Glucose-Assisted One-Pot Hydrothermal Synthesis of Hierarchical-Structured MoS2/C Quasi-Hollow Microspheres for High-Performance Lithium Ion Battery
Abstract
1. Introduction
2. Experimental Section
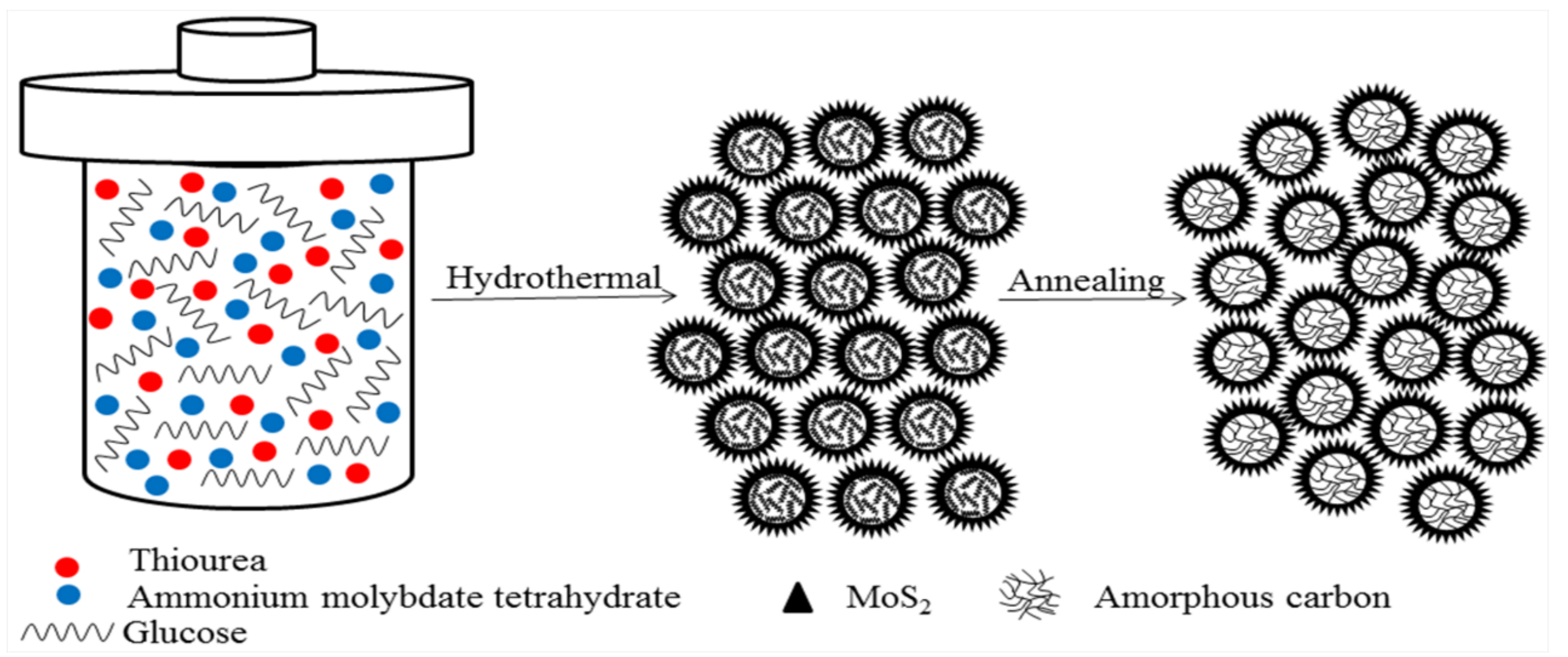
2.1. Synthesis of Hierarchical MoS2/C Quasi-Hollow Microspheres
2.2. Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
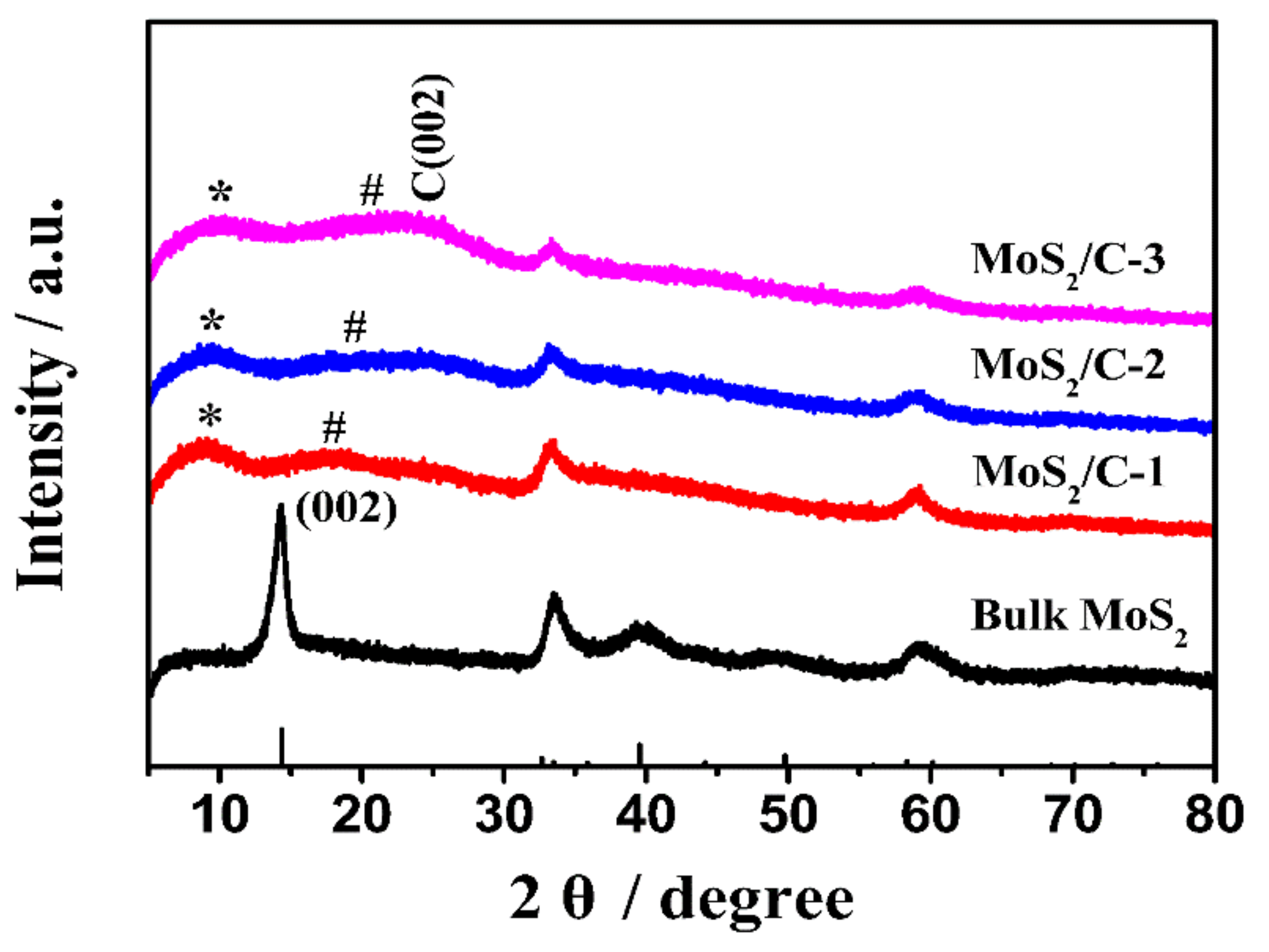
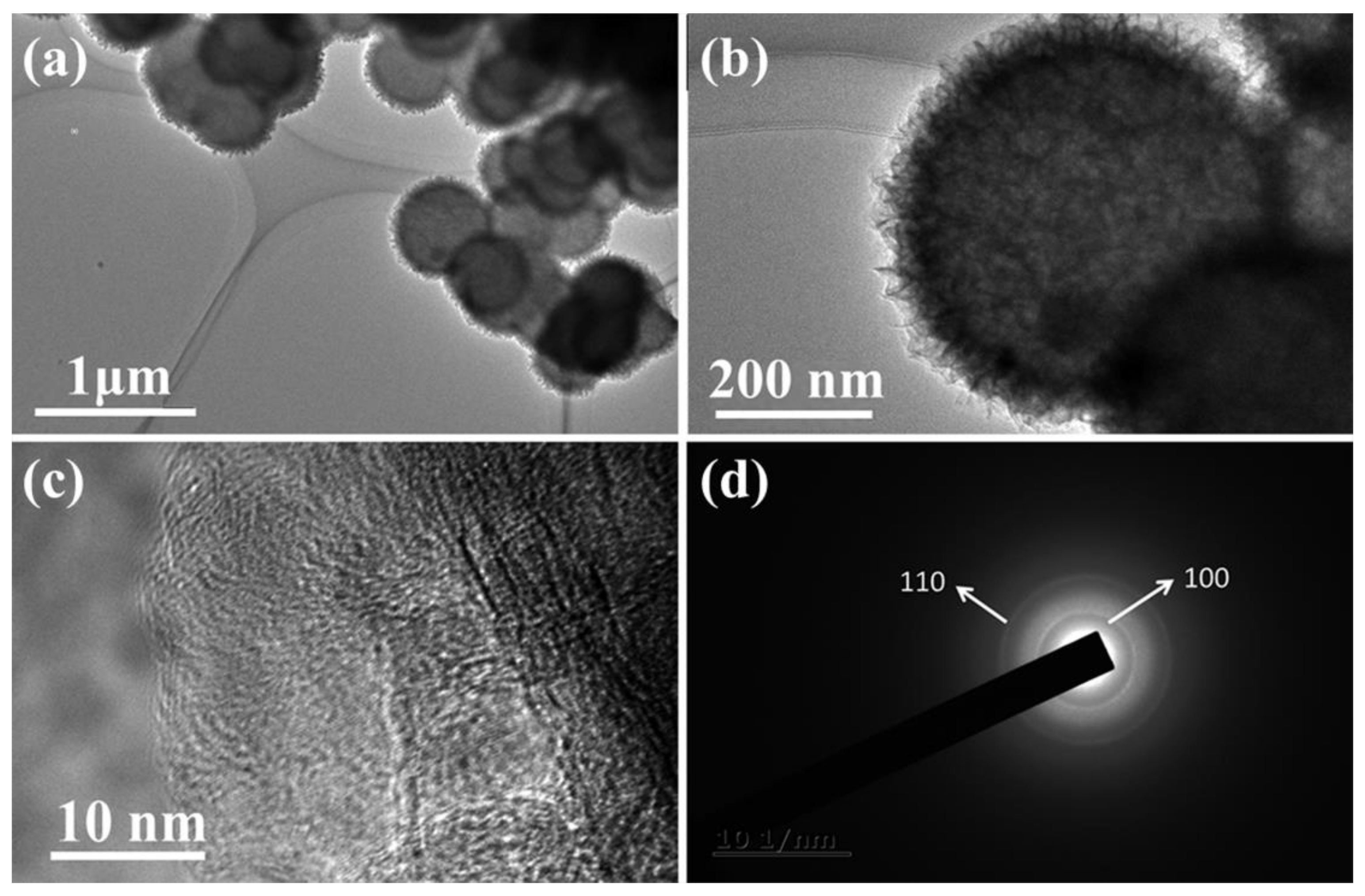
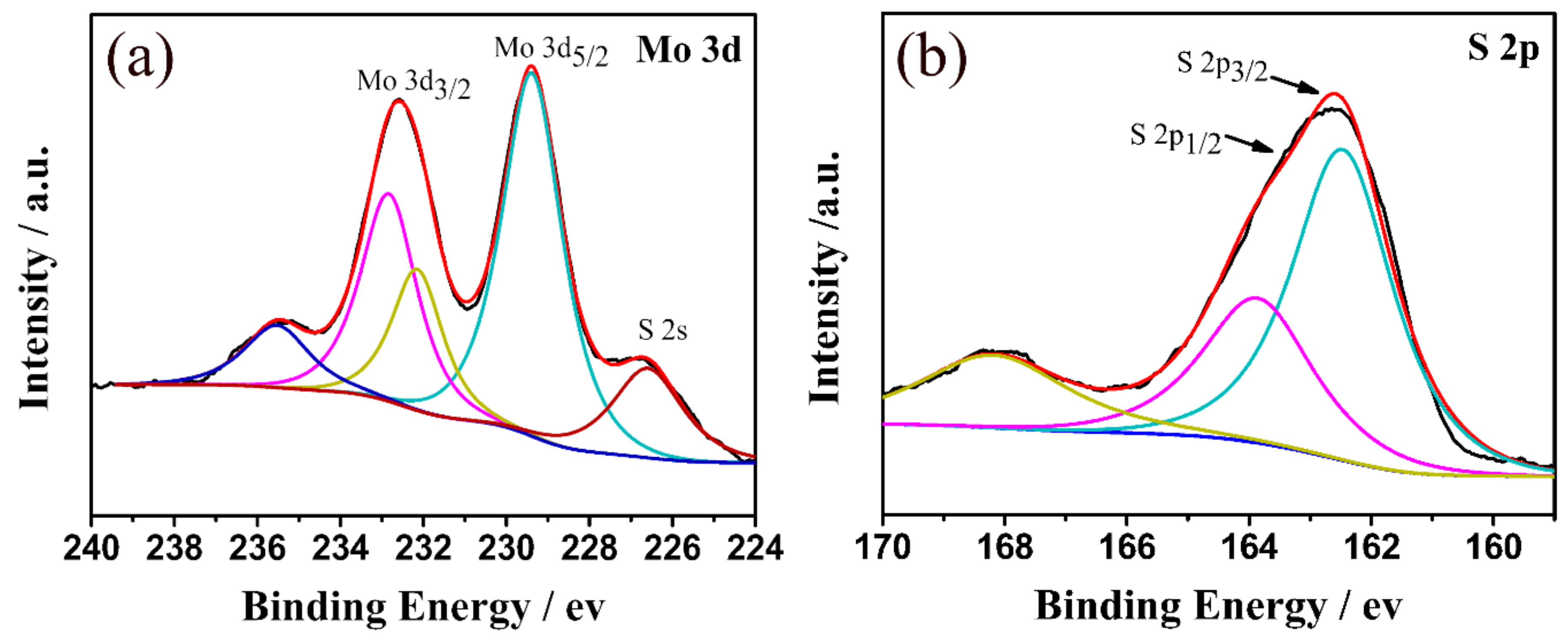
3.1. Characterization of Structure and Morphology
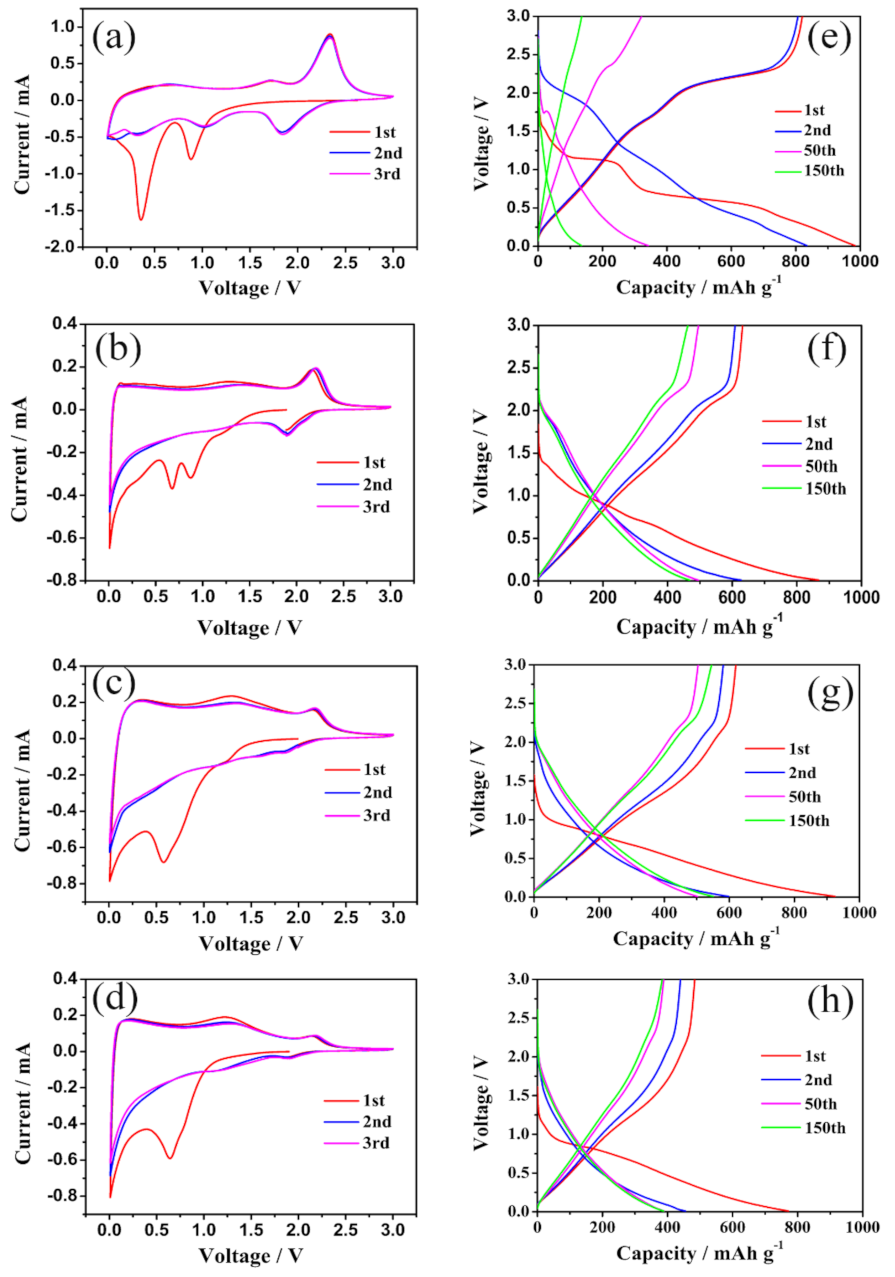
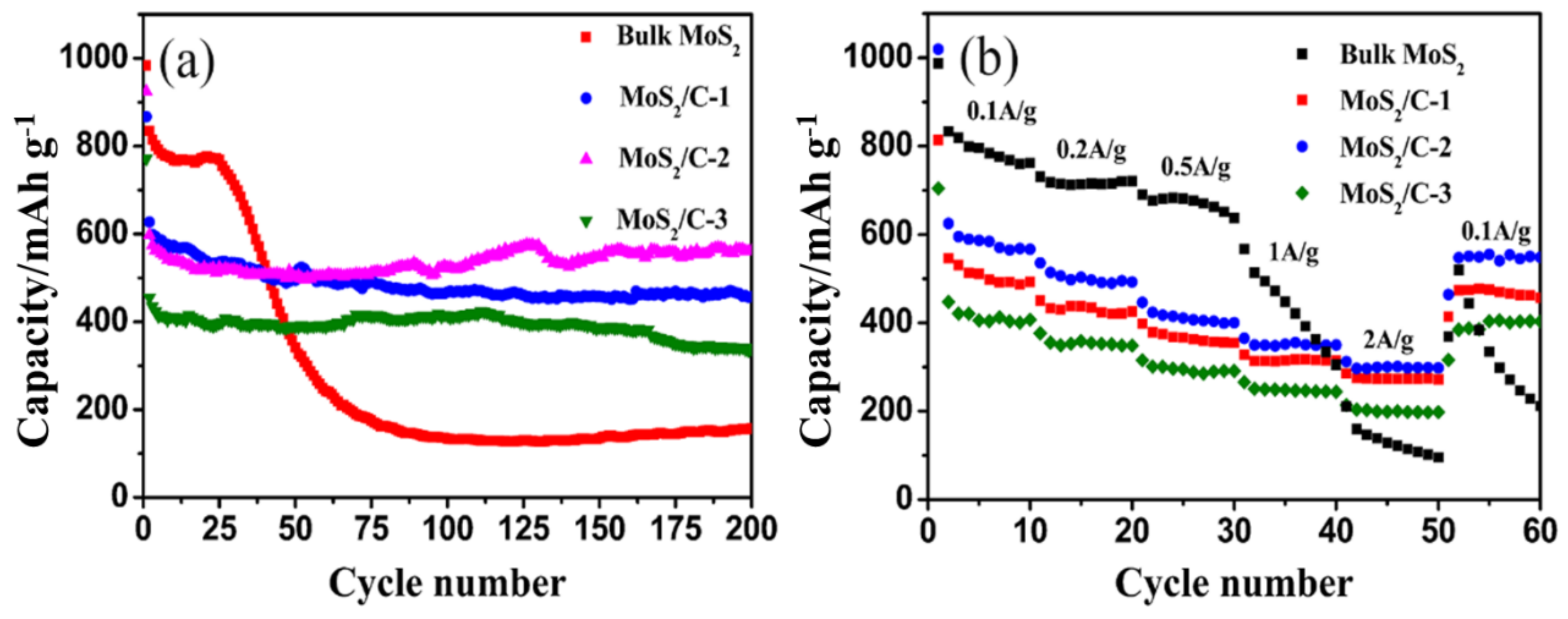
3.2. Electrochemical Performance Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Fan, Y.C.; Chen, X.; Legut, D.; Zhang, Q.F. Modeling and theoretical design of next-generation lithium metal batteries. Energy Storage Mater. 2019, 16, 169–193. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. Recent Advances in Energy Chemical Engineering of Next-Generation Lithium Batteries. Engineering 2018, 4, 831–847. [Google Scholar] [CrossRef]
- Hewathilake, H.; Karunarathne, N.; Wijayasinghe, A.; Balasooriya, N.W.B.; Arof, A.K. Performance of developed natural vein graphite as the anode material of rechargeable lithium ion batteries. Ionics 2017, 23, 1417–1422. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Jiao, L.; Chen, J. A graphene-like MoS2/graphene nanocomposite as a highperformance anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 13109–13115. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Mao, J.; Deng, J.; Gao, Q.; Tang, Y.; Schmidt, O.G. Hierarchical MoS2/Polyaniline Nanowires with Excellent Electrochemical Performance for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 1180–1184. