymer | ε (M−1 cm−1) | Solution | Film | Film after Thermal Treatment around 300 °C | ||||
|---|---|---|---|---|---|---|---|---|
| λmax (nm) | λmax (nm) | λonset (nm) | Eg (eV) | λmax (nm) | λonset (nm) | Eg (eV) | ||
| PBTP-11 | 16,800 | 397 | 425 | 564 | 2.19 | 498 | 666 | 1.86 |
| PBTDTP-11 | 59,900 | 464 | 475 | 585 | 2.11 | 491 | 656 | 1.89 |
| PFDTP-11 | 37,500 | 398 | 408 | 480 | 2.58 | 411 | 579 | 2.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. Murad, A.; Iraqi, A.; Aziz, S.B.; N. Abdullah, S.; Abdulwahid, R.T. Synthesis, Optical, Thermal and Structural Characteristics of Novel Thermocleavable Polymers Based on Phthalate Esters. Polymers 2020, 12, 2791. https://doi.org/10.3390/polym12122791
R. Murad A, Iraqi A, Aziz SB, N. Abdullah S, Abdulwahid RT. Synthesis, Optical, Thermal and Structural Characteristics of Novel Thermocleavable Polymers Based on Phthalate Esters. Polymers. 2020; 12(12):2791. https://doi.org/10.3390/polym12122791
Chicago/Turabian StyleR. Murad, Ary, A. Iraqi, Shujahadeen B. Aziz, Sozan N. Abdullah, and Rebar T. Abdulwahid. 2020. "Synthesis, Optical, Thermal and Structural Characteristics of Novel Thermocleavable Polymers Based on Phthalate Esters" Polymers 12, no. 12: 2791. https://doi.org/10.3390/polym12122791
APA StyleR. Murad, A., Iraqi, A., Aziz, S. B., N. Abdullah, S., & Abdulwahid, R. T. (2020). Synthesis, Optical, Thermal and Structural Characteristics of Novel Thermocleavable Polymers Based on Phthalate Esters. Polymers, 12(12), 2791. https://doi.org/10.3390/polym12122791







