Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Cellulose from Jute Fiber
2.3. Preparation of Poly(Methyl Acrylate)/Acrylonitrile (PMA/PAN)-Grafted Jute Cellulose
2.4. Synthesis of Poly(Hydroxamic Acid) Ligand or Poly(Amidoxime) Ligand
2.5. Adsorption and Isotherm Study by Poly(Hydroxamic Acid)/Poly(Amidoxime) (PHA/PA) Ligands
2.6. Kinetic Study
3. Results and Discussion
3.1. Reaction Mechanism
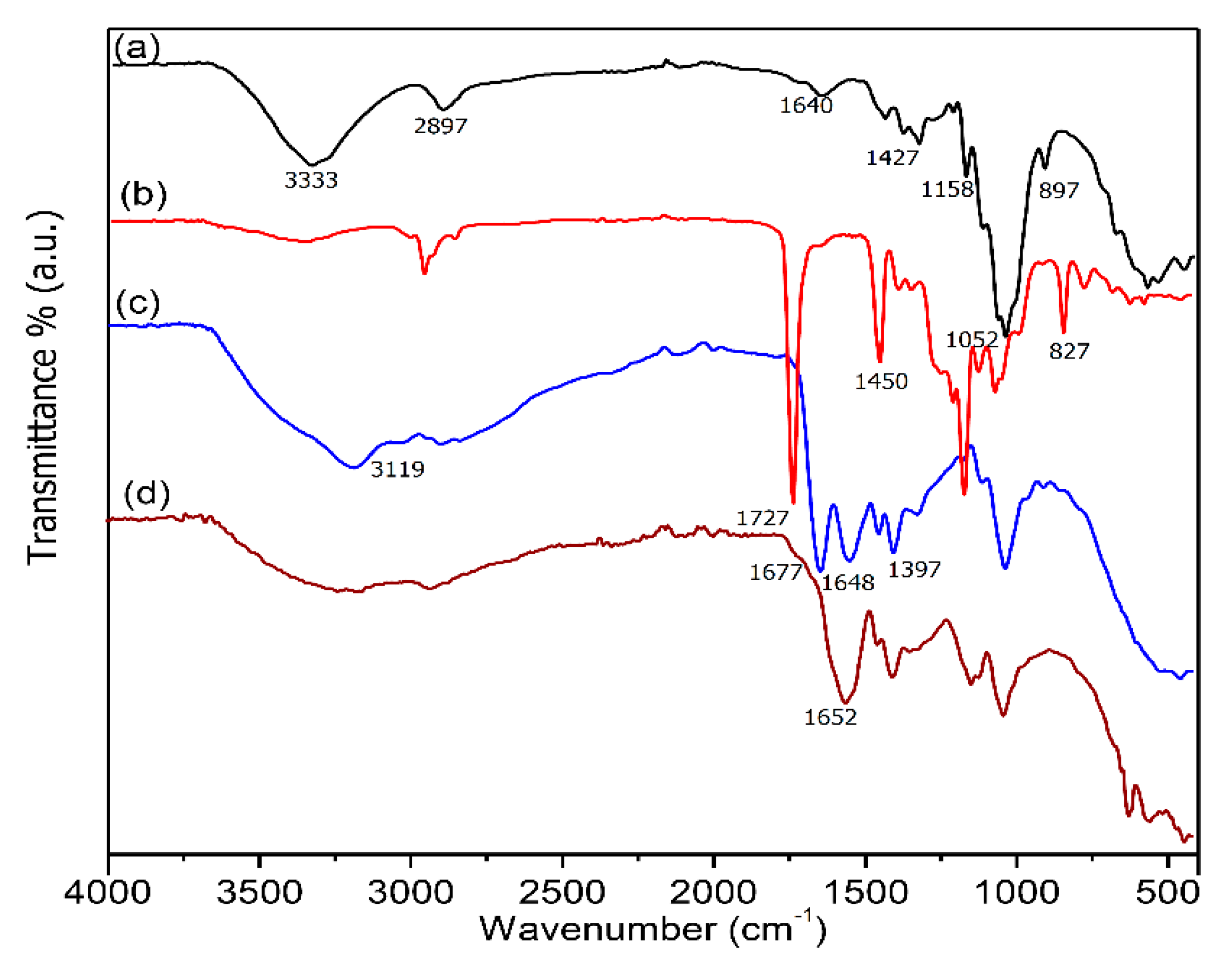
3.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Spectroscopy Analysis
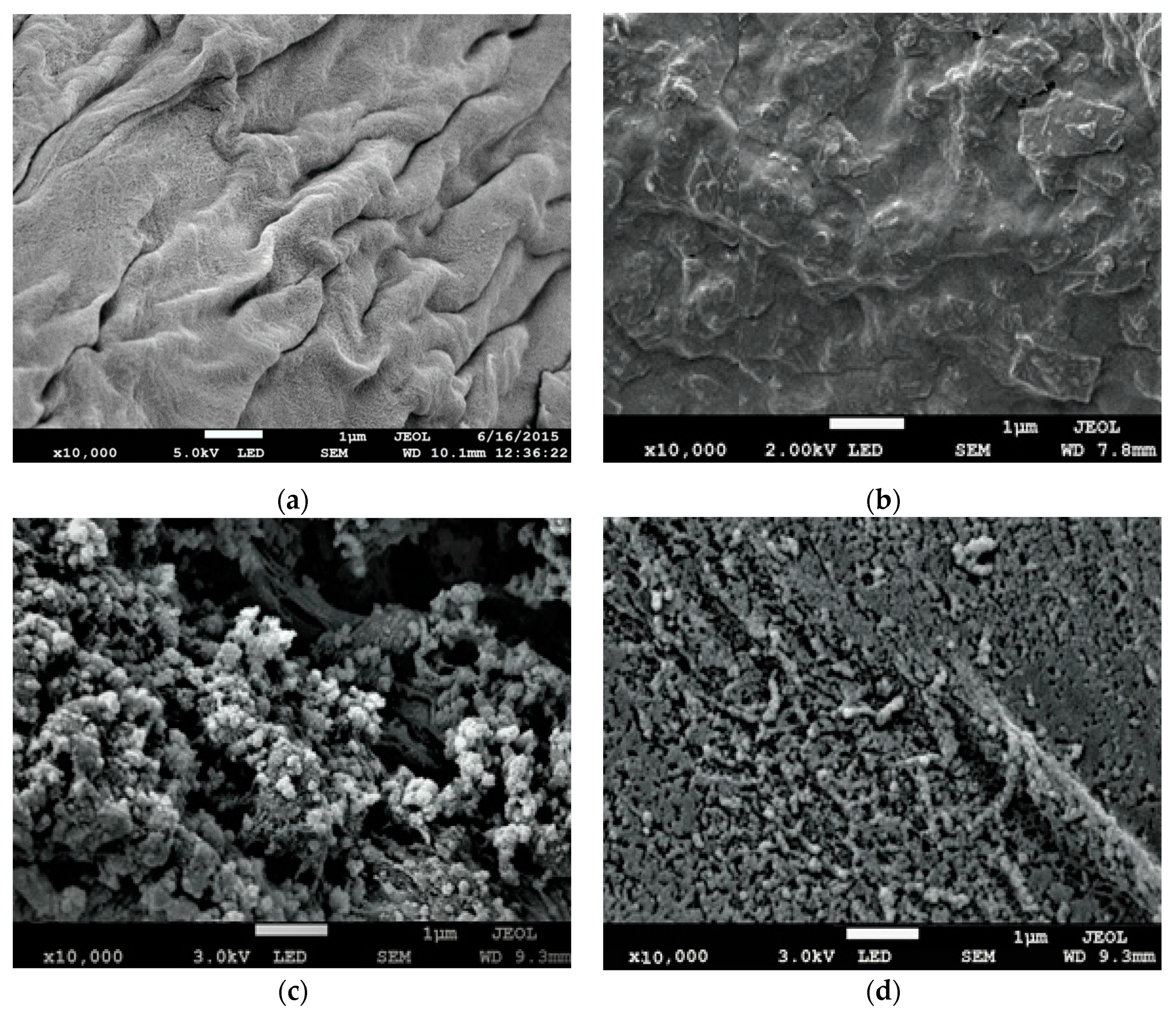
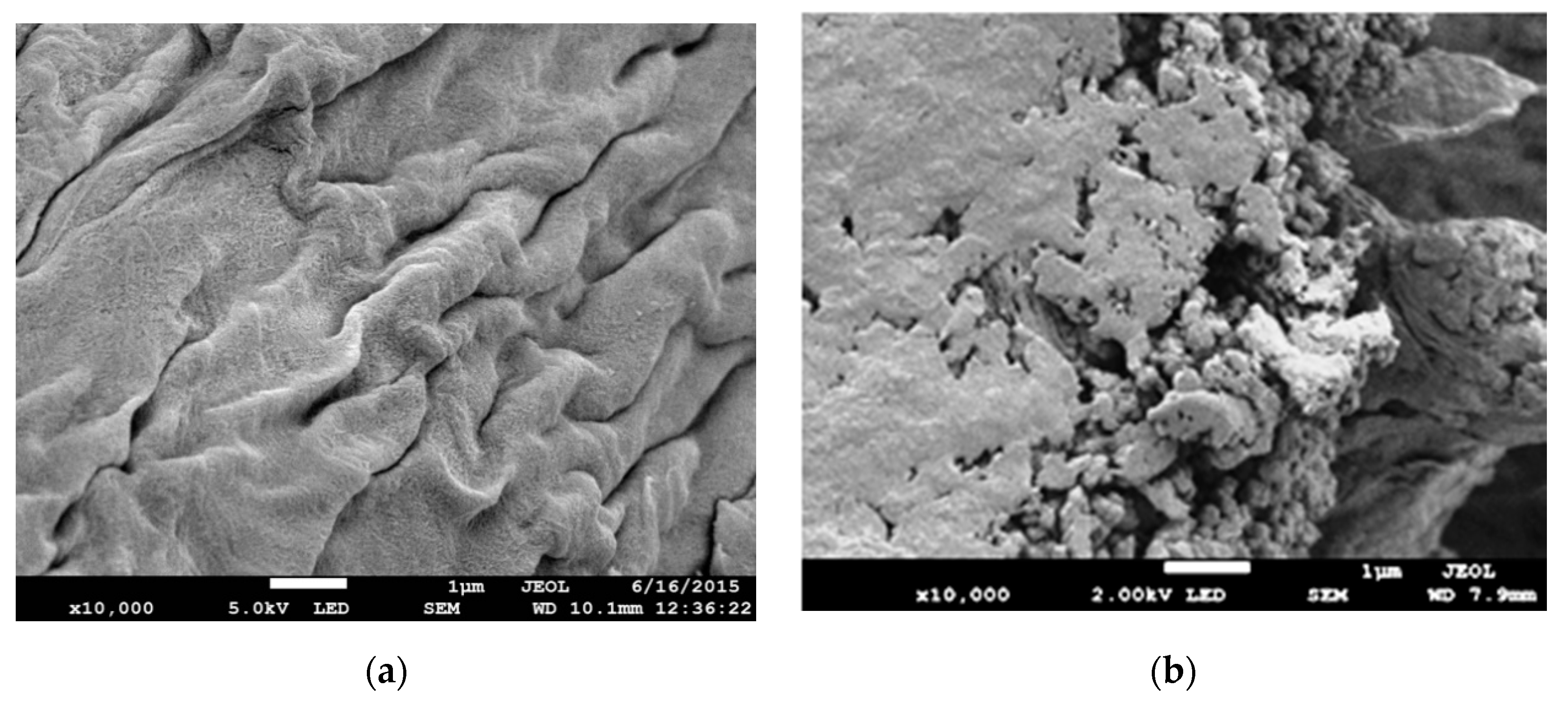
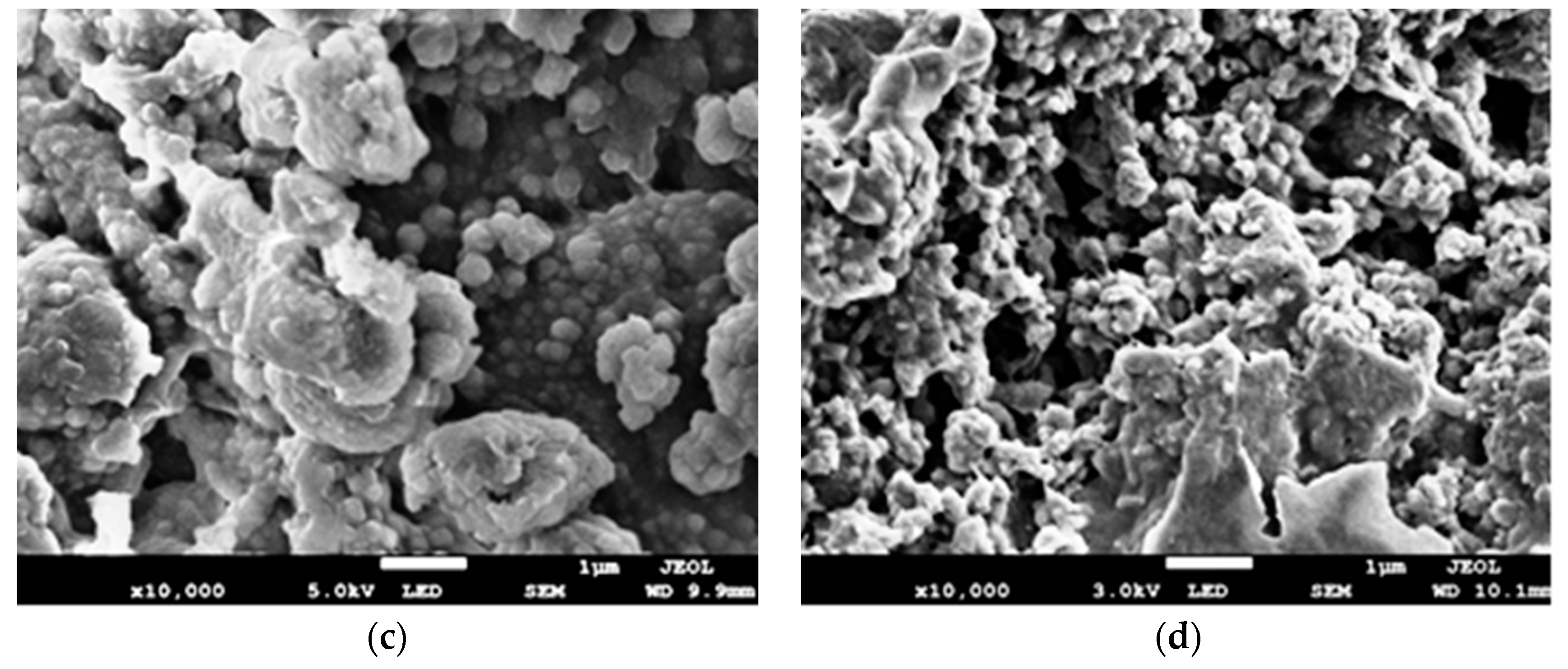
3.3. Field Emission Scanning Electron Microscopy (FE-SEM) Analysis
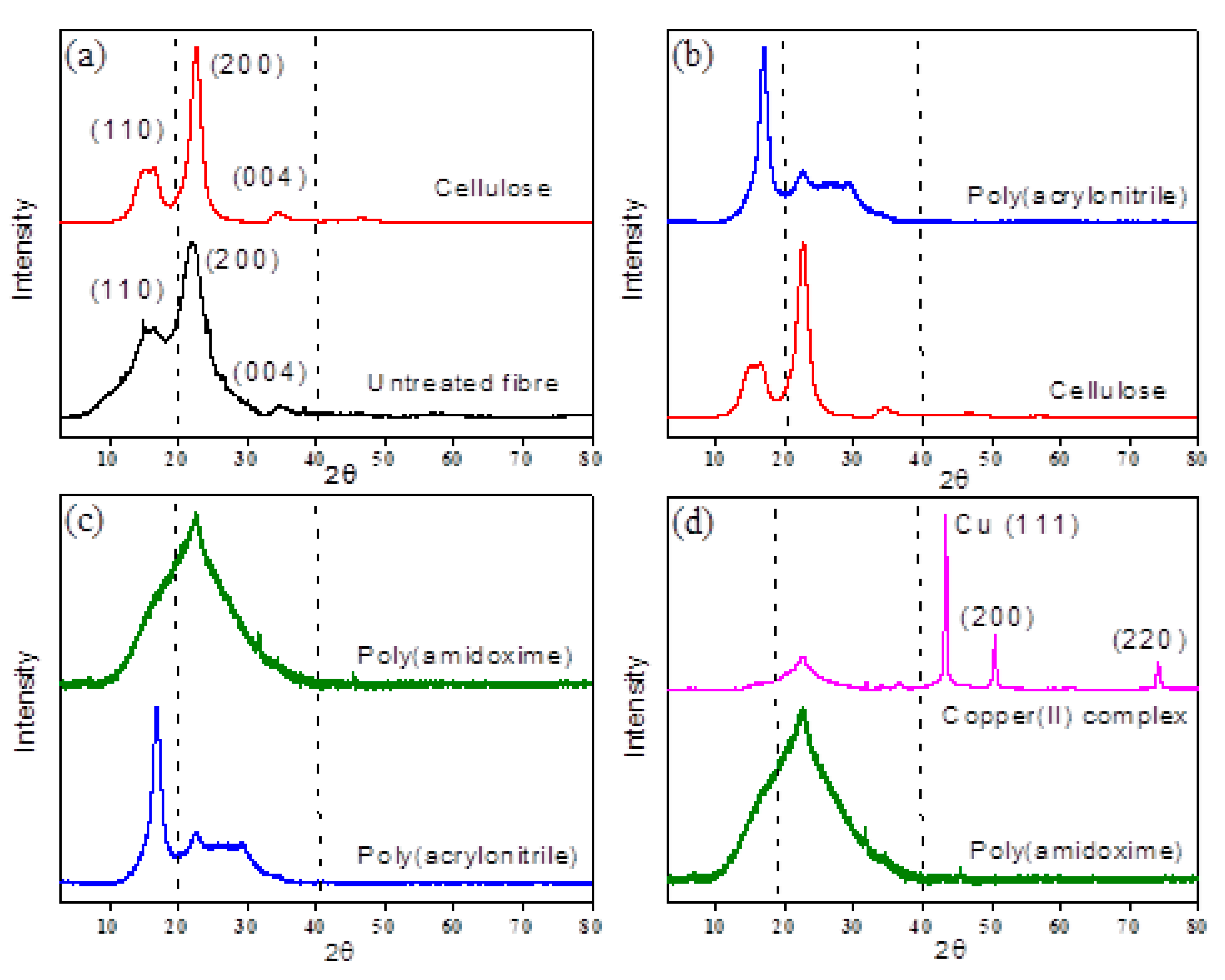
3.4. X-ray Diffraction (XRD) Analysis
3.5. Thermogravimetry Analysis
3.6. Adsorption Study of Poly(Hydroxamic Acid) and Poly(Amidoxime) Ligands
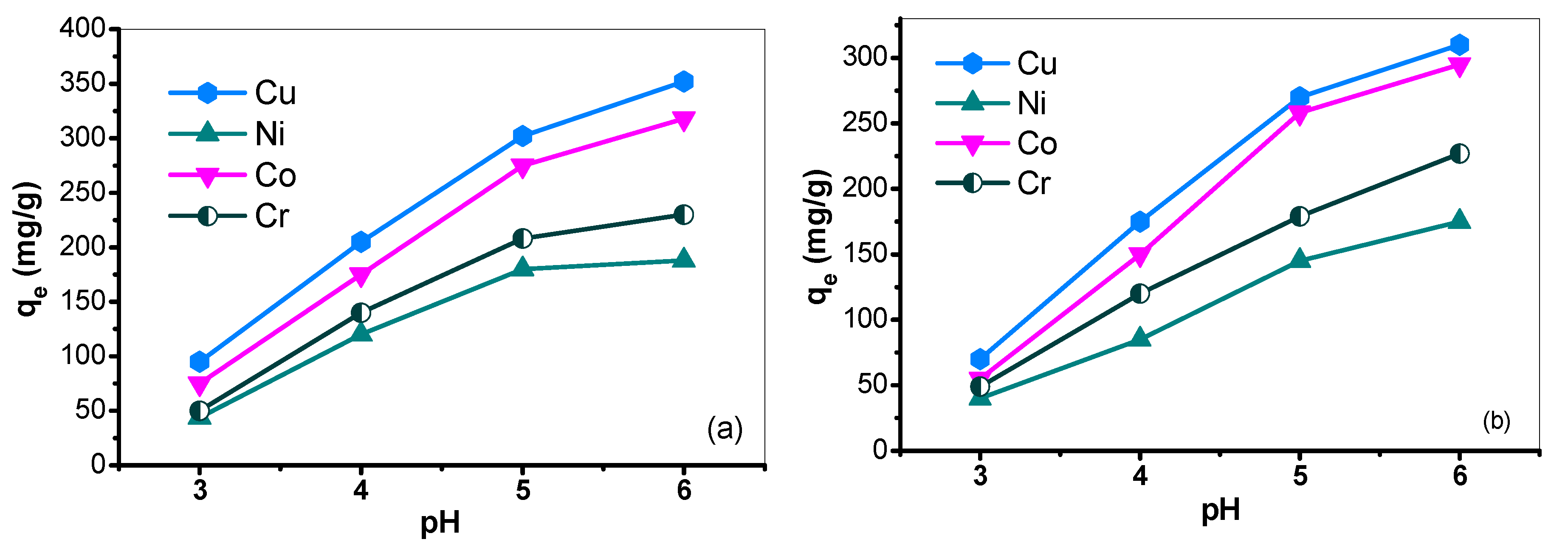
3.6.1. Effect of pH on the Adsorption of Metal Ions by PHA Ligand
3.6.2. Effect of pH on the Adsorption of Metal Ions by Poly(Amidoxime) (PA) Ligand
3.7. Adsorption Kinetic Studies
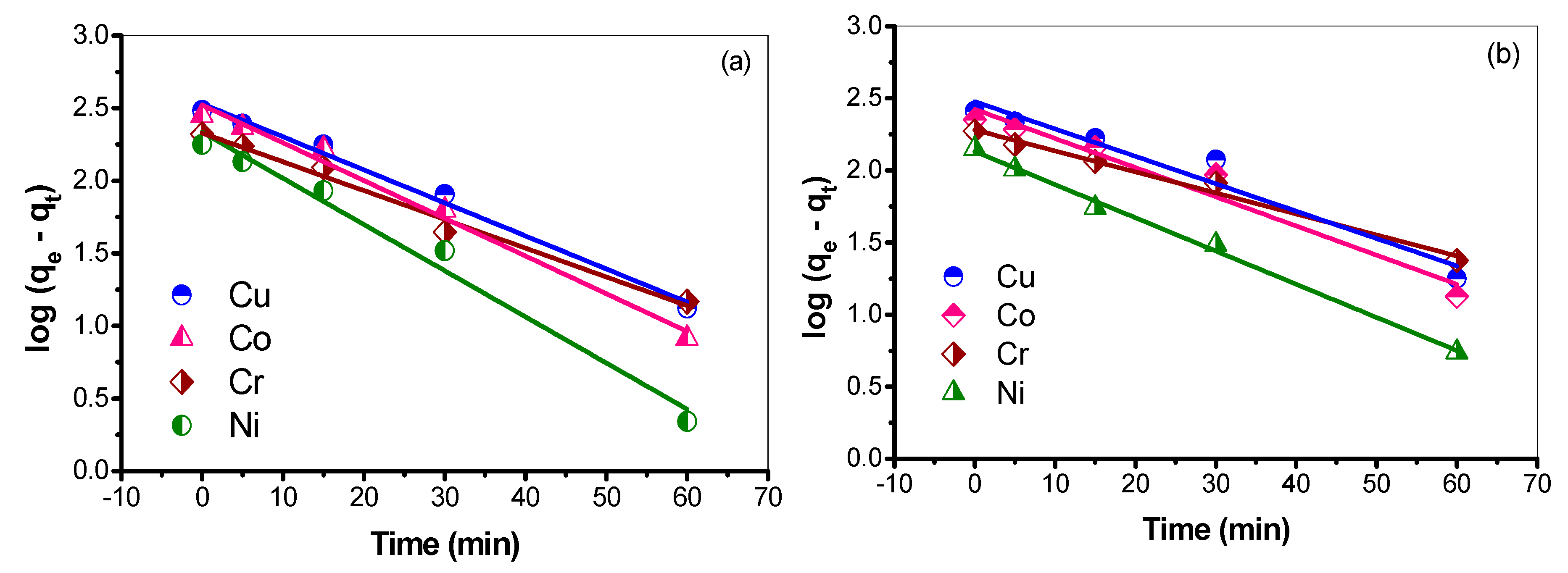
3.7.1. Pseudo First-Order Rate of Adsorption
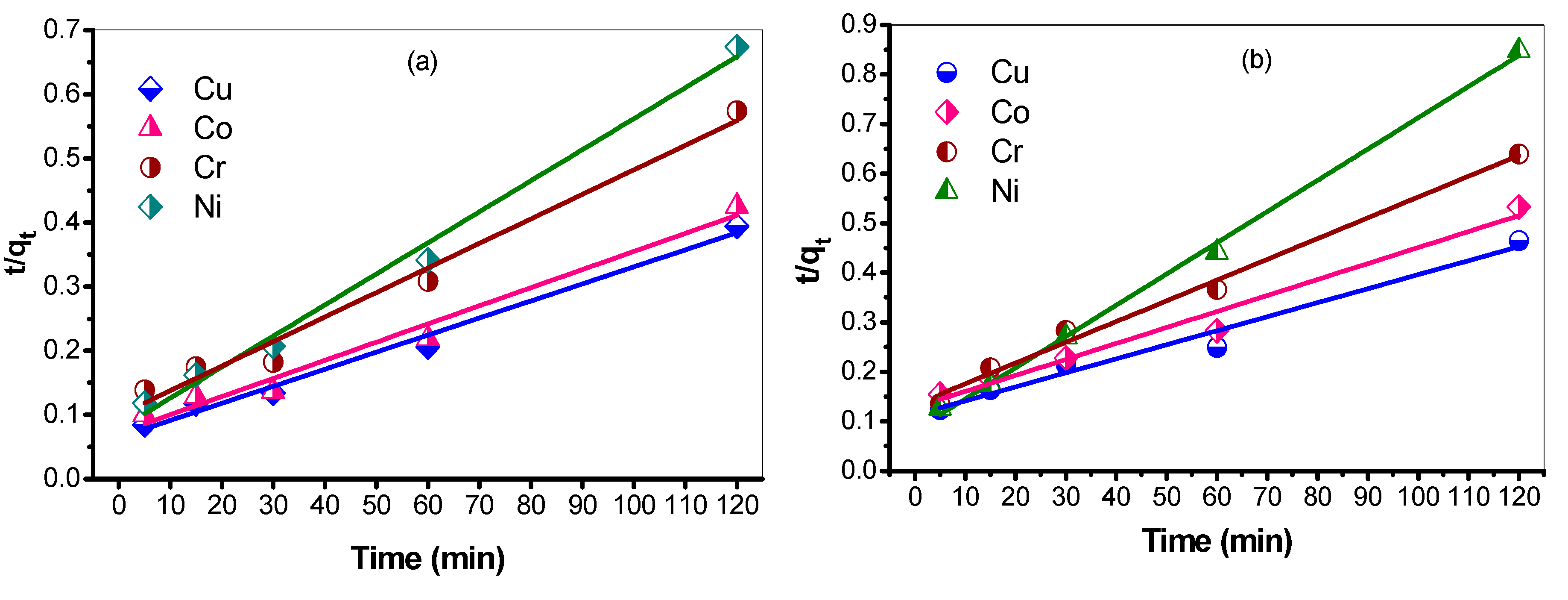
3.7.2. Pseudo Second-Order Rate of Reaction
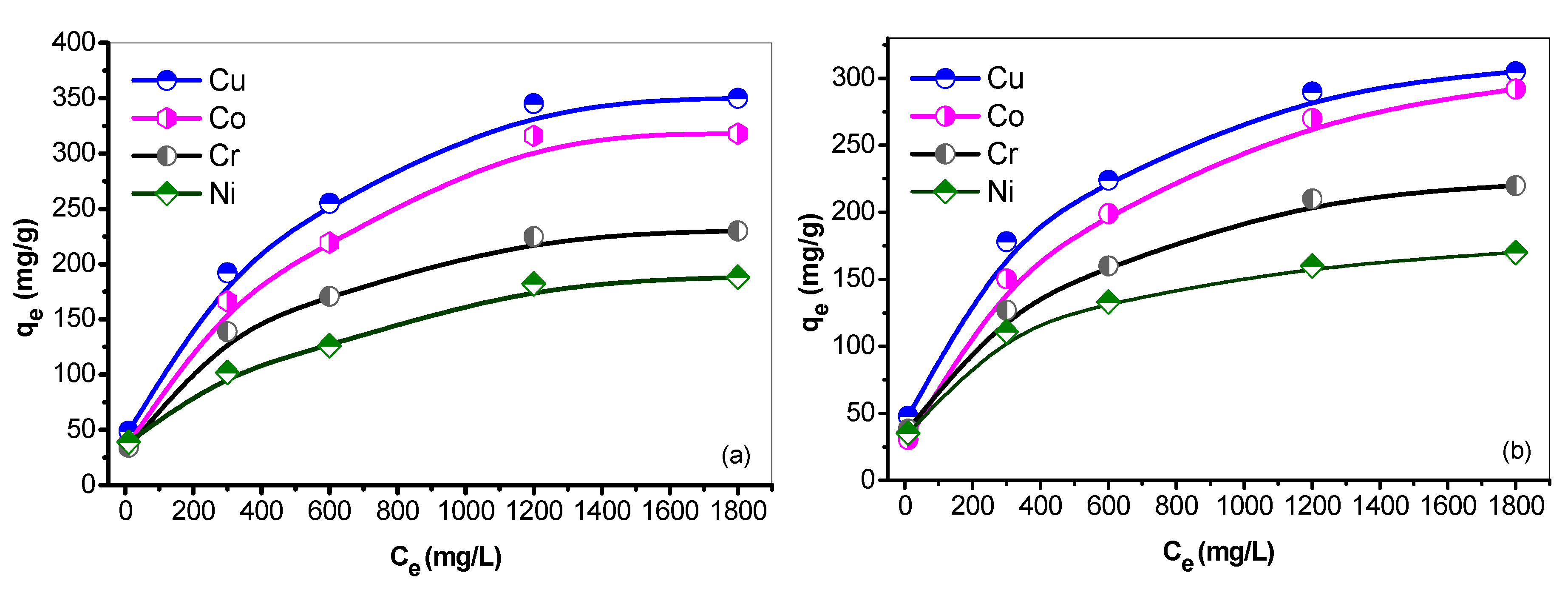
3.8. Sorption Isotherm
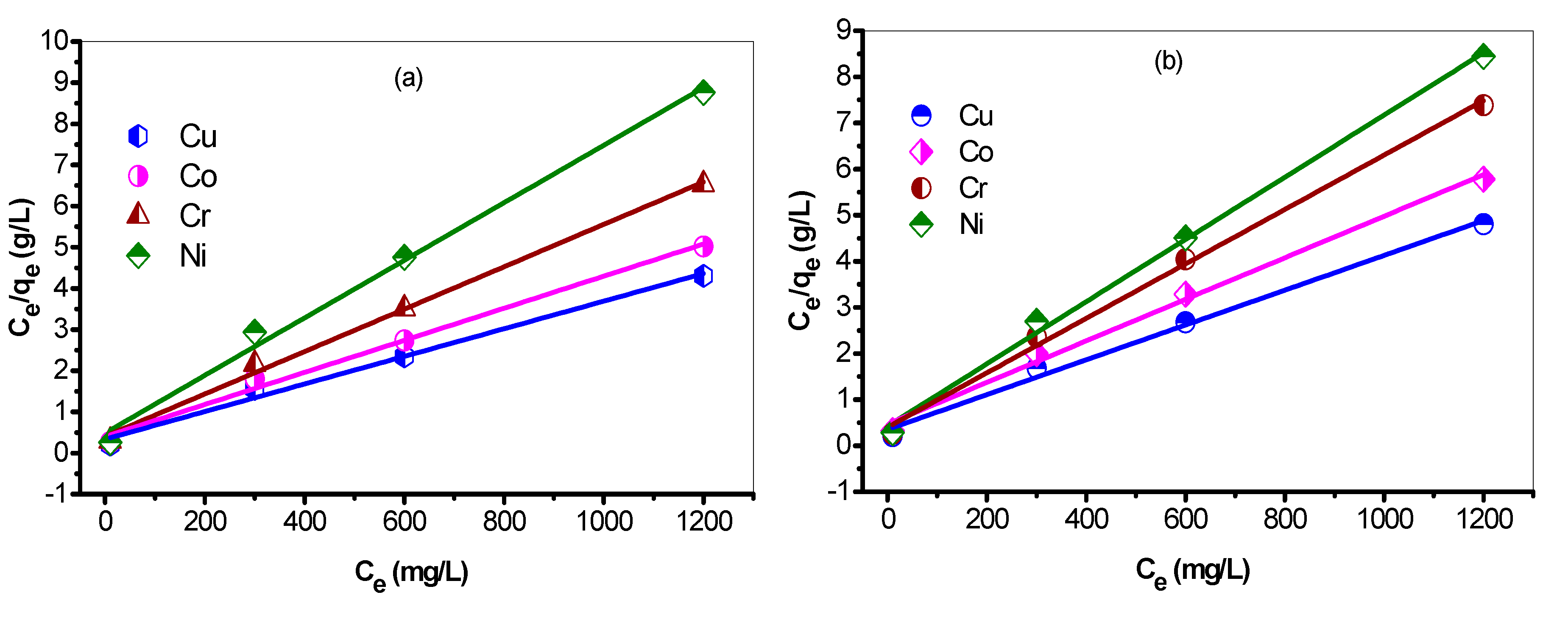
3.8.1. Linear Langmuir Adsorption Isotherm
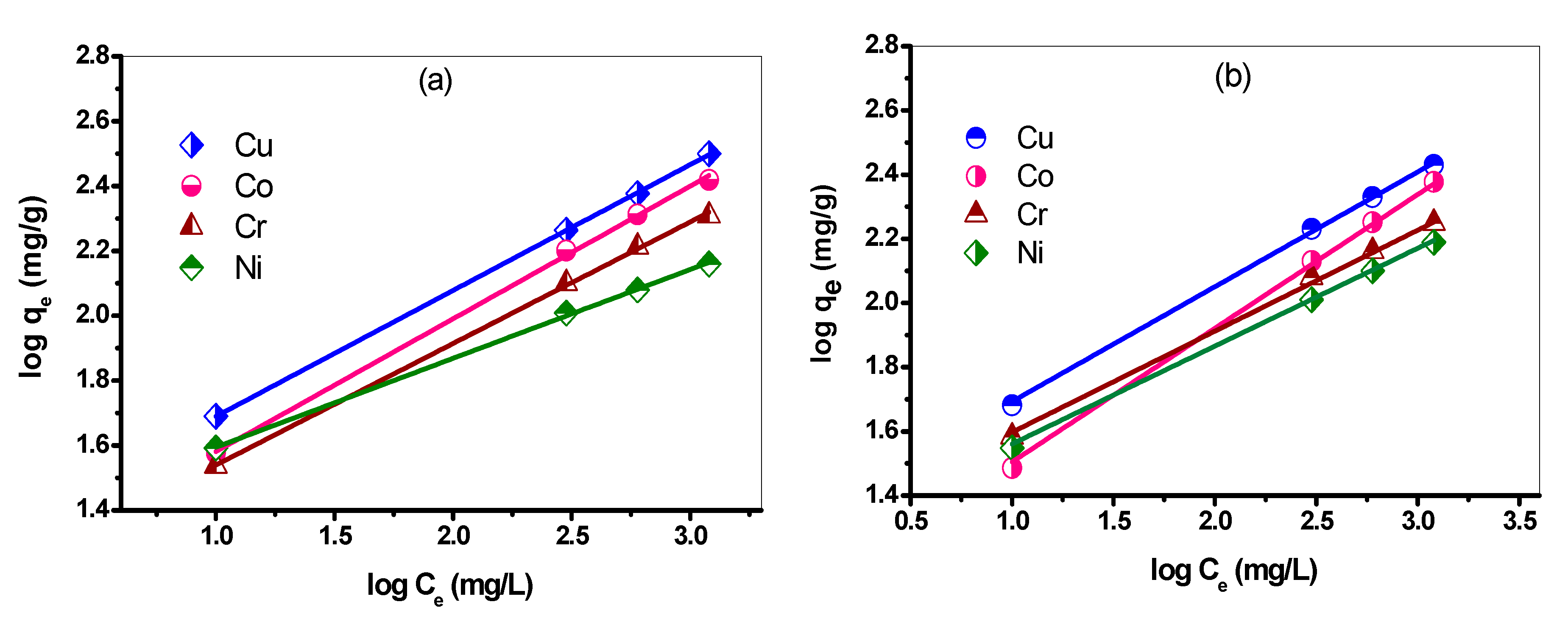
3.8.2. Linear Freundlich Adsorption Isotherm
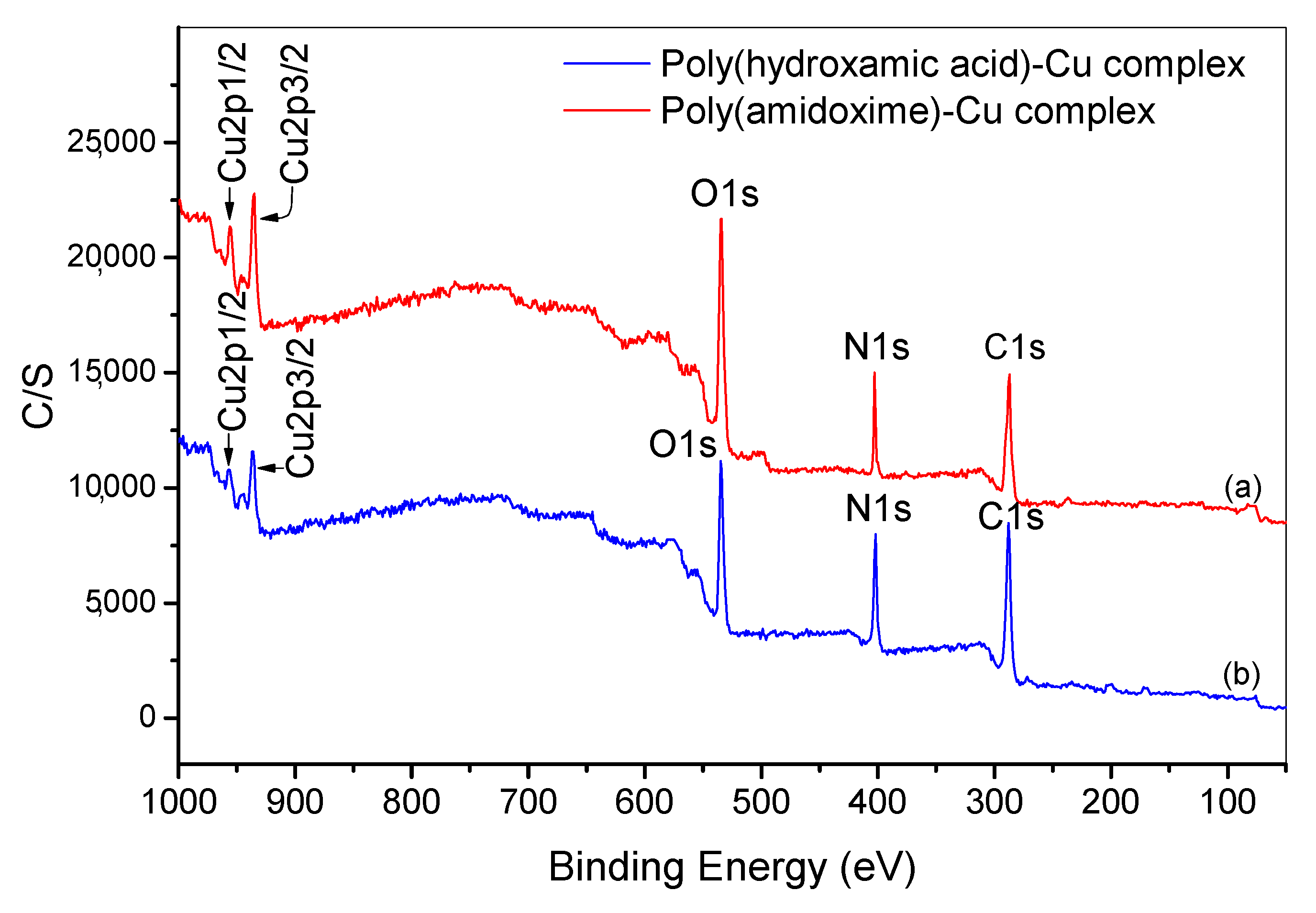
3.9. Investigation of Adsorption Mechanism by X-ray Photoelectron Spectroscopy (XPS) Analysis
3.10. Reusability Studies of Poly(Hydroxamic Acid)/Poly(Amidoxime) Ligands
3.11. Electroplating Wastewater Purification
3.11.1. Practical Application of PHA Ligand
3.11.2. Practical Application of PA Ligand
3.12. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Lakherwal, D. Adsorption of Heavy Metals: A Review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Gunatilake, S.K. Methods of Removing Heavy Metals for Industrial Wastewater. J. Multidiscip. Eng. Sci. Stud. 2015, 1, 12–18. [Google Scholar]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019, 179, 105151. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Teng, H.; Sun, Y.; Guo, J.; Xie, W. Chemical coagulation process for the removal of heavy metals from water: A review. Desalination Water Treat. 2016, 57, 1733–1748. [Google Scholar] [CrossRef]
