Effect of a Novel Flame Retardant on the Mechanical, Thermal and Combustion Properties of Poly(Lactic Acid)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
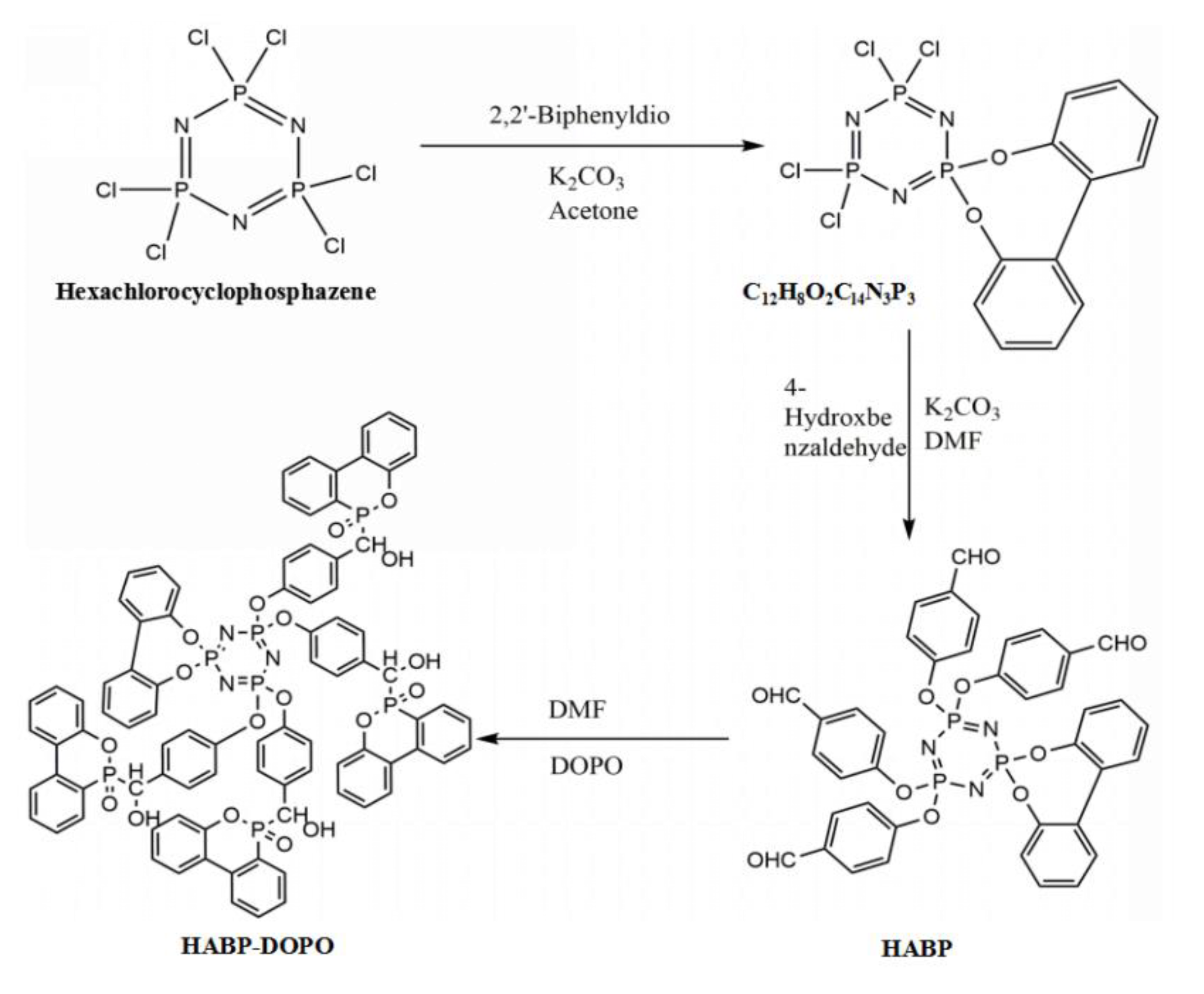
2.2. Synthesis of the Flame Retardant HABP-DOPO
2.3. Preparation of Flame Retardant PLA Blends
2.4. Measurements
3. Results and Discussion
3.1. Limiting Oxygen Index and Vertical Burning Rating Test
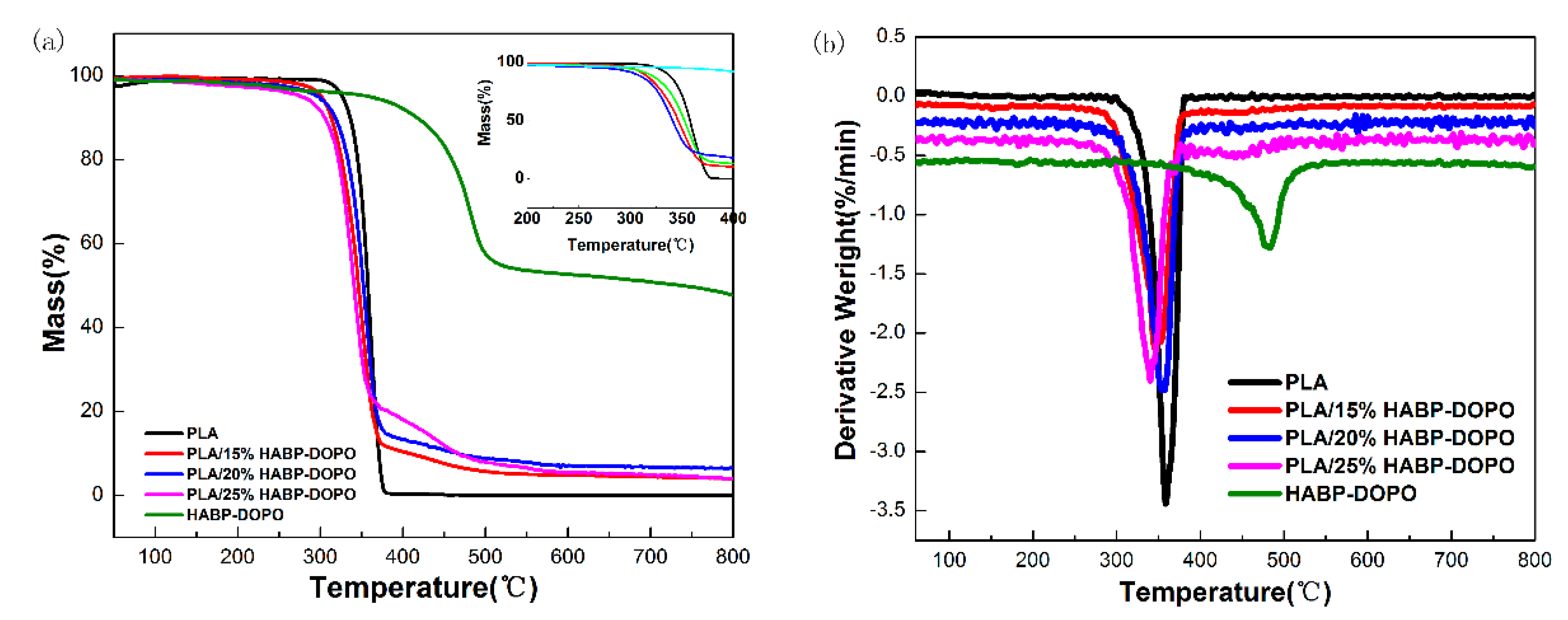
3.2. Thermogravimetric Analysis
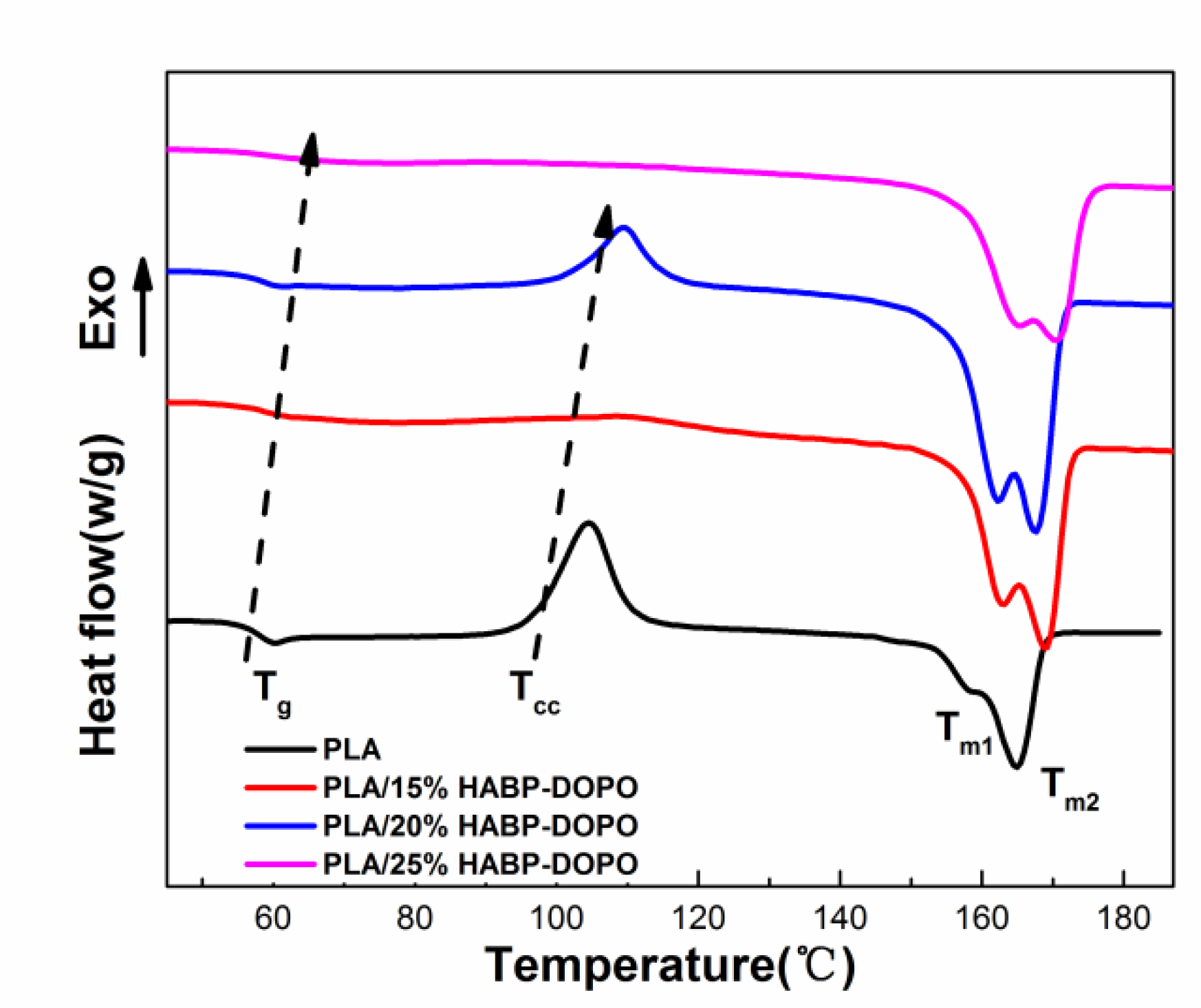
3.3. Differential Scanning Calorimetry Test
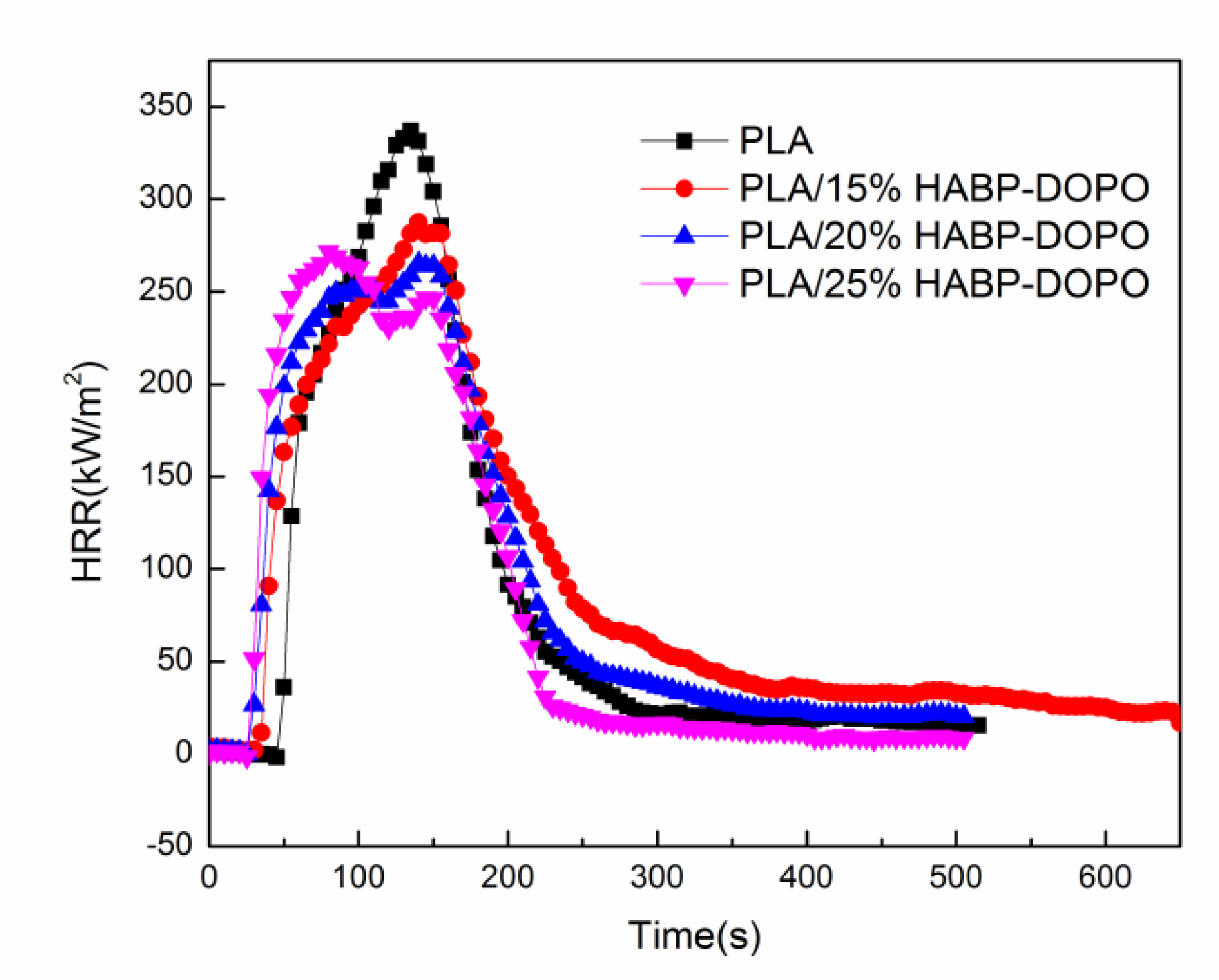
