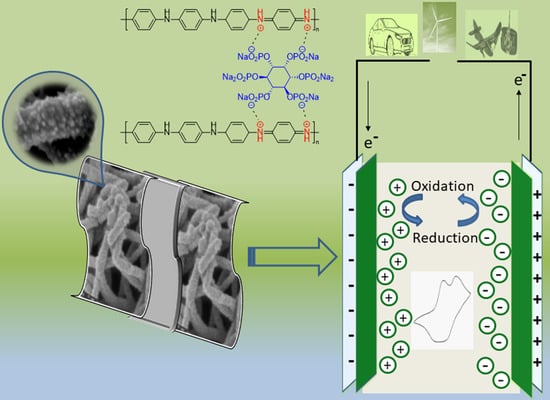
3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. General Procedure for the Synthesis of Sodium Phytate Doped PANI
2.3. Preparation of the Electrode Composite Materials
2.4. Manufacturing of the Supercapacitor Cell
3. Results and Discussion
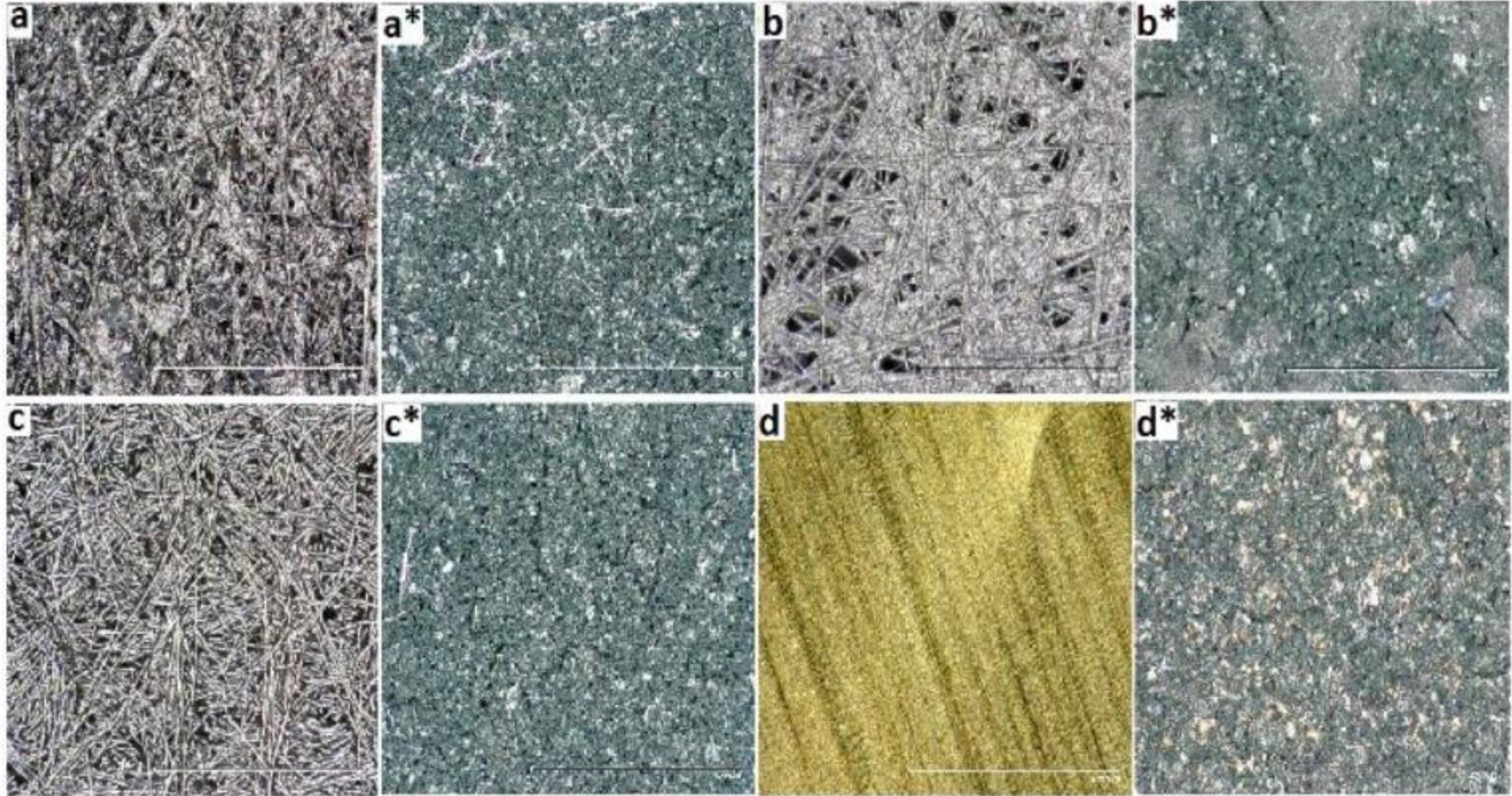
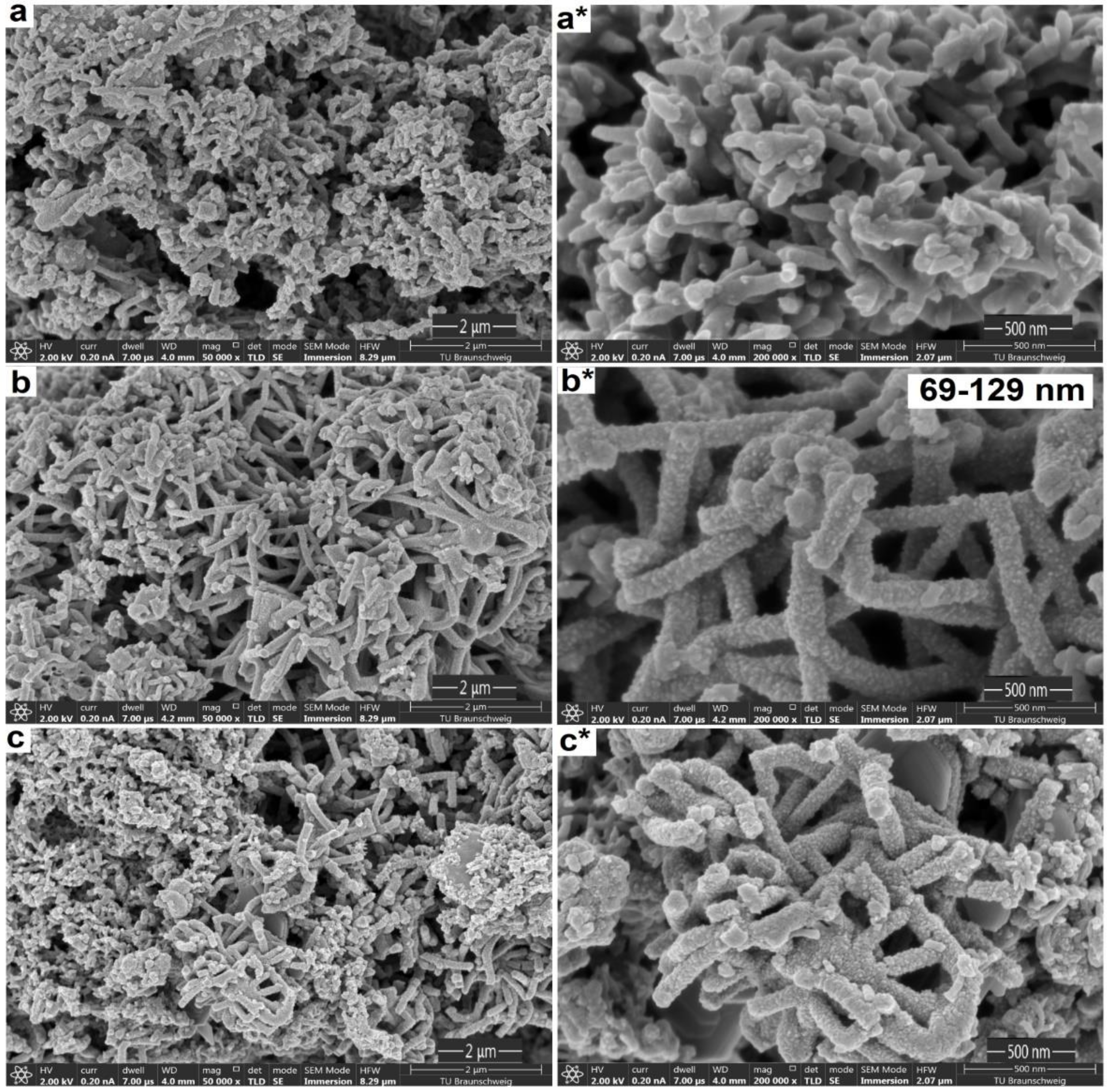
3.1. Structural and Morphological Analysis
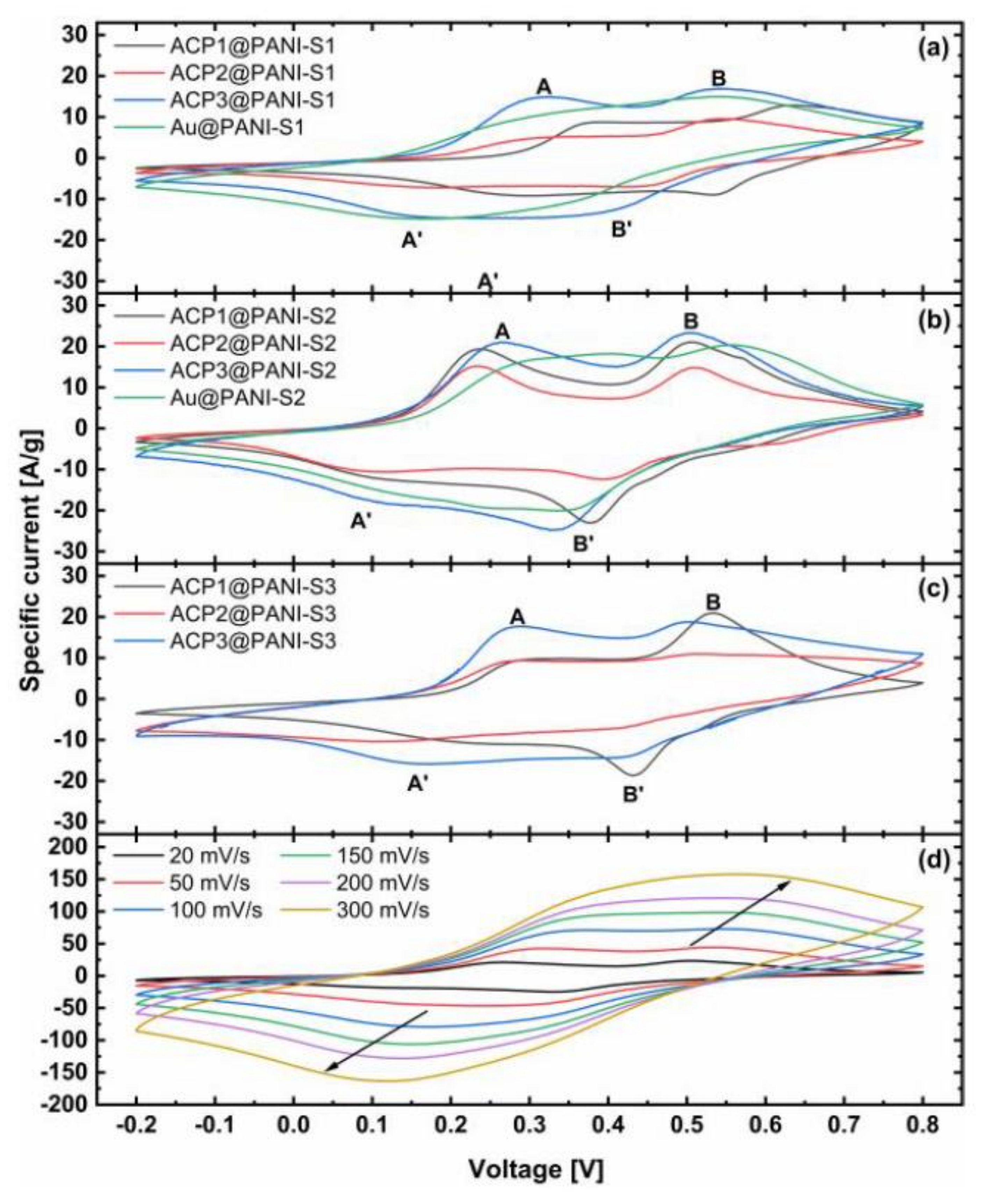
3.2. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Zhou, H.; Cheng, R.; Yu, W.; Huang, Y.; Duan, X. Highly Flexible Electronics from Scalable Vertical Thin Film Transistors. Nano Lett. 2014, 14, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-Molded Flexible, Ultrasensitive, and Highly Stable Electronic Skin for Monitoring Human Physiological Signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, O.; Khan, Z.U.; Wang, H.; Braun, S.; Evans, D.R.; Fabretto, M.; Talemi, P.H.; Dagnelund, D.; Arlin, J.B.; Geerts, Y.H.; et al. Semi-metallic polymers. Nat. Mater. 2014, 13, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Z.; Yuanab, S.; Zhang, X.-B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef]
- Yao, B.; Huang, L.; Zhang, J.; Gao, X.; Wu, J.; Cheng, Y.; Xiao, X.; Wang, B.; Li, Y.; Zhou, J. Flexible Transparent Molybdenum Trioxide Nanopaper for Energy Storage. Adv. Mater. 2016, 28, 6353–6358. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Wang, D.-W.; Shan, X.-Y.; Pei, S.; Li, F.; Cheng, H.-M. A Flexible Sulfur-Graphene-Polypropylene Separator Integrated Electrode for Advanced Li–S Batteries. Adv. Mater. 2015, 27, 641–647. [Google Scholar] [CrossRef]
- Liu, Q.-C.; Li, L.; Xu, J.-J.; Chang, Z.W.; Xu, D.; Yin, Y.-B.; Yang, X.Y.; Liu, T.; Jiang, Y.S.; Yan, J.-M.; et al. Flexible and Foldable Li–O2 Battery Based on Paper-Ink Cathode. Adv. Mater. 2015, 27, 8095–8101. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, S.; Wu, J.; Shi, X.; Zhu, B.; Meng, X. Wearable High-Performance Supercapacitors Based on Silver-Sputtered Textiles with FeCo2S4–NiCo2S4 Composite Nanotube-Built Multitripod Architectures as Advanced Flexible Electrodes. Adv. Energy Mater. 2017, 7, 1601234. [Google Scholar] [CrossRef]