- Bashkim, S.T.; Salih, T.G. Reverse osmosis removal of heavy metals from wastewater effluents using biowaste materials pretreatment. Pol. J. Environ. Stud. 2019, 28, 337–341. [Google Scholar]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2019, 8. [Google Scholar] [CrossRef]
- Gupta, V.K.; Carrott, P.J.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar]
- Rahman, M.L.; Sarkar, S.M.; Yusoff, M.M.; Kulkarni, A.K.D.; Chowdhury, Z.Z.; Ali, M.E. Poly(amidoxime) from polymer-grafted khaya cellulose: An excellent medium for the removal of transition metal cations from aqueous solution. BioResources 2016, 11, 6780–6800. [Google Scholar] [CrossRef]
- Yu, H.; Fu, G.; He, B. Preparation and adsorption properties of PAA-grafted cellulose adsorbent for low-density lipoprotein from human plasma. Cellulose 2006, 14, 99–107. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Graft modification of cellulose: Methods, properties and applications. Polymer 2015, 70, A1–A16. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarkar, S.M.; Yusoff, M.M.; Abdullah, M.H. Optical detection and efficient removal of transition metal ions from water using poly (hydroxamic acid) ligand. Sens. Actuators B Chem. 2017, 242, 595–608. [Google Scholar] [CrossRef]
- Rahman, M.L.; Fui, C.J.; Sarjadi, M.S.; Arshad, S.E.; Musta, B.; Abdullah, M.H.; Sarkar, S.M.; O’Reilly, E.J. Poly(amidoxime) ligand derived from waste palm fiber for the removal of heavy metals from electroplating wastewater. Environ. Sci. Pollut. Res. 2020, 27, 34541–34556. [Google Scholar] [CrossRef]
- Rahman, M.L.; Wong, Z.J.; Sarjadi, M.S.; Abdullah, M.H.; Heffernan, M.A.; Sarkar, M.S.; O’Reilly, E. Poly(hydroxamic acid) ligand from palm-based waste materials for removal of heavy metals from electroplating wastewater. J. Appl. Polym. Sci. 2020, 49671. [Google Scholar] [CrossRef]
- Dmitrii, S.B.; Nadezhda, A.B.; Vadim, Y.K. Coordination chemistry and metal-involving reactions of amidoximes: Relevance to the chemistry of oximes and oxime ligands. Coord. Chem. Rev. 2016, 313, 62–93. [Google Scholar]
- Rachel, C. Traversing the coordination chemistry and chemical biology of hydroxamic acids. Coord. Chem. Rev. 2008, 252, 1387–1408. [Google Scholar]