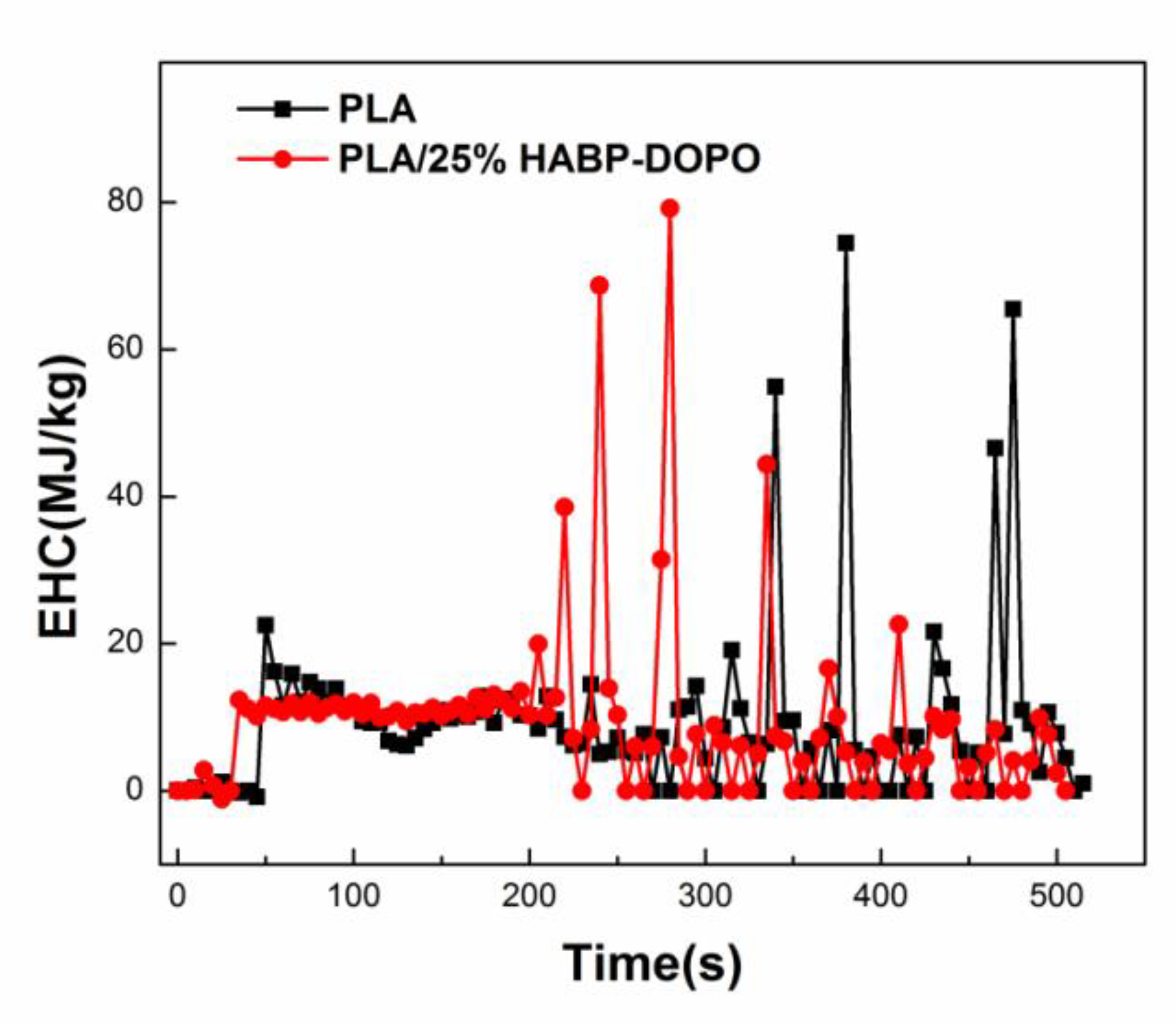
3.4. Cone Calorimetry Test
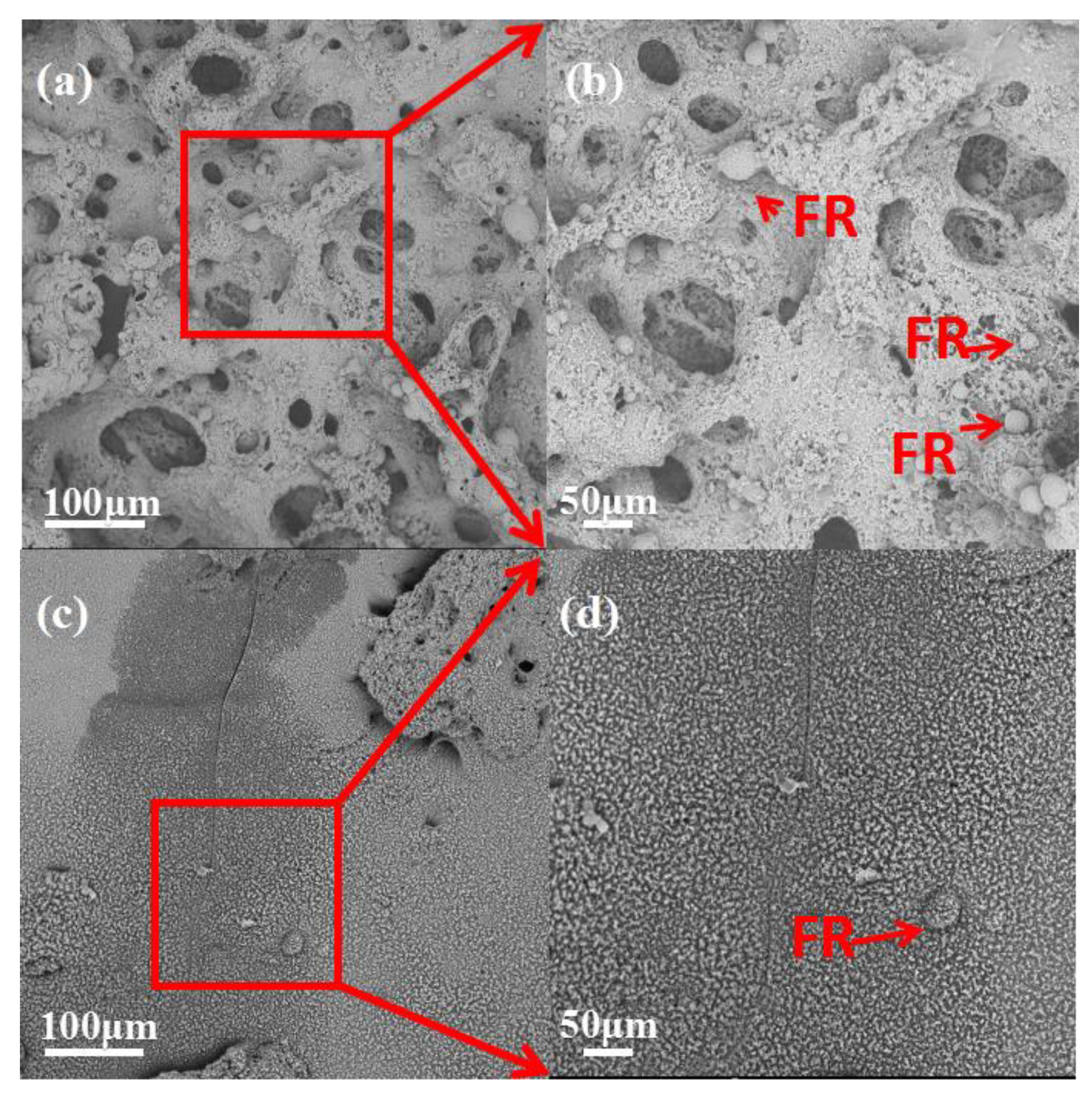
3.5. Morphologies of the Residue Char
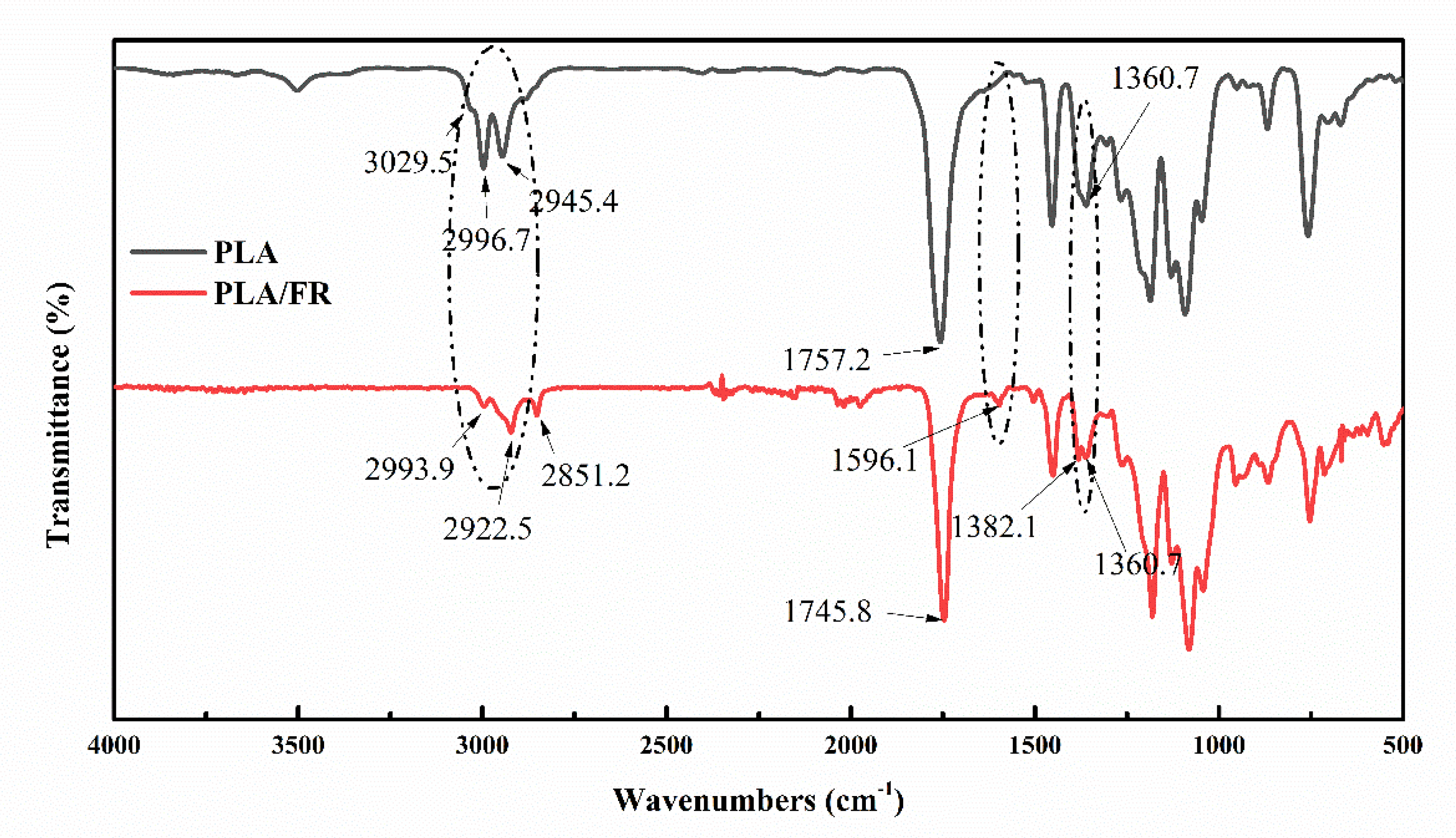
3.6. FTIR of PLA/HABP-DOPO Blends
3.7. Dispersion of Flame Retardant in PLA Matrix
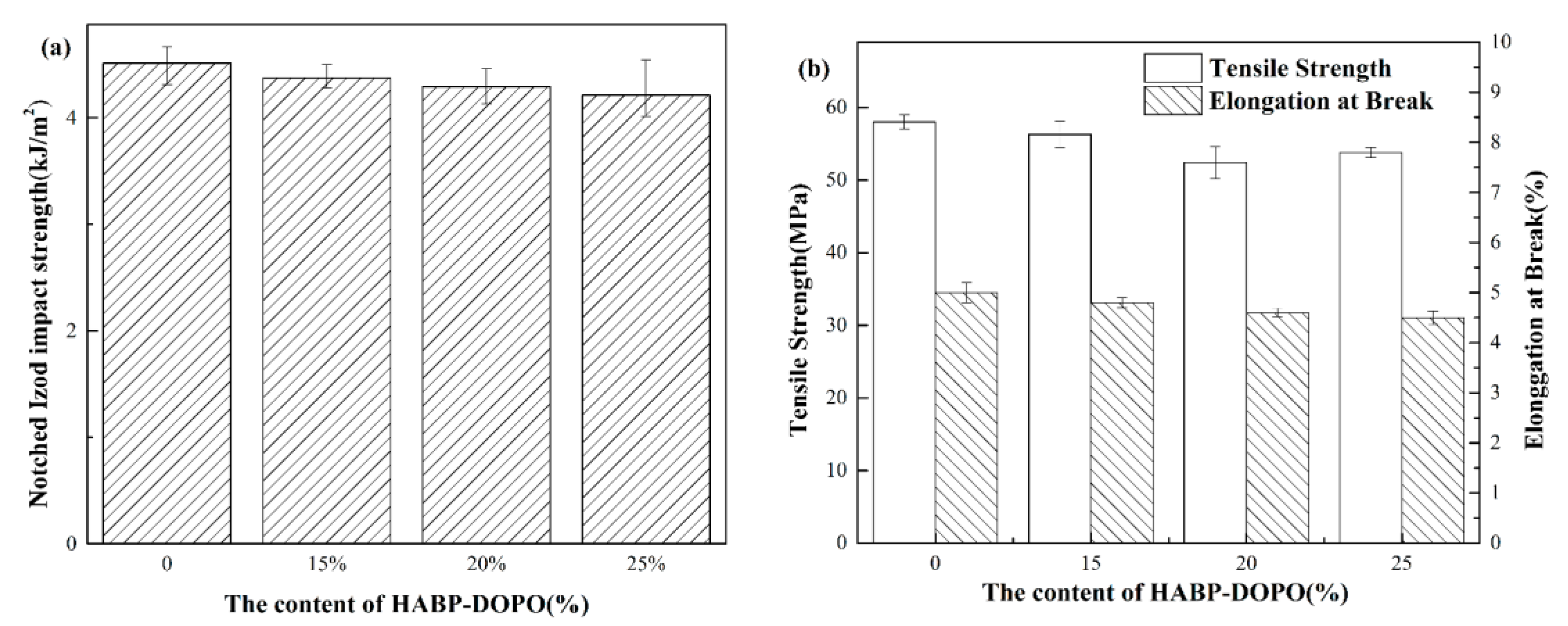
3.8. Mechanical Properties Test
3.9. Mechanism of Flame Retardant in PLA Matrix
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.; Kenny, J. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Emami, F.; Yazdi, S.J.M.; Na, D.H. Poly(lactic acid)/poly(lactic-co-glycolic acid) particulate carriers for pulmonary drug delivery. J. Pharm. Investig. 