- Song, Y.; Liu, T.-Y.; Yao, B.; Kou, T.-Y.; Feng, D.-Y.; Liu, X.-X.; Li, Y. Amorphous Mixed-Valence Vanadium Oxide/Exfoliated Carbon Cloth Structure Shows a Record High Cycling Stability. Small 2017, 13, 1700067. [Google Scholar] [CrossRef]
- Song, Y.; Liu, T.-Y.; Xu, X.-X.; Feng, D.-Y.; Li, Y.; Liu, X.-X. Pushing the Cycling Stability Limit of Polypyrrole for Supercapacitors. Adv. Funct. Mater. 2015, 25, 4626–4632. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Han, T.Y.-J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors Based on Three-Dimensional Hierarchical Graphene Aerogels with Periodic Macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zheng, D.; Zhai, T.; Liu, Z.; Huang, Y.; Xie, S.; Tong, Y. Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor. Energy Environ. Sci. 2011, 4, 2915–2921. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Xu, F.; Li, J.; Yuan, Y.; He, X.; et al. Lightweight, Superelastic, and Mechanically Flexible Graphene/Polyimide Nanocomposite Foam for Strain Sensor Application. ACS Nano 2015, 9, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Choi, J.W.; Cui, Y. Highly conductive paper for energy-storage devices. Proc. Natl. Acad. Sci. USA 2009, 106, 21490–21494. [Google Scholar] [CrossRef] [PubMed]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.; Zhao, Y.; Tsang, Y.H.; Lau, S.P.; Huang, H.; Chai, Y. Stretchable all-solid-state supercapacitor with wavy shaped polyaniline/graphene electrode. J. Mater. Chem. A 2014, 2, 9142–9149. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Q.; Wang, K.; Chen, J.; Chen, Z. Fabrication of Free-Standing Hierarchical Carbon Nanofiber/Graphene Oxide/Polyaniline Films for Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 200–209. [Google Scholar] [CrossRef]
- Yu, P.; Zhao, X.; Huang, Z.; Li, Y.; Zhang, Q. Free-standing three-dimensional graphene and polyaniline nanowire arrays hybrid foams for high-performance flexible and lightweight supercapacitors. J. Mater. Chem. A 2014, 2, 14413–14420. [Google Scholar] [CrossRef]
- Huang, F.; Lou, F.; Chen, D. Exploring Aligned-Carbon-Nanotubes@Polyaniline Arrays on Household Al as Supercapacitors. Chemsuschem 2012, 5, 888–895. [Google Scholar] [CrossRef]
