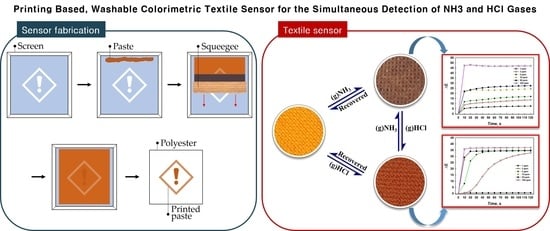
Colorimetric Textile Sensor for the Simultaneous Detection of NH3 and HCl Gases
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dye Synthesis
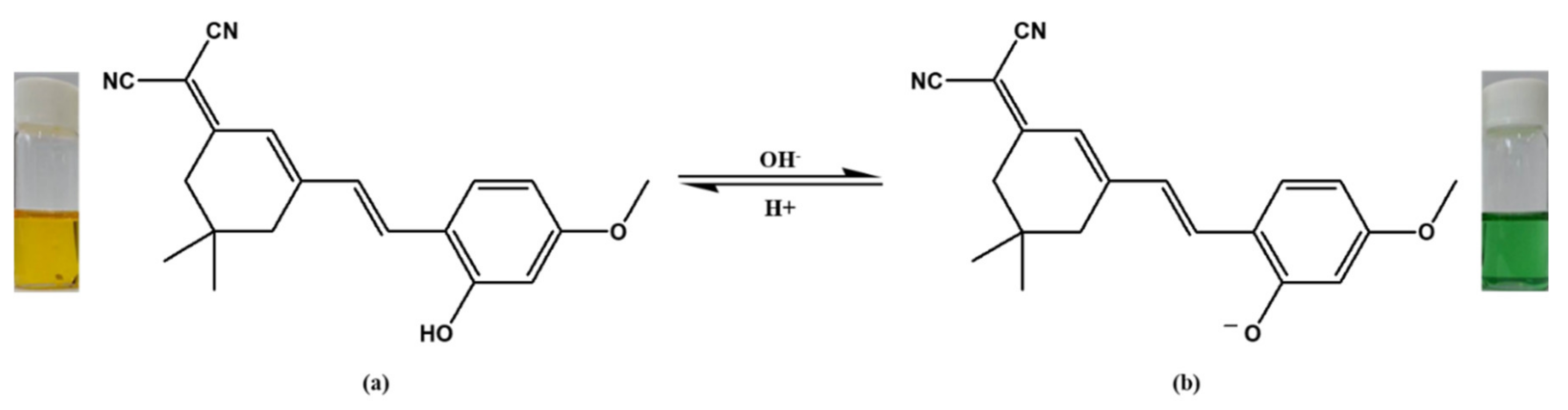
2.2.1. Synthesis of 2-{3-[2-(2-hydroxy-4-methoxy-phenyl)-vinyl]-5,5-dimethyl-cyclohex-2-enylidene}-malononitrile (Dye 3)
2.2.2. Synthesis of 5′,9′-dihydro-3H-spiro[isobenzofuran-1,15′-pyrano [2,3-b:6,5-b’]dicarbazol]-3-one (RhYK)
2.3. Fabrication of Colorimetric Textile Sensors
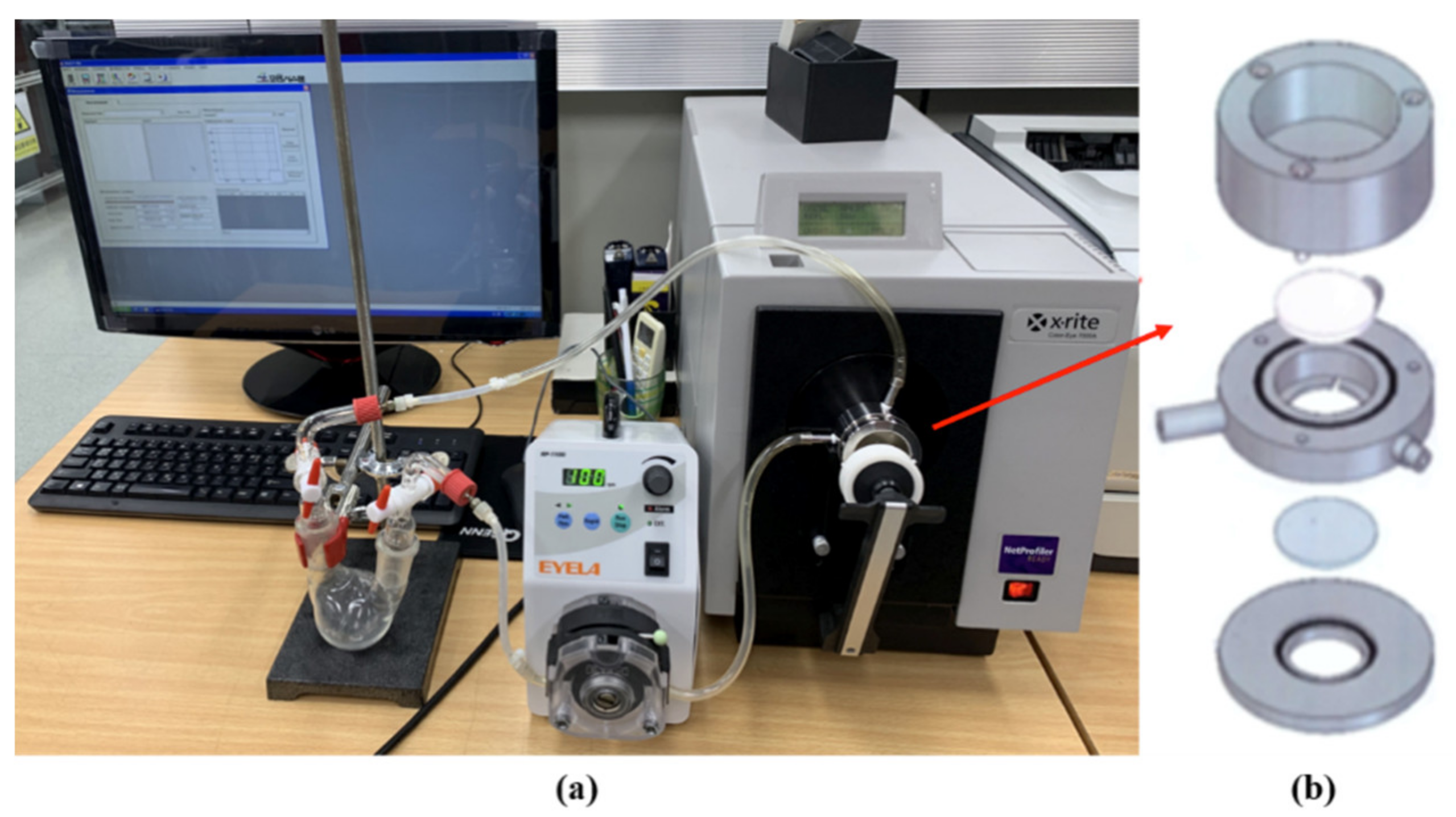
2.4. Characterization
2.5. Gas Detection Performance