2019, 49, 427–442. [Google Scholar] [CrossRef]
- Wang, L.-X.; Wang, N.; Zhou, Y.; Zhang, Y.; Li, Q.; Shen, C. Fabrication of open-porous PCL/PLA tissue engineering scaffolds and the relationship of foaming process, morphology, and mechanical behavior. Polym. Adv. Technol. 2019, 30, 2539–2548. [Google Scholar] [CrossRef]
- Zuo, L.; Fan, W.; Zhang, Y.; Zhang, L.; Gao, W.; Huang, Y.; Liu, T. Graphene/montmorillonite hybrid synergistically reinforced polyimide composite aerogels with enhanced flame-retardant performance. Compos. Sci. Technol. 2017, 139, 57–63. [Google Scholar] [CrossRef]
- Botiz, I.; Darling, S.B. Self-Assembly of Poly(3-hexylthiophene)-block-polylactide Block Copolymer and Subsequent Incorporation of Electron Acceptor Material. Macromolecules 2009, 42, 8211–8217. [Google Scholar] [CrossRef]
- Bocz, K.; Domonkos, M.; Igricz, T.; Kmetty, Á.; Bárány, T.; Marosi, G. Flame retarded self-reinforced poly(lactic acid) composites of outstanding impact resistance. Compos. Part. A: Appl. Sci. Manuf. 2015, 70, 27–34. [Google Scholar] [CrossRef]