- Haq, A.U.; Lim, L.; Yun, J.M.; Lee, W.J.; Han, T.H.; Kim, S.O. Direct Growth of Polyaniline Chains from N-Doped Sites of Carbon Nanotubes. Small 2013, 9, 3829–3833. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Luan, P.; Shao, Q.; Dong, H.; Li, J.; Chen, J.; Zhao, D.; Cai, L.; Zhou, W.; Chen, X.; et al. A “skeleton/skin” strategy for preparing ultrathin free-standing single-walled carbon nanotube/polyaniline films for high performance supercapacitor electrodes. Energy Environ. Sci. 2012, 5, 8726–8733. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.-F.; Hou, H. Electrospun carbon nanofibers surface-grown with carbon nanotubes and polyaniline for use as high-performance electrode materials of supercapacitors. RSC Adv. 2014, 4, 23622–23629. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.-F. High-performance porous electrodes for pseudosupercapacitors based on graphene-beaded carbon nanofibers surface-coated with nanostructured conducting polymers. J. Power Sources 2014, 262, 44–49. [Google Scholar] [CrossRef]
- Hu, H.; Liu, S.W.; Hanif, M. Three-dimentional cross-lingked carbon network wrapped with ordered polyaniline nanowires for high-performance pseudosupercapacitors. J. Power Sources 2014, 268, 451–458. [Google Scholar] [CrossRef]
- Ning, G.; Li, T.; Yan, J.; Xu, C.; Wei, T.; Fan, Z. Three-dimensional hybrid materials of fish scale-like polyaniline nanosheet arrays on graphene oxide and carbon nanotube for high-performance ultracapacitors. Carbon 2013, 54, 241–248. [Google Scholar] [CrossRef]
- Park, J.W.; Park, S.J.; Kwon, O.S.; Lee, C.; Jang, J. In Situ Synthesis of Graphene/Polyselenophene Nanohybrid Materials as Highly Flexible Energy Storage Electrodes. Chem. Mater. 2014, 26, 2354–2360. [Google Scholar] [CrossRef]
- Lin, H.; Li, L.; Ren, J.; Cai, Z.; Qiu, L.; Yang, Z.; Peng, H. Conducting polymer composite film incorporated with aligned carbon nanotubes for transparent, flexible, and efficient supercapacitor. Sci. Rep. 2013, 3, 1353. [Google Scholar] [CrossRef]
- Tobjörk, D.; Osterbacka, R. Paper Electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhong, J.; Cheng, X.; Yao, X.; Wang, B.; Li, W.; Wu, N.; Liu, K.; Hu, B.; Zhou, J. Paper-Based Active Tactile Sensor Array. Adv. Mater. 2015, 27, 7130–7136. [Google Scholar] [CrossRef]