2.6. Volatile Organic Compound (VOC) Test
2.7. Fastness Test
2.8. Hazardous Materials Test
2.9. Reversibility Test
3. Results and Discussion
3.1. Gas Detection by Textile Sensors Based on Single Dyes
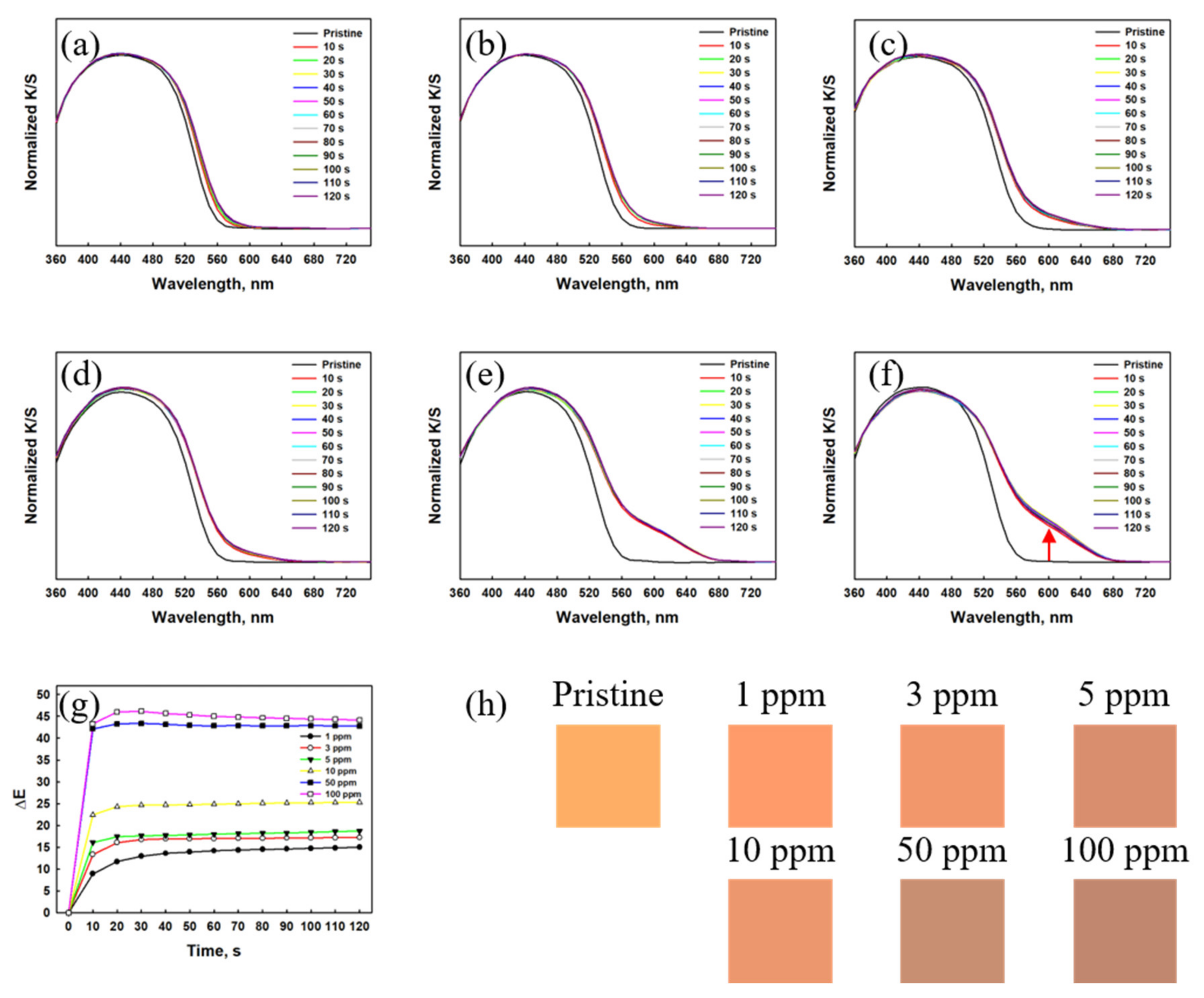
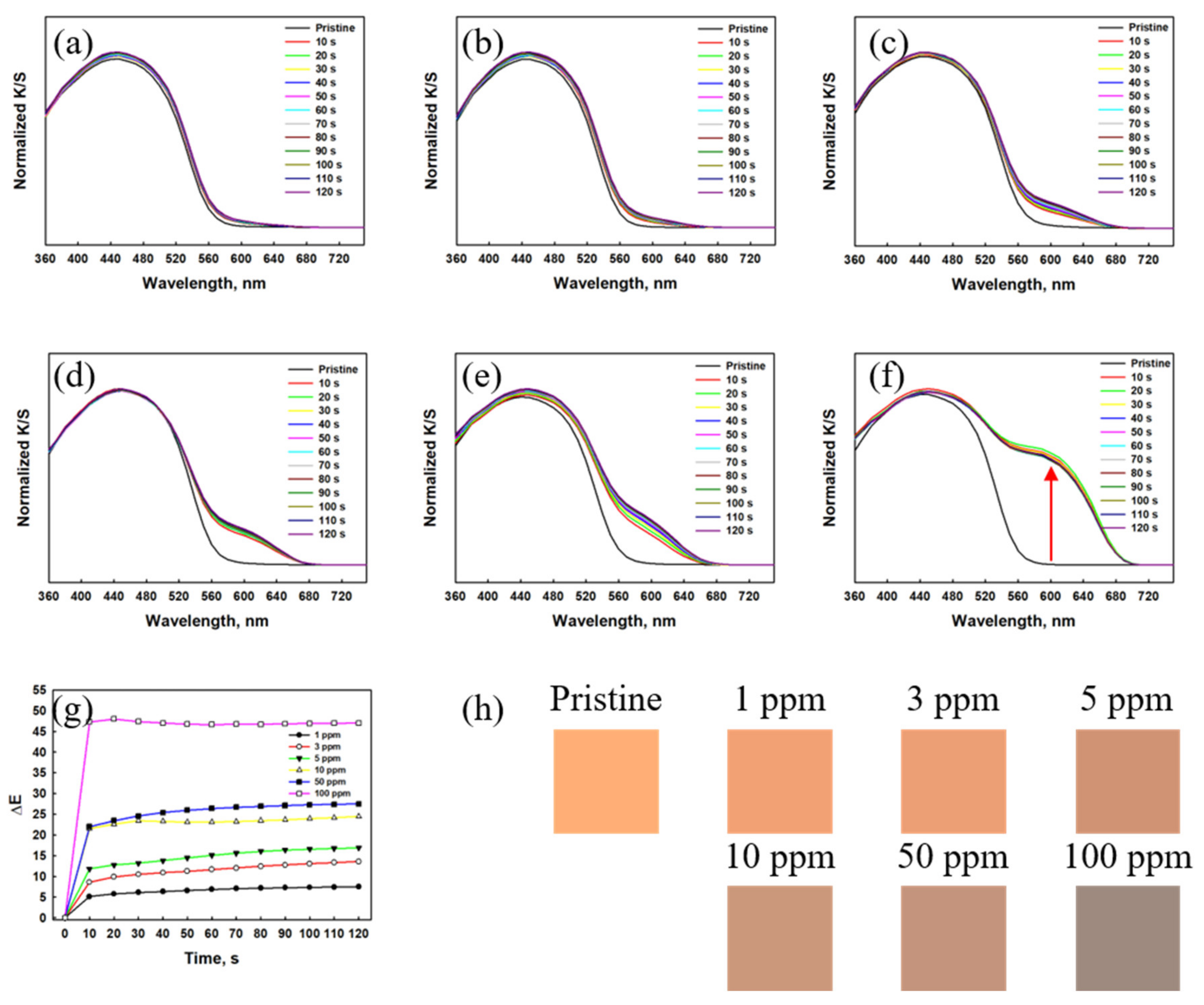
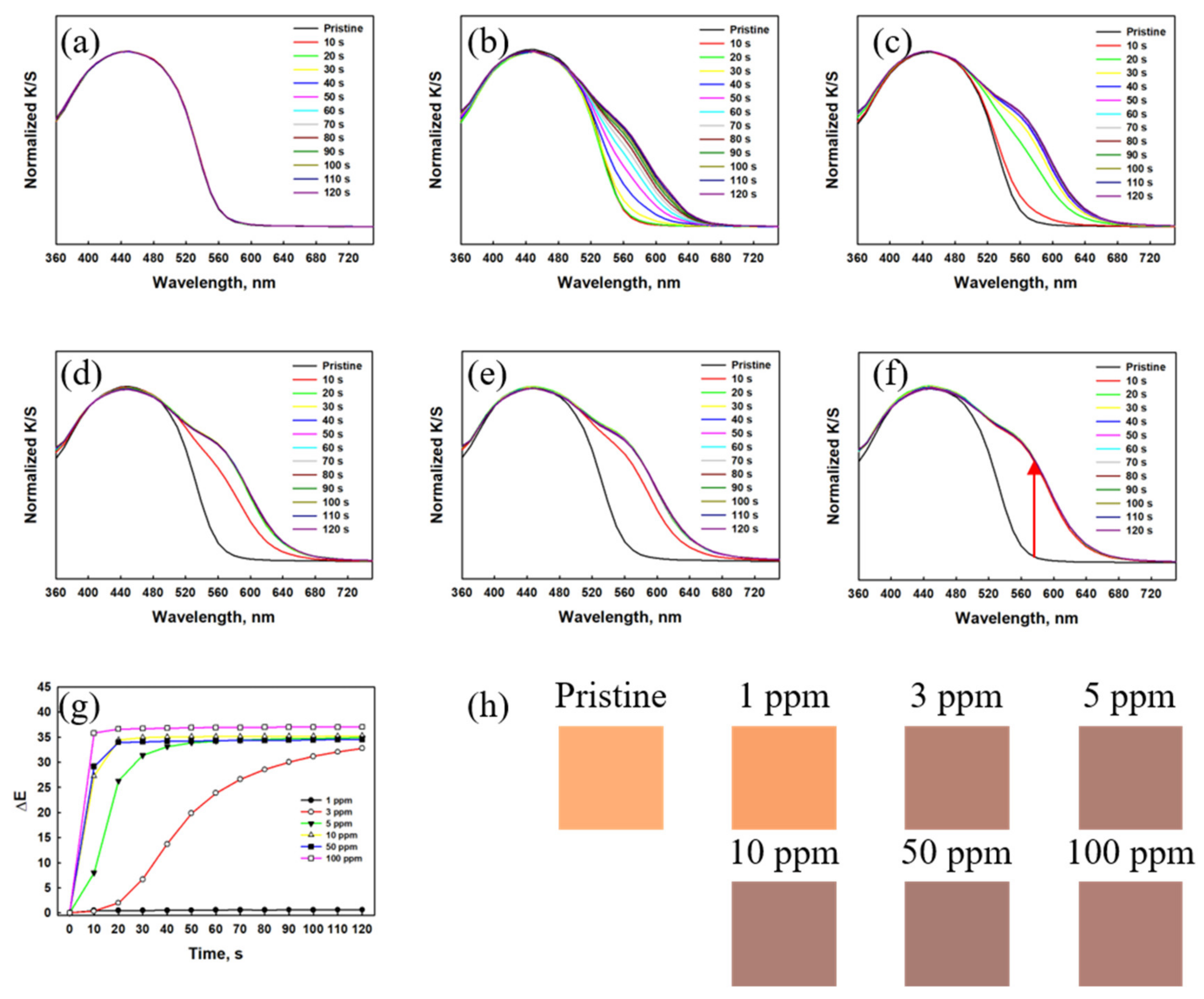
3.1.1. Gas Detection Performance of the Textile Sensor Based on Dye 3
3.1.2. Gas Detection Performance of the Textile Sensor Based on RhYK
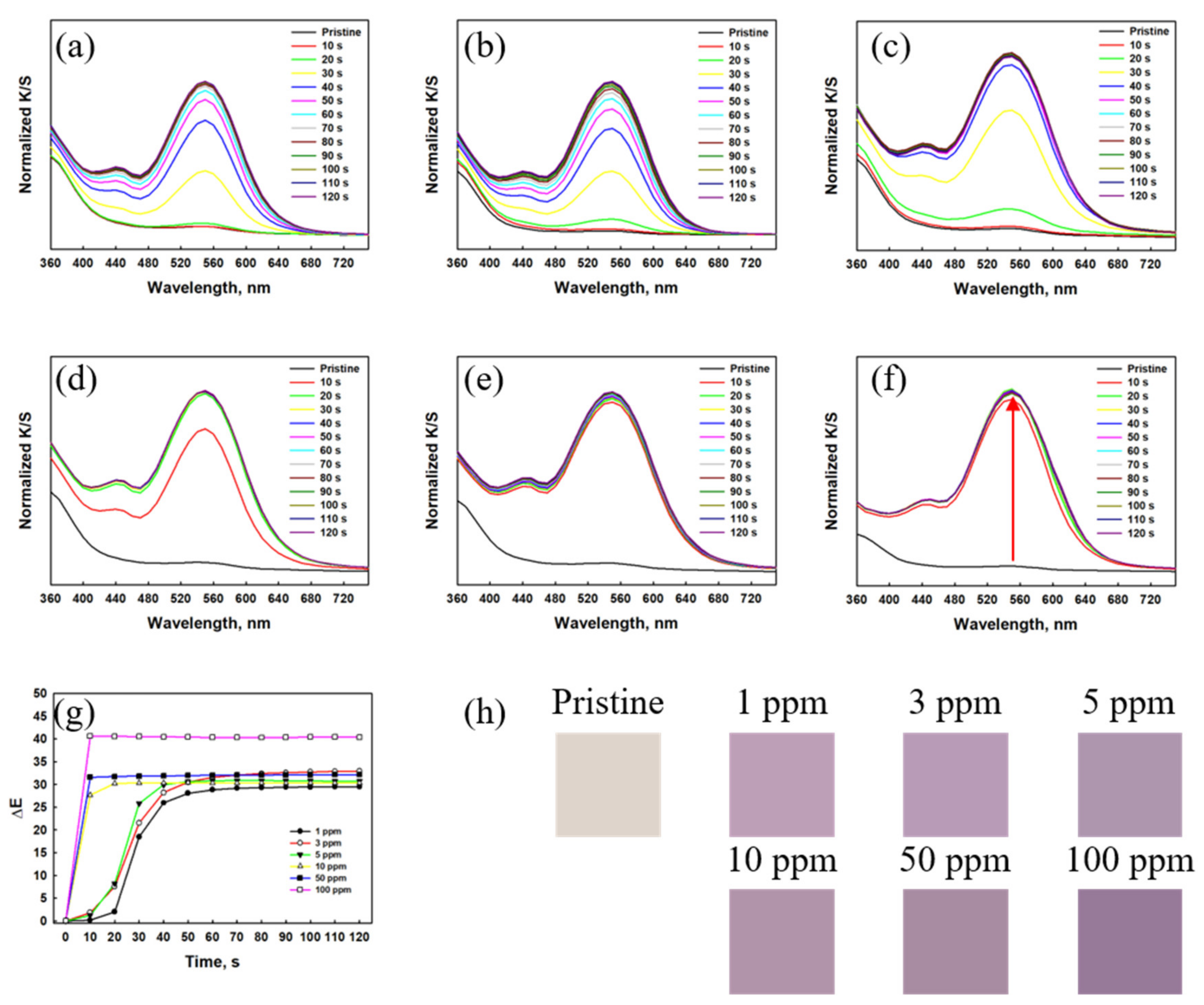
3.2. Dual-Gas Detection by Textile Sensors Based on Mixed Dyes (Dye 3/RhYK)