- Rahman, M.L.; Mandal, B.H.; Sarkar, S.M.; Yusoff, M.M.; Arshad, S.; Musta, B. Synthesis of poly(hydroxamic acid) ligand from polymer grafted corn-cob cellulose for transition metals extraction. Polym. Adv. Technol. 2016, 27, 1625–1636. [Google Scholar] [CrossRef]
- Rahman, M.L.; Rohani, N.; Mustapa, N.; Yusoff, M.M. Synthesis of polyamidoxime chelating ligand from polymer-grafted corn-cob cellulose for metal extraction. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarkar, S.M.; Yusoff, M.M.; Abdullah, M.H. Efficient removal of transition metal ions using poly(amidoxime) ligand from polymer grafted kenaf cellulose. RSC Adv. 2016, 6, 745–757. [Google Scholar] [CrossRef]
- Sultana, T.; Mandal, B.H.; Rahman, M.L.; Sarkar, S.M. Bio-Waste Corn-cob Cellulose Supported Poly(amidoxime) Palladium Nanoparticles for Suzuki-Miyaura Cross-Coupling Reactions. ChemistrySelect 2016, 1, 4108–4112. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, M.L.; Yusoff, M.M.; Sarkar, S.M. Highly active bio-waste cellulose supported poly(amidoxime) palladium(II) complex for Heck reactions. J. Clean. Prod. 2017, 149, 1045–1050. [Google Scholar] [CrossRef]
- Mandal, B.H.; Rahman, M.L.; Mohd Hasbi, A.R.; Sarkar, S.M. Highly active kenaf bio-cellulose-based Poly(hydroxamic acid) copper catalyst for Aza-Michael addition and click reactions. ChemistrySelect 2016, 1, 2750–2756. [Google Scholar] [CrossRef]
- Rahman, M.L.; Biswas, T.K.; Sarkar, S.M.; Yusoff, M.M.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Adsorption of rare earth metals from water using a kenaf cellulose-based poly(hydroxamic acid) ligand. J. Mol. Liq. 2017, 243, 616–623. [Google Scholar] [CrossRef]
- Larkin, P.J. IR and Raman Spectra-Structure Correlations: Characteristics Group Frequencies. In IR and Raman Spectroscopy Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–115. [Google Scholar]
- Rahman, M.L.; Mandal, B.H.; Sarkar, S.M.; Wahab, N.A.A.; Yusoff, M.M.; Arshad, S.E.; Musta, B. Synthesis of poly(hydroxamic acid) ligand from polymer grafted khaya cellulose for transition metals extraction. Fibers Polym. 2016, 17, 521–532. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarkar, S.M.; Farid, E.M.; Arshad, S.E.; Sarjadi, M.S.; Wid, N. Synthesis of tapioca cellulose-based poly(amidoxime) ligand for removal of heavy metal ions. J. Macromol. Sci. Part B 2018, 57, 83–99. [Google Scholar] [CrossRef]