- Comiskey, B.; Albert, J.D.; Yoshizawa, H.; Jacobson, J. An electrophoretic ink for all-printed reflective electronic displays. Nature 1998, 394, 253–255. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, Y.-S.; Wu, J.; Liu, Z.; Song, J.; Kim, H.S.; Huang, Y.Y.; Hwang, K.-C.; Rogers, J.A. Ultrathin Silicon Circuits with Strain-Isolation Layers and Mesh Layouts for High-Performance Electronics on Fabric, Vinyl, Leather, and Paper. Adv. Mater. 2009, 21, 3703–3707. [Google Scholar] [CrossRef]
- Subramanian, V.; Frechet, J.M.J.; Chang, P.C.; Huang, D.C.; Lee, J.B.; Molesa, S.E.; Murphy, A.R.; Redinger, D.R.; Volkman, S.K. Progress Toward Development of All-Printed RFID Tags. Mater. Process. Devices. Proc. IEEE 2005, 93, 1330–1338. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, L.; Li, M.; Yan, M.; Ge, S.; Yu, J.; Song, X.; Cao, B. Flexible paper-based ZnO nanorod light-emitting diodes induced multiplexed photoelectrochemical immunoassay. Chem. Commun. 2014, 50, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhu, H.; Zhong, Q.; Dai, J.; Li, W.; Jang, S.-H.; Yao, Y.; Henderson, D.; Hu, Q.; Hu, L.; et al. Self-Powered Human-Interactive Transparent Nanopaper Systems. ACS Nano 2015, 9, 7399–7406. [Google Scholar] [CrossRef]
- Zheng, G.; Cui, Y.; Karabulut, E.; Wågberg, L.; Zhu, H.; Hu, L. Nanostructured paper for flexible energy and electronic devices. MRS Bull. 2013, 38, 320–325. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Lai, W.Y.; Pang, H.; Huang, W. Flexible supercapacitors based on paper substrates: A new paradigm for low-cost energy storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef]
- Lin, Y.; Gritsenko, D.; Liu, Q.; Lu, X.; Xu, J. Recent Advancements in Functionalized Paper-Based Electronics. ACS Appl. Mater. Interfaces 2016, 8, 20501–20515. [Google Scholar] [CrossRef]
- Hua, L.; Cui, Y. Energy and environmental nanotechnology in conductive paper and textiles. Energy Environ. Sci. 2012, 5, 6423–6435. [Google Scholar] [CrossRef]