3.2.1. NH3 Gas Detection by the Mixed-Dye Sensor
3.2.2. HCl Gas Detection by the Mixed-Dye Textile Sensor
3.3. Effect of VOCs on Textile Sensors
3.4. Wash Fastness of Textile Sensors
3.5. Hazardous Materials in Textile Sensors
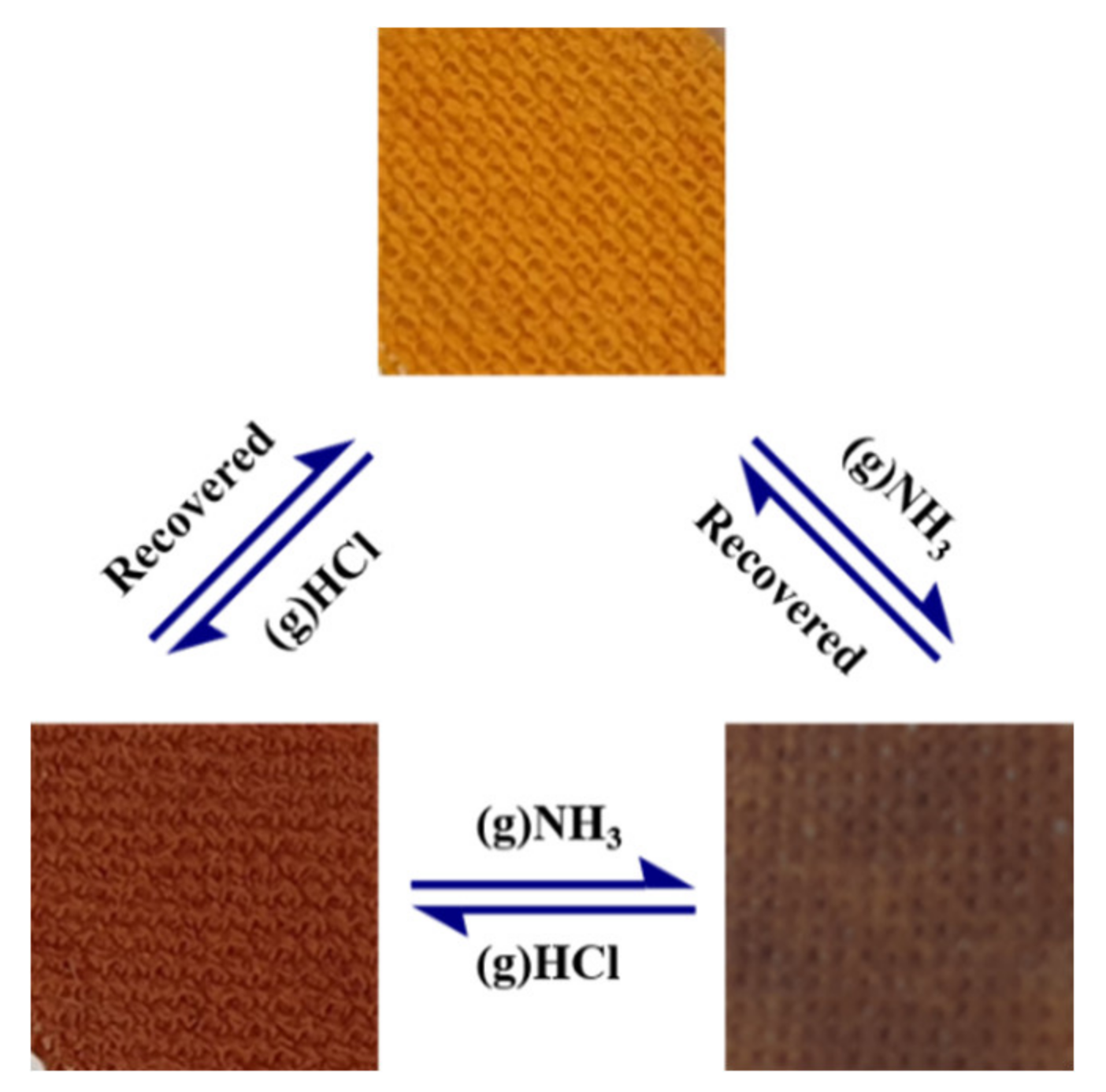
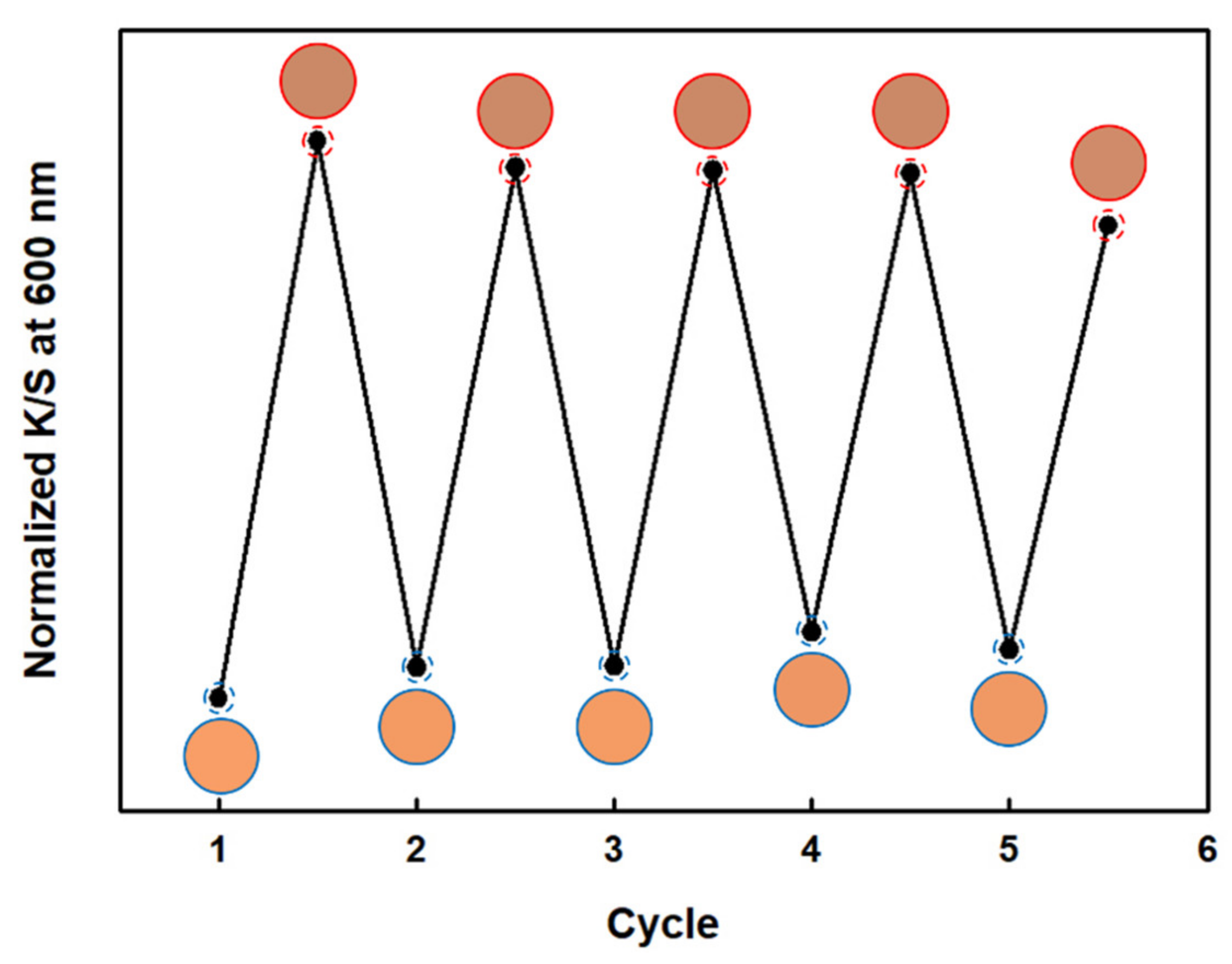
3.6. Reversibility of Textile Sensors
3.6.1. Reversibility with NH3 Gas
3.6.2. Reversibility with HCl Gas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chebabe, D.; Chikh, Z.A.; Hajjaji, N.; Srhiri, A.; Zucchi, F. Corrosion inhibition of Armco iron in 1 M HCl solution by alkyltriazoles. Corros. Sci. 2003, 45, 309–320. [Google Scholar] [CrossRef]