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.H.; Gao, S.; Chen, Q.; Peng, L.M.; Liu, K.H.; Wei, X.L. Superlubricity between MoS2 Monolayers. Adv. Mater. 2017, 29, 1701474. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Meng, X.G.; Yu, L.; Deng, D.H.; Bao, X.H. Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications. Chem. Rev. 2019, 119, 1806–1854. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.R.; Xu, W.X.; Wang, C.; Wang, F.B.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef]
- Merki, D.; Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef]
- Wang, H.Q.; Pan, Q.C.; Wu, Q.; Zhang, X.H.; Huang, Y.G.; Lushington, A.; Li, Q.Y.; Sun, X.L. Ultrasmall MoS2 embedded in carbon nanosheets-coated Sn/SnOx as anode material for high-rate and long life Li-ion batteries. J. Mater. Chem. A 2017, 5, 4576–4582. [Google Scholar] [CrossRef]
- Sun, D.; Ye, D.L.; Liu, P.; Tang, Y.G.; Guo, J.; Wang, L.Z.; Wang, H.Y. MoS2/Graphene Nanosheets from Commercial Bulky MoS2 and Graphite as Anode Materials for High Rate Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 11. [Google Scholar] [CrossRef]
- Liu, Y.C.; Fan, L.Z.; Jiao, L.F. Graphene intercalated in graphene-like MoS2: A promising cathode for rechargeable Mg batteries. J. Power Sources 2017, 340, 104–110. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Lou, X.W. Glucose-Assisted Growth of MoS2 Nanosheets on CNT Backbone for Improved Lithium Storage Properties. Chem. A Eur. J. 2011, 17, 13142–13145. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, R.; Yang, S.; Ma, H.; Liang, J.; Chen, J. Rechargeable Mg Batteries with Graphene-like MoS2 Cathode and Ultrasmall Mg Nanoparticle Anode. Adv. Mater. 2011, 23, 640–643. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, L.; Wu, Q.; Du, J.; Zhao, Y.; Si, Y.; Wang, Y.; Yuan, H. Sandwich-structured graphene-like MoS2/C microspheres for rechargeable Mg batteries. J. Mater. Chem. A 2013, 1, 5822–5826. [Google Scholar] [CrossRef]
- Choi, S.H.; Ko, Y.N.; Lee, J.-K.; Kang, Y.C. 3D MoS2-Graphene Microspheres Consisting of Multiple Nanospheres with Superior Sodium Ion Storage Properties. Adv. Funct. Mater. 2015, 25, 1780–1788. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Vuong, V.D.; Phong, T.M.; Le, T.V. Fabrication of MoS2 Nanoflakes Supported on Carbon Nanotubes for High Performance Anode in Lithium-Ion Batteries (LIBs). J. Nanomater. 2019, 2019, 7. [Google Scholar] [CrossRef]
- Ette, P.M.; Chithambararaj, A.; Prakash, A.S.; Ramesha, K. MoS2 Nanoflower-Derived Interconnected CoMoO4 Nanoarchitectures as a Stable and High Rate Performing Anode for Lithium-Ion Battery Applications. ACS Appl. Mater. Interfaces 2020, 12, 11511–11521. [Google Scholar] [CrossRef]
- Tang, W.J.; Wang, X.L.; Zhong, Y.; Xie, D.; Zhang, X.Q.; Xia, X.H.; Wu, J.B.; Gu, C.D.; Tu, J.P. Hierarchical MoS2/Carbon Composite Microspheres as Advanced Anodes for Lithium/Sodium-Ion Batteries. Chem. A Eur. J. 2018, 24, 11220–11226. [Google Scholar] [CrossRef]