- Zheng, G.; Hu, L.; Wu, H.; Xie, X.; Cui, Y. Paper supercapacitors by a solvent-free drawing method. Energy Environ. Sci. 2011, 4, 3368–3373. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Kou, T.; Song, Y.; Liu, T.; Li, Y. Paper-Based Electrodes for Flexible Energy Storage Devices. Adv. Sci. 2017, 4, 1700107. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Rahman, S.; Rose, P.; Shah, A.-U.-H.A.; Krewer, U.; Bilal, S. An Amazingly Simple, Fast and Green Synthesis Route to Polyaniline Nanofiberes for Efficient Energy Storage. Polymers 2020, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lu, S.; Wang, S.; Zhang, X.; He, S.; He, T. High-performance polyaniline counter electrode electropolymerized in presence of sodium dodecyl sulfate for dye-sensitized solar cells. J. Power Sources 2014, 253, 300–304. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Effect of viscosity and electrical conductivity on the morphology and fiber diameter in melt electrospinning of polypropylene. Text. Res. J. 2013, 83, 606–617. [Google Scholar] [CrossRef]
- Tian, R.Z.R.; Liu, J.; Voigt, A.J.; Xu, F.H.; McDermot, J.M. Dendritic growth of cubically ordered nanoporous materials through self-assembly. Nano Lett. 2003, 3, 89–92. [Google Scholar] [CrossRef]
- Tran, H.D.; Arcy, J.M.D.; Wang, Y.; Beltramo, P.J.; Strong, V.A.; Kaner, R.B. The oxidation of aniline to produce “polyaniline”: A process yielding many different nanoscale structures. J. Mater. Chem. 2011, 21, 3534–3550. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Pan, L.; Yu, G. 3D nanostructured conductive polymer hydrogels for high- performance electrochemical devices. Energy Environ. Sci. 2013, 6, 2856–2870. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, R.H.; Zhao, W.; Liu, N.; Wang, H.; Tee, K.B.C.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Khdary, N.H.; Abdesalam, M.E.; Enany, G.E.L. Mesoporous polyaniline films for high performance supercapacitors. J. Electrochem. Soc. 2014, 161, G63–G68. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Wang, G. Polyaniline nanofibers with a high specific surface area and an improved pore structure for supercapacitors. J. Power Sources 2015, 294, 16–21. [Google Scholar] [CrossRef]
- Jungkyun, O.; Yun, K.K.; Jun, L.S.; Jyongsik, J. Highly porous structured polyaniline nanocomposites for scalable and flexible high-performance supercapacitors. Nanoscale 2019, 11, 6462–6470. [Google Scholar] [CrossRef]