- Ma, N.; Liu, D.; Liu, Y.; Sui, G. Extraction and Characterization of Nanocellulose from Xanthoceras Sorbifolia Husks. Int. J. Nanosci. Nanoeng. 2015, 2, 43–50. [Google Scholar]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on Native Cellulose and Microcrystalline Cellulose I Structure Studied by X-Ray Diffraction (WAXD): Comparison between Measurement Techniques. Lenzinger Berichte 2011, 89, 118–131. [Google Scholar]
- Singh, V.; Kumari, P.; Pandey, S.; Narayan, T. Removal of chromium (VI) using poly(methylacrylate) functionalized guar gum. Bioresour. Technol. 2009, 100, 1977–1982. [Google Scholar] [CrossRef]
- Islam, M.S.; Mandal, H.B.; Biswas, T.K.; Rahman, M.L.; Rashid, S.S.; Tan, S.H.; Sarkar, S.M. Poly(hydroxamic acid) Functionalized Copper Catalyzed C-N Bond Formation Reactions. RSC Adv. 2016, 6, 56450–56457. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Ye, G.; Xu, J.; Jiang, M. Polyamidoxime/Poly(vinyl alcohol) Composite Chelating Fiber Prepared by Emulsion Spinning and Its Adsorption Properties for Metal Ions. Ind. Eng. Chem. Res. 2015, 54, 12367–12373. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Lekshmi, G.S.; Shainy, F. Synthesis and characterization of amidoxime modified chitosan/bentonite composite for the adsorptive removal and recovery of uranium from seawater. J. Colloid Interface Sci. 2018, 534, 248–261. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Xu, J. Microwave-assisted conversion of lignin. In Production of Biofuels and Chemicals with Microwave; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–82. [Google Scholar]
- El-Bahy, S.M.; El-Bahy, Z.M. Synthesis and characterization of polyamidoxime chelating resin for adsorption of Cu (II), Mn (II) and Ni (II) by batch and column study. J. Environ. Chem. Eng. 2016, 4, 276–286. [Google Scholar] [CrossRef]
- Ribeiro, R.F.; Pardini, L.C.; Alves, N.P.; Brito, C.A.R., Jr. Thermal Stabilization study of polyacrylonitrile fiber obtained by extrusion. Polimeros 2015, 25, 523–530. [Google Scholar] [CrossRef]
- Kiani, G.R.; Sheikhloie, H.; Arsalani, N. Heavy metal ion removal from aqueous solutions by functionalized polyacrylonitrile. Desalination 2011, 269, 266–270. [Google Scholar] [CrossRef]
- Siew, S.Y.W.; Rahman, M.L.; Arshad, S.E.; Surugau, N.L.; Musta, B. Synthesis and characterization of poly(hydroxamic acid)-poly(amidoxime) chelating ligands from polymer-grafted acacia cellulose. J. Appl. Polym. Sci. 2012, 124, 4443–4451. [Google Scholar]