- Feng, C.; Ma, J.; Li, H.; Zeng, R.; Guo, Z.; Liu, H. Synthesis of molybdenum disulfide (MoS2) for lithium ion battery applications. Mater. Res. Bull. 2009, 44, 1811–1815. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Bai, Y.L.; Wu, X.Y.; Wei, X.; Wang, K.X.; Chen, J.S. MoS2 nanoflakes integrated in a 3D carbon framework for high-performance sodium-ion batteries. J. Alloy. Compd. 2019, 797, 1126–1132. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Wan, Z.M.; Shao, J.; Yun, J.J.; Zheng, H.Y.; Gao, T.; Shen, M.; Qu, Q.T.; Zheng, H.H. Core-Shell Structure of Hierarchical Quasi-Hollow MoS2 Microspheres Encapsulated Porous Carbon as Stable Anode for Li-Ion Batteries. Small 2014, 10, 4975–4981. [Google Scholar] [CrossRef]
- Li, J.Y.; Hou, Y.; Gao, X.F.; Guan, D.S.; Xie, Y.Y.; Chen, J.H.; Yuan, C. A three-dimensionally interconnected carbon nanotube/layered MoS2 nanohybrid network for lithium ion battery anode with superior rate capacity and long-cycle-life. Nano Energy 2015, 16, 10–18. [Google Scholar] [CrossRef]
- Guo, Y.; Li, S.; Fang, Q.Y.; Zuo, J.L.; Liu, M.; Zhang, J. An integrated electrode based on nanoflakes of MoS2 on carbon cloth for enhanced lithium storage. Rsc. Adv. 2020, 10, 9335–9340. [Google Scholar] [CrossRef]
- Hu, S.; Chen, W.; Uchaker, E.; Zhou, J.; Cao, G.Z. Mesoporous Carbon Nanofibers Embedded with MoS2 Nanocrystals for Extraordinary Li-Ion Storage. Chem. A Eur. J. 2015, 21, 18248–18257. [Google Scholar] [CrossRef]
- Wang, X.X.; Tian, J.H.; Cheng, X.; Na, R.; Wang, D.D.; Shan, Z.Q. Chitosan-Induced Synthesis of Hierarchical Flower Ridge-like MoS2/N-Doped Carbon Composites with Enhanced Lithium Storage. ACS Appl. Mater. Interfaces 2018, 10, 35953–35962. [Google Scholar] [CrossRef]