- Yang, J.-P.; Liao, Q.; Zhou, J.; Jiang, X.; Wang, X.-H.; Zhang, Y.; Jiang, S.-D.; Yan, S.-K.; Li, L. What Determines the Lamellar Orientation on Substrates? Macromolecules 2011, 44, 3511–3516. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Lin, Y.-H.; Guo, W.; Chuang, T.-H.; Chang, S.-C.; Wanga, S.-F.; Don, T.-M. Flammability and tensile properties of polylactide nanocomposites with short carbon fibers. J. Mater. Sci. 2014, 50, 1605–1612. [Google Scholar] [CrossRef]
- Sun, F.; Yu, T.; Hu, C.; Li, Y. Influence of functionalized graphene by grafted phosphorus containing flame retardant on the flammability of carbon fiber/epoxy resin (CF/ER) composite. Compos. Sci. Technol. 2016, 136, 76–84. [Google Scholar] [CrossRef]
- Ji, J.; Wang, L.; Yu, H.; Chen, Y.; Zhao, Y.; Zhang, H.; Amer, W.A.; Sun, Y.; Huang, L.; Saleem, M. Chemical Modifications of Chitosan and Its Applications. Polym. Technol. Eng. 2014, 53, 1494–1505. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Zammarano, M.; Gilman, J.W.; Shields, J.R.; Boday, D.J. Synthesis and characterization of isosorbide-based polyphosphonates as biobased flame-retardants. Polym. Chem. 2014, 5, 5139–5146. [Google Scholar] [CrossRef]