- Gao, X.; Jing, X.; Li, Y.; Zhu, J.; Zhang, M. Synthesis, and characterization of phosphorized polyaniline doped with phytic acid and its anticorrosion properties for Mg-Li alloy. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 24–35. [Google Scholar] [CrossRef]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Ogata, C.; Kurogi, R.; Awaya, A.; Hatakeyama, K.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y. All-Graphene Oxide Flexible Solid-State Supercapacitors with Enhanced Electrochemical Performance. ACS Appl. Mat. Interfaces 2017, 9, 26151–26160. [Google Scholar] [CrossRef]
- Basnayaka, P.A.; Ram, M.K.; Stefanakos, E.K.; Kumar, A. Supercapacitors based on graphene–polyaniline derivative nanocomposite electrode materials. Electrochim. Acta 2013, 92, 376–382. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mat. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Lyu, L.; Chai, H.; Seong, K.-D.; Lee, C.; Kang, J.; Zhang, W.; Piao, Y. Yeast-derived N-doped carbon microsphere/polyaniline composites as high performance pseudocapacitive electrodes. Electrochim. Acta 2018, 291, 256–266. [Google Scholar] [CrossRef]
- Wei-Dua, X.W.; Suna, X.; Zhan, J.; Zhang, H.; Zhao, X. Nitrogendoped hierarchical porous carbon using biomass-derived activated carbon/carbonized polyaniline composites for supercapacitor electrodes. J. Electroanal. Chem. 2018, 827, 213–222. [Google Scholar] [CrossRef]
- Lebedeva1, M.V.; Ayupov, A.B.; Yeletsky, P.M.; Parmon, V.N. Rice Husk Derived Activated Carbon/Polyaniline Composites as Active Materials for Supercapacitors. Int. J. Electrochem. Sci. 2018, 13, 3674–3690. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.M.; Hua, Q.; Zhao, Y.; Yin, H.; Yuan, J.; Dai, Z.; Zheng, L.; Tang, J. Synergistically reinforced capacitive performance from a hierarchically structured composite of polyaniline and cellulose-derived highly porous carbons. Mat. Lett. 2019, 62–65. [Google Scholar] [CrossRef]
- Yang, N.; Lin, X.-Q.; Qiu-Feng, L.; Jin, Y.-Q.; Yang, H. Polyaniline-modified renewable biocarbon composites as an efficient hybrid electrode for supercapacitors. Ionics 2019, 25, 5459–5472. [Google Scholar] [CrossRef]