- Naciye, T. Stability of metal chelates of some hydroxamic acid ligands. J. Chem. Eng. Data 2011, 56, 2337–2342. [Google Scholar]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, F.; Wei, T.; Zhang, C.; Xiao, H. Hydrophobic modification of bagasse cellulose fibers with cationic latex: Adsorption kinetics and mechanism. Chem. Eng. J. 2016, 302, 33–43. [Google Scholar] [CrossRef]
- Appel, J. Freundlich’s Adsorption Isotherm. Surf. Sci. 1973, 39, 237–244. [Google Scholar] [CrossRef]
- Fabián, F.L.; Fernando, L.V.; Prócoro, G.M.; Silvia, L.S.; Elsa, N.A.G.; Arturo, I.M.; Gildardo, H.M.; Raúl, H.M.; Manuel, A.Á.G.; Ixchel, R.P.V.; et al. Heavy metal pollution in drinking water-a global risk for human health: A review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar]
- Lin, L.; Jin, P.X.; Yu, J.L.; Qin, Z.; Ju, M.Y. Three-dimensional macroporous cellulose-based bioadsorbents for efficient removal of nickel ions from aqueous solution. Cellulose 2016, 23, 723–736. [Google Scholar]
- Barsbay, M.; Güven, O. Surface modification of cellulose via conventional and controlled radiation-induced grafting. Radiat. Phys. Chem. 2019, 160, 1–8. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Wang, A. Preparation of carboxymethyl cellulose-g-poly (acrylamide)/attapulgite porous monolith with an eco-friendly pickering-mipe template for Ce(III) and Gd(III) adsorption. Front. Chem. 2020, 8, 398. [Google Scholar] [CrossRef]
- Somia, H.; Ayman, K.; Tony, C.; Jonathan, C.; Prince, N.A.; José, M.G.F.; De Karine, O.V.; Isabelle, C.; François, J.; Haouache, S.; et al. Selective radical depolymerization of cellulose to glucose induced by high frequency ultrasound. Chem. Sci. 2020, 11, 664–2669. [Google Scholar]
- Gupta, K.C.; Sahoo, S. Co (III) acetylacetonate complex initiated grafting of N-vinyl pyrrolidone on cellulose in aqueous media. J. Appl. Polym. Sci. 2001, 81, 2286–2296. [Google Scholar] [CrossRef]