- Bhawsar, J.; Jain, P.K.; Jain, P. Experimental and computational studies of Nicotiana tabacum leaves extract as green corrosion inhibitor for mild steel in acidic medium. Alex. Eng. J. 2015, 54, 769–775. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Sharma, A.; Kumar, J.; Tomar, M.; Umar, A.; Gupta, V. Metal clusters activated SnO2 thin film for low level detection of NH3 gas. Sens. Actuators B 2014, 194, 410–418. [Google Scholar] [CrossRef]
- Goertz, O.; Popp, A.; Kolbenschlag, J.; Vogelpohl, J.; Daigeler, A.; Ring, A.; Lehnhardt, M.; Hirsch, T. Intravital pathophysiological comparison of acid- and alkali-burn injuries in a murine model. J. Surg. Res. 2013, 182, 347–352. [Google Scholar] [CrossRef]
- Kozawa, S.; Kakizaki, E.; Muraoka, E.; Koketsu, H.; Setoyama, M.; Yukawa, N. An autopsy case of chemical burns by hydrochloric acid. Leg. Med. 2009, 11, S535–S537. [Google Scholar] [CrossRef]
- Amshel, C.E.; Fealk, M.H.; Phillips, B.J.; Caruso, D.M. Anhydrous ammonia burns case report and review of the literature. Burns 2000, 26, 493–497. [Google Scholar] [CrossRef]
- Ly, A.; Luo, Y.; Cavaillès, G.; Olivier, M.-G.; Debliquy, M.; Lahem, D. Ammonia sensor based on vapor phase polymerized polypyrrole. Chemosensors 2020, 8, 38. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Fujii, S. HCl gas sensor coating based on poly(N-isopropylacrylamide) nanoparticles prepared from water-methanol binary solvent. Sensors 2018, 18, 3283. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration, Permissible Exposure Limits/OSHA Annotated Table Z-1. 9 January 2017. Available online: https://www.osha.gov/dsg/annotated-pels/tablez-1.html#ppm1 (accessed on 25 September 2020).