- Hui, J.; Wei, D.; Chen, J.; Yang, Z. Polyaniline Nanotubes/Carbon Cloth Composite Electrode by Thermal Acid Doping for High-Performance Supercapacitors. Polymers 2019, 11, 2053. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Shah, A.-U.-H.A.; Krewer, U.; Bilal, S. Study on Direct Synthesis of Energy Efficient Multifunctional Polyaniline–Graphene Oxide Nanocomposite and Its Application in Aqueous Symmetric Supercapacitor Devices. Nanomaterials 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Gu, H.; Dong, L. Synthesis and electrochemical performance of polyaniline @MnO2/graphene ternary composites for electrochemical supercapacitors. J. Power Sources 2016, 303, 175–181. [Google Scholar] [CrossRef]
- Tabrizi, A.G.; Arsalani, N.; Mohammadi, A.; Ghadimi, L.S.; Ahadzadeh, I.; Namazi, H. A new route for the synthesis of polyaniline nanoarrays on graphene oxide for high-performance supercapacitors. Electrochim. Acta 2018, 265, 379–390. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Choia, D.J.; Boscab, A.; Pedrosb, J.; Martínezb, J.; Barrancoe, V.; Rojoe, J.M.; Yoof, J.J.; Kima, Y.-H.; Calle, F. Improvement of the adhesion between polyaniline and commercial carbon paper by acid treatment and its application in supercapacitor electrodes. Compos. Interfaces 2016, 23, 133–143. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, S.; Vongehr, S.; Syed, J.A.; Wang, X.; Meng, X. High-Performance Flexible SolidState Carbon Cloth Supercapacitors Based on Highly Processible N-Graphene Doped Polyacrylic Acid/Polyaniline Composites. Sci. Rep. 2016, 6, 12883. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Shah, A.-U.-H.A.; Bilal, S. Achieving Ultrahigh Cycling Stability and Extended Potential Window for Supercapacitors through Asymmetric Combination of Conductive Polymer Nanocomposite and Activated Carbon. Polymers 2019, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, Y.; Lu, X.; Wen, Z.; Li, Z.; Hou, H.; Song, Y. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Reduced Graphene Oxide Sheets for Supercapacitors. Sci. Rep. 2013, 3, 3568. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, J.; Wei, Z. Conducting polyaniline nanowire arrays for high performance supercapacitors. J. Phys. Chem. C 2010, 114, 8062–8067. [Google Scholar] [CrossRef]