- Ouajai, S.; Hodzic, A.; Shanks, R.A. Morphological and grafting modification of natural cellulose fibers. J. Appl. Polym. Sci. 2004, 94, 2456–2465. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. A modified cellulose adsorbent for the removal of nickel (II) from aqueous solutions. J. Chem. Technol. Biotechnol. 2006, 81, 1820–1828. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. A chelating cellulose adsorbent for the removal of Cu (II) from aqueous solutions. J. Appl. Polym. Sci. 2006, 99, 2888–2897. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nima, J.; Divya, P.L. Adsorption of chromium (VI) from aqueous solutions by glycidylmethacrylate-grafted-densified cellulose with quaternary ammonium groups. Appl. Surf. Sci. 2013, 279, 441–449. [Google Scholar] [CrossRef]
- Zheng, L.; Dang, Z.; Yi, X.; Zhang, H. Equilibrium and kinetic studies of adsorption of Cd (II) from aqueous solution using modified corn stalk. J. Hazard. Mater. 2010, 176, 650–656. [Google Scholar] [CrossRef]
- Hajeeth, T.; Vijayalakshmi, K.; Gomathi, T.; Sudha, P.N. Removal of Cu (II) and Ni (II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int. J. Biol. Macromol. 2013, 62, 59–65. [Google Scholar] [CrossRef]





| Adsorbent | Adsorbate | Pseudo First Order | Experimental qe (mg/g) | Difference (mg/g) | ||
|---|---|---|---|---|---|---|
| qt (mg/g) | Kads (g/mg min) | R2 | ||||
| PHA | Cu | 340 | 0.0227 | 0.987 | 352 | 12 |
| Co | 332 | 0.0259 | 0.984 | 318 | −14 | |
| Cr | 213 | 0.0198 | 0.982 | 230 | 17 | |
| Ni | 216 | 0.0318 | 0.977 | 188 | −28 | |
| PA | Cu | 300 | 0.0190 | 0.935 | 310 | 10 |
| Co | 265 | 0.0202 | 0.948 | 295 | 30 | |
| Cr | 190 | 0.0145 | 0.982 | 227 | 37 | |
| Ni | 135 | 0.0230 | 0.895 | 175 | 40 | |
| Adsorbent | Adsorbate | Pseudo Second-Order | Experimental qe (mg/g) | Difference (mg/g) | ||
|---|---|---|---|---|---|---|
| qt (mg/g) | k2 (g/mg min) × 10−4 | R2 | ||||
| PHA | Cu | 375 | 0.0647 | 0.983 | 352 | −23 |
| Co | 353 | 0.0719 | 0.971 | 318 | −35 | |
| Cr | 261 | 0.0995 | 0.974 | 230 | −30 | |
| Ni | 206 | 0.0777 | 0.989 | 188 | −18 | |
| PA | Cu | 353 | 0.1129 | 0.970 | 310 | −43 |
| Co | 309 | 0.1280 | 0.971 | 295 | −14 | |
| Cr | 238 | 0.1337 | 0.987 | 227 | −11 | |
| Ni | 158 | 0.0820 | 0.997 | 175 | 17 | |
| Adsorbent | Adsorbate | Langmuir | Experimental qe (mg/g) | Difference (mg/g) | ||
|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/g) | R2 | ||||
| PHA | Cu | 299 | 0.00033 | 0.986 | 352 | 53 |
| Co | 257 | 0.00039 | 0.989 | 318 | 60 | |
| Cr | 195 | 0.00040 | 0.984 | 230 | 35 | |
| Ni | 144 | 0.00048 | 0.980 | 188 | 44 | |
| PA | Cu | 264 | 0.00035 | 0.988 | 310 | 45 |
| Co | 221 | 0.00046 | 0.981 | 295 | 74 | |