- Qi, J.; Xu, X.; Liu, X.; Lau, K.T. Fabrication of textile based conductometric polyaniline gas sensor. Sens. Actuators B 2014, 202, 732–740. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Choi, N.J.; Kim, B.H.; Jun, Y.; Lee, H.K. Ultrasensitive and highly selective graphene-based single yarn for use in wearable gas sensor. Sci. Rep. 2015, 5, 10904. [Google Scholar] [CrossRef]
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.; Xiang, L.; Xie, D.; et al. Reduced graphene oxide/mesoporous ZnO NSs hybrid fibers for flexible, stretchable, twisted, and wearable NO2 E-textile gas sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. A carbon nanotube based ammonia sensor on cotton textile. Appl. Phys. Lett. 2013, 102, 193104. [Google Scholar] [CrossRef]
- Subbiah, D.K.; Mani, G.K.; Babu, K.J.; Das, A.; Rayappan, J.B. Nanostructured ZnO on cotton fabrics—A novel flexible gas sensor & UV filter. J. Clean. Prod. 2018, 194, 372–382. [Google Scholar]
- Yun, Y.J.; Hong, W.G.; Kim, H.J.; Jun, Y.; Lee, H.K. E-textile gas sensors composed of molybdenum disulfide and reduced graphene oxide for high response and reliability. Sens. Actuators B 2017, 248, 829–835. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K. Coloration and application of pH-sensitive dyes on textile materials. Color. Technol. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- Boerman, J.-K.; van Harberden, J.-K.; Pannek, C.; Schmitt, K.; Tarantik, K.R.; Bauersfeld, M.-L.; Wöllenstein, J. Improvement methods for colorimetric gas sensor for use in indoor livestock farming. Proceedings 2018, 2, 769. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K.; Brancatelli, G.; Rosace, G.; Van Damme, E.; De Vos, W. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of Methyl Red into a sol–gel matrix. Sens. Actuators B 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Staneva, D.; Betcheva, R.; Chovelon, J.-M. Optical sensor for aliphatic amines based on the simultaneous colorimetric and fluorescence responses of smart textile. J. Appl. Polym. Sci. 2007, 106, 1950–1956. [Google Scholar] [CrossRef]
- Owyeung, R.E.; Panzer, M.J.; Sonkusale, S.R. Colorimetric gas sensing washable threads for smart textiles. Sci. Rep. 2019, 9, 5607. [Google Scholar] [CrossRef]
- Schoolaert, E.; Hoogenboom, R.; De Clerck, K. Colorimetric nanofibers as optical sensors. Adv. Funct. Mater. 2017, 27, 1702646. [Google Scholar] [CrossRef]
- Van der Schueren, L.; Mollet, T.; Ceylan, Ö.; De Clerck, K. The development of polyamide 6.6 nanofibres with a pH-sensitive function by electrospinning. Eur. Polym. J. 2010, 46, 2229–2239. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, W.; Zhan, N.; Xiao, N.; Wang, S.; Li, Y.; Yang, Q. A novel two-nozzle electrospinning process for preparing microfiber reinforced pH-sensitive nano-membrane with enhanced mechanical property. Eur. Polym. J. 2011, 47, 2228–2233. [Google Scholar] [CrossRef]
- Agarwal, A.; Raheja, A.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B 2012, 161, 1097–1101. [Google Scholar] [CrossRef]
- Kim, S.-H.; Bae, J.-S. Halochromic chemosensor prepared by pyran-based nanofibers. Fibers Polym. 2013, 14, 1981–1984. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.E.; Karaca, B.B. Investigation of a natural pH-indicator dye for nanofibrous wound dressings. In Proceedings of the IOP Conference Series: Materials Science and Engineering, AUTEX, Istanbul, Turkey, 20–22 June 2018; IOP Publishing: Bristol, UK, 2018; Volume 460, p. 012020. [Google Scholar]
- Geltmeyer, J.; Vancoillie, G.; Steyaert, I.; Breyne, B.; Cousins, G.; Lava, K.; Hoogenboom, R.; De Buysser, K.; De Clerck, K. Dye modification of nanofibrous silicon oxide membranes for colorimetric HCl and NH3 sensing. Adv. Funct. Mater. 2016, 26, 5987–5996. [Google Scholar] [CrossRef]
- Jeevarathinam, A.S.; Varathan, E.; Ravindran, E.; Somanathan, N.; Subramanian, V.; Mandal, A.B.; Sudha, J.D.; Ramakrishnan, R. A solution processable fluorene–fluorenone oligomer with aggregation induced emission enhancement. Chem. Commun. 2013, 49, 10742–10744. [Google Scholar]