- Anwer, S.; Huang, Y.X.; Li, B.S.; Govindan, B.; Liao, K.; Cantwell, W.J.; Wu, F.; Chen, R.J.; Zheng, L.X. Nature-Inspired, Graphene-Wrapped 3D MoS2 Ultrathin Microflower Architecture as a High-Performance Anode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 22323–22331. [Google Scholar] [CrossRef]
- Zhang, X.E.; Chen, X.; Ren, H.J.; Diao, G.W.; Chen, M.; Chen, S.W. Bowl-like C@MoS2 Nanocomposites as Anode Materials for Lithium-Ion Batteries: Enhanced Stress Buffering and Charge/Mass Transfer. ACS Sustain. Chem. Eng. 2020, 8, 10065–10072. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Ting, J.M. High performance asymmetric supercapacitor having novel 3D networked polypyrrole nanotube/N-doped graphene negative electrode and core-shelled MoO3/PPy supported MoS2 positive electrode. Electrochim. Acta 2019, 320, 11. [Google Scholar]
- Zhang, S.; Yu, X.; Yu, H.; Chen, Y.; Gao, P.; Li, C.; Zhu, C. Growth of Ultrathin MoS2 Nanosheets with Expanded Spacing of (002) Plane on Carbon Nanotubes for High-Performance Sodium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2014, 6, 21880–21885. [Google Scholar] [CrossRef]
- Zhao, B.C.; Zhou, J.F.; Si, J.G.; Fang, Z.T.; Li, K.Z.; Ma, H.Y.; Dai, J.M.; Zhu, X.B.; Sun, Y.P. Glucose-Induced Synthesis of 1T-MoS2/C Hybrid for High-Rate Lithium-Ion Batteries. Small 2019, 15, 1805420. [Google Scholar]
- Hu, S.; Chen, W.; Zhou, J.; Yin, F.; Uchaker, E.; Zhang, Q.F.; Cao, G.Z. Preparation of carbon coated MoS2 flower-like nanostructure with self-assembled nanosheets as high-performance lithium-ion battery anodes. J Mater. Chem. A 2013, 24, 125–130. [Google Scholar] [CrossRef]