| Cr | 169 | 0.00039 | 0.984 | 227 | 58 | |
| Ni | 148 | 0.00042 | 0.985 | 175 | 27 | |
| Adsorbent | Adsorbate | Freundlich | ||
|---|---|---|---|---|
| 1/n | KL(L/mg) | R2 | ||
| PHA | Cu | 1.32 | 0.378 | 0.999 |
| Co | 1.18 | 0.403 | 0.998 | |
| Cr | 1.18 | 0.367 | 0.999 | |
| Ni | 1.32 | 0.271 | 0.998 | |
| PA | Cu | 1.33 | 0.357 | 0.999 |
| Co | 1.08 | 0.416 | 0.997 | |
| Cr | 1.28 | 0.314 | 0.998 | |
| Ni | 1.25 | 0.304 | 0.997 | |
| Metal Ions | Sample 1 | Sample 2 | ||||
|---|---|---|---|---|---|---|
| Before Treatment (ppm) | After Treatment (ppm) | % Removal | Before Treatment (ppm) | After Treatment (ppm) | % Removal | |
| Fe3+ | 32.0095 | 1.0012 | 96.87 | 1.4637 | 0.0198 | 98.64 |
| Cu2+ | 23.0493 | 0.4256 | 98.15 | 85.7627 | 1.5681 | 98.17 |
| Pb2+ | 0.0207 | 0.0020 | 90.33 | 0.1331 | 0.0156 | 88.27 |
| Zn2+ | 0.0421 | 0.0080 | 80.99 | 0.1037 | 0.0084 | 91.89 |
| Cr3+ | 0.0268 | 0.0011 | 95.89 | 0.0857 | 0.0031 | 96.38 |
| Mn2+ | 0.1296 | 0.0096 | 92.59 | 0.0055 | 0.0013 | 76.36 |
| Ni2+ | 0.0182 | 0.0012 | 93.40 | 0.4421 | 0.0364 | 91.76 |
| Ba2+ | 0.0053 | 0.0017 | 67.92 | - | - | - |
| Ca2+ | 0.5517 | 0.3948 | 28.43 | 0.8918 | 0.6987 | 21.65 |
| Rb+ | 0.0305 | 0.0182 | 40.32 | 0.0109 | 0.0066 | 39.44 |
| K+ | 0.0783 | 0.0545 | 30.39 | 0.2791 | 0.1878 | 32.71 |
| Na+ | 15.2312 | 12.3812 | 18.71 | 9.7411 | 8.2497 | 15.31 |
| V4+ | 0.0086 | 0.0012 | 86.04 | - | - | - |
| Al3+ | 0.0505 | 0.0274 | 45.74 | 0.0063 | 0.0024 | 61.90 |
| Ag+ | - | - | - | 0.0322 | 0.0121 | 62.42 |
| Mg2+ | - | - | - | 0.0879 | 0.0612 | 30.37 |
| Metal Ions | Sample 1 | Sample 2 | ||||
|---|---|---|---|---|---|---|
| Before Treatment (ppm) | After Treatment (ppm) | % Removal | Before Treatment (ppm) | After Treatment (ppm) | % Removal | |
| Fe3+ | 32.0095 | 1.2452 | 96.10 | 1.4637 | 0.0162 | 98.89 |
| Cu2+ | 23.0493 | 0.4601 | 98.00 | 85.7627 | 1.4215 | 98.34 |
| Pb2+ | 0.0207 | 0.0021 | 89.85 | 0.1331 | 0.0162 | 87.82 |
| Zn2+ | 0.0421 | 0.0084 | 80.04 | 0.1037 | 0.0079 | 92.38 |
| Cr3+ | 0.0268 | 0.0018 | 93.28 | 0.0857 | 0.0039 | 95.44 |
| Mn2+ | 0.1296 | 0.0082 | 93.67 | 0.0055 | 0.0021 | 61.81 |
| Ni2+ | 0.0182 | 0.0024 | 86.81 | 0.4421 | 0.0372 | 91.58 |
| Ba2+ | 0.0053 | 0.0022 | 58.49 | - | - | - |
| Ca2+ | 0.5517 | 0.3554 | 35.58 | 0.8918 | 0.7145 | 19.88 |
| Rb+ | 0.0305 | 0.0161 | 47.21 | 0.0109 | 0.0056 | 48.62 |