- Suleymanov, A.A.; Doll, M.; Ruggi, A.; Scopelliti, R.; Fadaei-Tirani, F.; Severin, K. Synthesis of tetraarylethene luminogens by C− H vinylation of aromatic compounds with triazenes. Angew. Chem. Int. Ed. 2020, 59, 9957–9961. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.M.; Noh, H.L.; Gwon, S.-Y.; Park, Y.K.; Cho, N.; Lee, W.; Kim, S.-H.; Kim, J.H. Some properties of a new D-π-A dye based on hydroxyl-methoxybenzene donor and isophorone acceptor moiety: Effects of anion, ethylamine and temperature. Dyes Pigment. 2018, 159, 158–165. [Google Scholar] [CrossRef]
- Park, Y.K.; Oh, B.M.; Jo, A.R.; Han, J.H.; Lim, J.Y.; Oh, H.J.; Lim, S.J.; Kim, J.H.; Lee, W.S. Fabrication of colorimetric textile sensor based on rhodamine dye for acidic gas detection. Polymers 2020, 12, 431. [Google Scholar] [CrossRef]
- Oh, H.J.; Yeang, B.J.; Park, Y.K.; Choi, H.J.; Kim, J.H.; Kang, Y.S.; Bae, Y.; Kim, J.Y.; Lim, S.J.; Lee, W.; et al. Washable colorimetric nanofiber nonwoven for ammonia gas detection. Polymers 2020, 12, 1585. [Google Scholar] [CrossRef]
- Nobbs, J.H. Kubelka–Munk theory and the prediction of reflectance. Rev. Prog. Color. Relat. Top. 1985, 15, 66–75. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- ISO. Textiles—Tests for Colour Fastness—Part C10: Colour Fastness to Washing with Soap or Soap and Soda; ISO 105-C10:2006; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- KSA. Test Method for Determination of Aryl Amine Level on the Dyestuff and Dyed Products; KS K 0147:2015; Korean Standards Association: Seoul, Korea, 2015. [Google Scholar]
- KSA. Test Method for the Determination of Allergenous Dyes in Textiles; KS K 0736:2014; Korean Standards Association: Seoul, Korea, 2014. [Google Scholar]
- KSA. Textiles—Determination of Formaldehyde—Part 1: Free and Hydrolized Formaldehyde (Water Extraction Method); KS K ISO 14184:2009; Korean Standards Association: Seoul, Korea, 2009. [Google Scholar]
- KSA. Textiles—Determination of pH of Aqueous Extract; KS K ISO 3071:2009; Korean Standards Association: Seoul, Korea, 2009. [Google Scholar]
- Kessler, M.A.; Wolfbeis, O.S. New highly fluorescent ketocyanine polarity probes. Spectrochim. Acta Part A 1991, 47, 187–192. [Google Scholar] [CrossRef]
- Soller, B.R. Design of intravascular fiber optic blood gas sensors. IEEE Eng. Med. Biol. Mag. 1994, 13, 327–335. [Google Scholar] [CrossRef]
- Van der Schueren, L.; Hemelsoet, K.; Van Speybroeck, V.; De Clerck, K. The influence of a polyamide matrix on the halochromic behaviour of the pH-sensitive azo dye Nitrazine Yellow. Dyes Pigm. 2012, 94, 443–451. [Google Scholar] [CrossRef]




| Dye | Change | Staining | |||||
|---|---|---|---|---|---|---|---|
| A | C | N | P | Ac | W | ||
| Dye 3 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| RhYK | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| Arylamines (mg/kg) | Allergenic Disperse Dyes (mg/kg) | Formaldehyde (mg/kg) | pH | Total Pb (mg/kg) | Total Cd (mg/kg) |
|---|---|---|---|---|---|
| <5 | <20 | Not detected | 7.2 | <10 | <10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.K.; Oh, H.J.; Bae, J.H.; Lim, J.Y.; Lee, H.D.; Hong, S.I.; Son, H.S.; Kim, J.H.; Lim, S.J.; Lee, W. Colorimetric Textile Sensor for the Simultaneous Detection of NH3 and HCl Gases. Polymers 2020, 12, 2595. https://doi.org/10.3390/polym12112595
Park YK, Oh HJ, Bae JH, Lim JY, Lee HD, Hong SI, Son HS, Kim JH, Lim SJ, Lee W. Colorimetric Textile Sensor for the Simultaneous Detection of NH3 and HCl Gases. Polymers. 2020; 12(11):2595. https://doi.org/10.3390/polym12112595
Chicago/Turabian StylePark, Young Ki, Hyun Ju Oh, Jong Hyuk Bae, Jee Young Lim, Hee Dong Lee, Seok Il Hong, Hyun Sik Son, Jong H. Kim, Seung Ju Lim, and Woosung Lee. 2020. "Colorimetric Textile Sensor for the Simultaneous Detection of NH3 and HCl Gases" Polymers 12, no. 11: 2595. https://doi.org/10.3390/polym12112595
APA StylePark, Y. K., Oh, H. J., Bae, J. H., Lim, J. Y., Lee, H. D., Hong, S. I., Son, H. S., Kim, J. H., Lim, S. J., & Lee, W. (2020). Colorimetric Textile Sensor for the Simultaneous Detection of NH3 and HCl Gases. Polymers, 12(11), 2595. https://doi.org/10.3390/polym12112595