| Materials | Current Density a | Csp | Year | Reference |
|---|---|---|---|---|
| PANI-carbon microspheres | 1.0 Ag−1 | 500 Fg−1 | 2018 | [60] |
| PANI-biomas porous carbon | 1.0 Ag−1 | 402 Fg−1 | 2018 | [61] |
| Activated carbon PANI | 0.2 Ag−1 | 465 Fg−1 | 2018 | [62] |
| PANI-cellulose derived porous carbon | 1.0 Ag−1 | 765 Fg−1 | 2019 | [63] |
| Activated carbon PANI | 1.0 Ag−1 | 520 Fg−1 | 2019 | [64] |
| PANI-carbon cloth | 25 mAcm−2 | 438 Fg−1 | 2019 | [65] |
| PANI-GO nano-composite | 10 Ag−1 | 658 Fg−1 | 2020 | [66] |
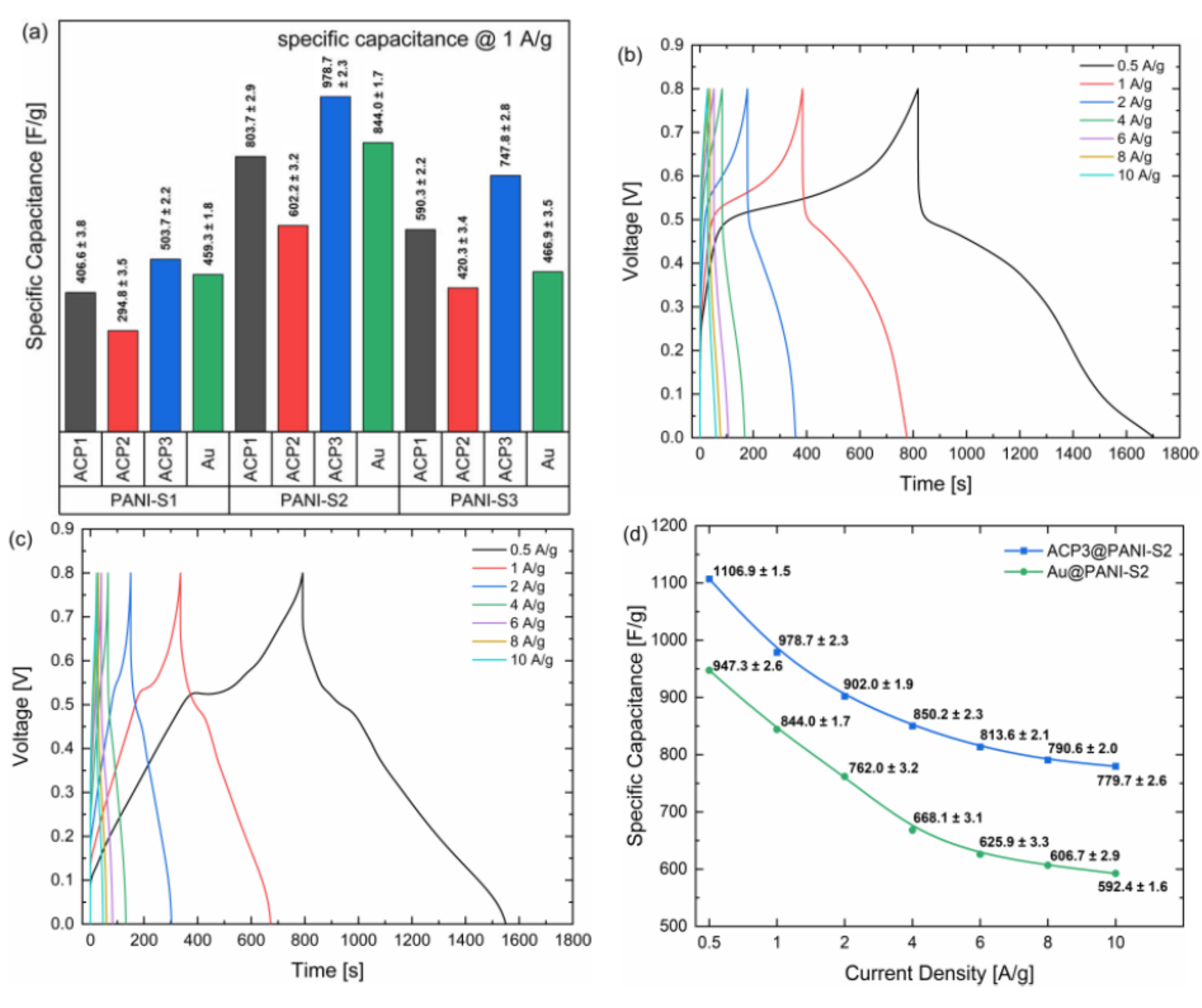
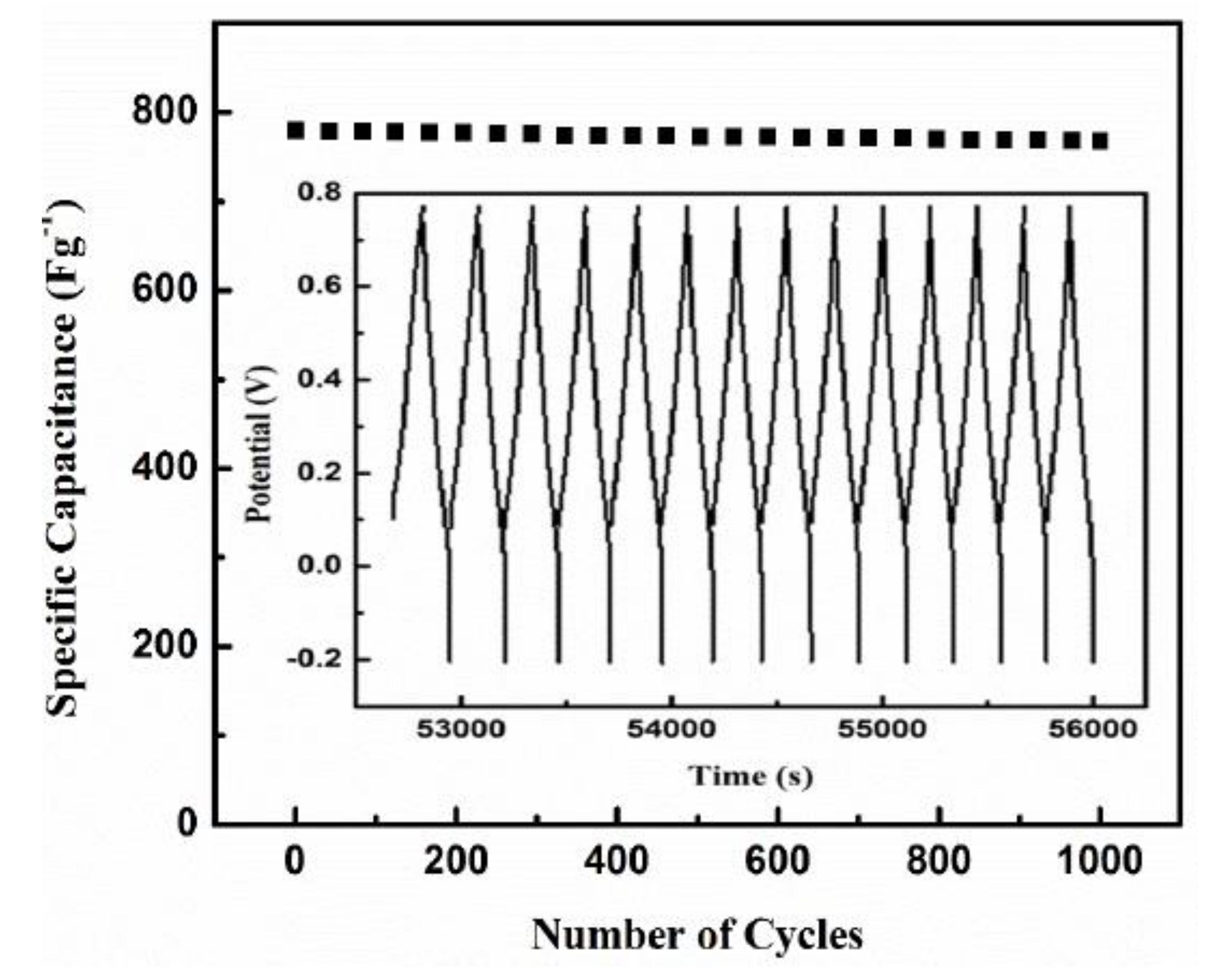
| ACP3-coated sodium phytate doped PANI-S2 | 1.0 Ag−1 10.0 Ag−1 | 978.7 ± 2.3 Fg−1 779.7 ± 2.6 Fg−1 | Present work | |
| Materials | Current Density a | Csp | Year | Reference |
|---|---|---|---|---|
| Commercial carbon paper coated PANI | 0.1 Ag−1 | 174 Fg−1 | 2016 | [70] |
| PANI carbon cloth | 1.0 Ag−1 | 68 Fg−1 | 2016 | [71] |
| PANI activated carbon nanocomposite | 1.0 Ag−1 | 142 Fg−1 | 2019 | [72] |
| Activated carbon-PANI | 1.0 Ag−1 | 520 Fg−1 | 2019 | [64] |
| PANI carbon cloth | 25 mAcm−2 | 247 Fg−1 | 2019 | [65] |
| PANI-GO nano-composite | 1.0 Ag−1 | 264 Fg−1 | 2020 | [66] |
| ACP-coated sodium phytate doped PANI | 0.5 Ag−1 1.0 Ag−1 | 503.1 ± 2.4 Fg−1 402.1 ± 3.3 Fg−1 | Present work | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.u.; Röse, P.; Surati, M.; Shah, A.u.H.A.; Krewer, U.; Bilal, S. 3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors. Polymers 2020, 12, 2705. https://doi.org/10.3390/polym12112705
Rahman Su, Röse P, Surati M, Shah AuHA, Krewer U, Bilal S. 3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors. Polymers. 2020; 12(11):2705. https://doi.org/10.3390/polym12112705
Chicago/Turabian StyleRahman, Sami ur, Philipp Röse, Mit Surati, Anwar ul Haq Ali Shah, Ulrike Krewer, and Salma Bilal. 2020. "3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors" Polymers 12, no. 11: 2705. https://doi.org/10.3390/polym12112705
APA StyleRahman, S. u., Röse, P., Surati, M., Shah, A. u. H. A., Krewer, U., & Bilal, S. (2020). 3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors. Polymers, 12(11), 2705. https://doi.org/10.3390/polym12112705