- Bindumadhavan, K.; Srivastava, S.K.; Mahanty, S. MoS2-MWCNT hybrids as a superior anode in lithium-ion batteries. Chem. Commun. 2013, 49, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ma, C.; Ge, B.; Chen, Y.; Xu, Z.; Zhu, C.; Li, C.; Ouyang, Q.; Gao, P.; Li, J.; et al. Three-Dimensional Hierarchical Architectures Constructed by Graphene/MoS2 Nanoflake Arrays and Their Rapid Charging/Discharging Properties as Lithium-Ion Battery Anodes. Chem. A Eur. J. 2013, 19, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chen, W.; Ma, L.; Li, H.; Li, H.; Huang, F.; Xu, Z.; Zhang, Q.; Lee, J.-Y. Graphene-like MoS2/amorphous carbon composites with high capacity and excellent stability as anode materials for lithium ion batteries. J. Mater. Chem. 2011, 21, 6251–6257. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.F.; Wu, Q.H.; Li, W.L.; Shen, C.; Ni, L.B.; Yan, H.; Diao, G.W.; Chen, M. Petal-like MoS2 Nanosheets Space-Confined in Hollow Mesoporous Carbon Spheres for Enhanced Lithium Storage Performance. ACS Nano 2017, 11, 8429–8436. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.C.; Chen, T.; Chen, W.X.; Wang, Z.; Chang, K.; Ma, L.; Huang, F.H.; Chen, D.Y.; Lee, J.Y. Graphene-Like MoS2/Graphene Composites: Cationic Surfactant-Assisted Hydrothermal Synthesis and Electrochemical Reversible Storage of Lithium. Small 2013, 9, 3693–3703. [Google Scholar] [CrossRef]
- Shao, J.; Qu, Q.; Wan, Z.; Gao, T.; Zuo, Z.; Zheng, H. From Dispersed Microspheres to Interconnected Nanospheres: Carbon-Sandwiched Monolayered MoS2 as High-Performance Anode of Li-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 22927–22934. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.K.; Mitra, S. High-Rate and High-Energy-Density Lithium-Ion Battery Anode Containing 2D MoS2 Nanowall and Cellulose Binder. ACS Appl. Mater. Interfaces 2013, 5, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ren, Y.; Yang, H.; Xu, Q. Fabrication of 3D Hierarchical MoS2/Polyaniline and MoS2/C Architectures for Lithium-Ion Battery Applications. ACS Appl. Mater. Interfaces 2014, 6, 14644–14652. [Google Scholar] [CrossRef]
- McCreary, K.M.; Hanbicki, A.T.; Robinson, J.T.; Cobas, E.; Culbertson, J.C.; Friedman, A.L.; Jernigan, G.G.; Jonker, B.T. Large-Area Synthesis of Continuous and Uniform MoS2 Monolayer Films on Graphene. Adv. Funct. Mater. 2014, 24, 6449–6454. [Google Scholar] [CrossRef]
- Jie, X.; Wang, X.; Yang, X.Q.; Xun, S.; Gao, L.; Phillip, K.K.; Liu, J.; Lemmon, J.P. Electrochemically Induced High Capacity Displacement Reaction of PEO/MoS2/Graphene Nanocomposites with Lithium. Adv. Funct. Mater. 2011, 21, 2840–2846. [Google Scholar]
- Wang, M.; Li, G.; Xu, H.; Qian, Y.; Yang, J. Enhanced lithium storage performances of hierarchical hollow MoS2 nanoparticles assembled from nanosheets. ACS Appl. Mater. Interfaces 2013, 5, 1003–1008. [Google Scholar] [CrossRef]
- He, G.; Evers, S.; Liang, X.; Cuisinier, M.; Garsuch, A.; Nazar, L.F. Tailoring porosity in carbon nanospheres for lithium-sulfur battery cathodes. ACS Nano 2013, 7, 10920–10930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, Y.; Liu, Y.; Luo, C.; Wang, C. Comparison of electrochemical performances of olivine NaFePO4 in sodium-ion batteries and olivine LiFePO4 in lithium-ion batteries. Nanoscale 2013, 5, 780–787. [Google Scholar] [CrossRef]