| K+ | 0.0783 | 0.0553 | 29.37 | 0.2791 | 0.2015 | 27.80 |
| Na+ | 15.2312 | 11.2587 | 26.08 | 9.7411 | 8.5891 | 11.82 |
| V4+ | 0.0086 | 0.0024 | 72.09 | - | - | - |
| Al3+ | 0.0505 | 0.0261 | 48.31 | 0.0063 | 0.0027 | 57.14 |
| Ag+ | - | - | - | 0.0322 | 0.0129 | 59.93 |
| Mg2+ | - | - | - | 0.0879 | 0.0705 | 19.79 |
| Supported Materials | Modifying Agents/Ligands Functional Groups | Adsorbate | Adsorption qe (mg g−1) | References |
|---|---|---|---|---|
| Cellulose | Glycidyl methacrylate (Imidazole) | Cu2+ Ni2+ Pb2+ | 70 49 85 | [53,54,55] |
| Cellulose | Glycidylmethacrylate (GMA) onto titanium dioxide cellulose (TDC) followed by amination and ethylation reactions (Amino) | Cr3+ | 123 | [56] |
| Cellulose | Acrylonitrile N,N-methylenebisacrylamide (Amino) | Cd2+ | 21 | [57] |
| Cellulose | Acrylic acid (Carboxyl) | Cu2+ Ni2+ | 328 276 | [58] |
| Cellulose | Amidoxime from grafted kenaf cellulose | Cu2+ Fe3+ Mn2+ Cr3+ Ni2+ | 326 273 241 228 204 | [21] |
| Cellulose | Hydroxamic acid from grafted kenaf cellulose | Cu2+ Co2+ Cr3+ Fe3+ Ni2+ | 305 256 254 275 198 | [14] |
| Cellulose | Amidoxime from grafted khaya cellulose | Cu2+ Fe3+ Co2+ Cr3+ Ni2+ | 282 254 221 229 202 | [11] |
| Cellulose | Amidoxime from grafted palm cellulose | Cu2+ Fe3+ Co2+ Ni2+ Pb2+ | 260 210 168 172 272 | [15] |
| Cellulose | Hydroxamic acid from grafted jute cellulose | Cu2+ Co2+ Cr3+ Ni2+ | 352 318 230 188 | This study |
| Cellulose | Amidoxime from grafted jute cellulose | Cu2+ Co2+ Cr3+ Ni2+ | 310 295 227 175 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.L.; Fui, C.J.; Ting, T.X.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater. Polymers 2020, 12, 2521. https://doi.org/10.3390/polym12112521
Rahman ML, Fui CJ, Ting TX, Sarjadi MS, Arshad SE, Musta B. Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater. Polymers. 2020; 12(11):2521. https://doi.org/10.3390/polym12112521
Chicago/Turabian StyleRahman, Md Lutfor, Choong Jian Fui, Tang Xin Ting, Mohd Sani Sarjadi, Sazmal E. Arshad, and Baba Musta. 2020. "Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater" Polymers 12, no. 11: 2521. https://doi.org/10.3390/polym12112521
APA StyleRahman, M. L., Fui, C. J., Ting, T. X., Sarjadi, M. S., Arshad, S. E., & Musta, B. (2020). Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater. Polymers, 12(11), 2521. https://doi.org/10.3390/polym12112521