- Cao, X.H.; Shi, Y.M.; Shi, W.H.; Rui, X.H.; Yan, Q.Y.; Kong, J.; Zhang, H. Preparation of MoS2-Coated Three-Dimensional Graphene Networks for High-Performance Anode Material in Lithium-Ion Batteries. Small 2013, 9, 3433–3438. [Google Scholar] [CrossRef] [PubMed]


| Materials | Current Density mA g−1 | Cyclic Number | Specific Capacity mAh g−1 | References |
|---|---|---|---|---|
| C@MoS2 microsphere | 100 | 100 | 652 | [25] |
| Bowl-like C@ MoS2 | 100 | 100 | 798 | [31] |
| Flower-like MoS2/C | 100 | 50 | 834 | [35] |
| MoS2-MWCNT | 100 | 30 | 938 | [36] |
| MoS2/C nanoflowers | 100 | 50 | 888.1 | [43] |
| MoS2/C-2 | 100 | 200 | 598 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tan, J.; Li, X.; Zhang, C. Glucose-Assisted One-Pot Hydrothermal Synthesis of Hierarchical-Structured MoS2/C Quasi-Hollow Microspheres for High-Performance Lithium Ion Battery. Polymers 2021, 13, 837. https://doi.org/10.3390/polym13050837
Liu X, Tan J, Li X, Zhang C. Glucose-Assisted One-Pot Hydrothermal Synthesis of Hierarchical-Structured MoS2/C Quasi-Hollow Microspheres for High-Performance Lithium Ion Battery. Polymers. 2021; 13(5):837. https://doi.org/10.3390/polym13050837
Chicago/Turabian StyleLiu, Xingang, Jiang Tan, Xi Li, and Chuhong Zhang. 2021. "Glucose-Assisted One-Pot Hydrothermal Synthesis of Hierarchical-Structured MoS2/C Quasi-Hollow Microspheres for High-Performance Lithium Ion Battery" Polymers 13, no. 5: 837. https://doi.org/10.3390/polym13050837
APA StyleLiu, X., Tan, J., Li, X., & Zhang, C. (2021). Glucose-Assisted One-Pot Hydrothermal Synthesis of Hierarchical-Structured MoS2/C Quasi-Hollow Microspheres for High-Performance Lithium Ion Battery. Polymers, 13(5), 837. https://doi.org/10.3390/polym13050837