- González, A.; Dasari, A.; Herrero, B.; Plancher, E.; Santarén, J.; Esteban, A.; Lim, S.-H. Fire retardancy behavior of PLA based nanocomposites. Polym. Degrad. Stab. 2012, 97, 248–256. [Google Scholar] [CrossRef]
- Kanat, M.; Eren, T. Synthesis of phosphorus-containing flame retardants and investigation of their flame retardant behavior in textile applications. J. Appl. Polym. Sci. 2019, 136, 47935–47948. [Google Scholar] [CrossRef]
- Adner, D.; Helmy, M.; Otto, T.; Schellenberg, J.; Schadewald, A. A macromolecular halogen-free flame retardant and its effect on the properties of thermoplastic polyesters. Fire Mater. 2018, 43, 169–174. [Google Scholar] [CrossRef]
- Wang, J.; Ai, K.; Lu, L. Flame-retardant porous hexagonal boron nitride for safe and effective radioactive iodine capture. J. Mater. Chem. A 2019, 7, 16850–16858. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Wu, Y. Synergistic effect of aluminum hypophosphite and intumescent flame retardants in polylactide. Polym. Adv. Technol. 2015, 26, 255–265. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, S.; Liu, G. A THEIC-based polyphosphate melamine intumescent flame retardant and its flame retardancy properties for polylactide. J. Anal. Appl. Pyrolysis 2016, 122, 24–34. [Google Scholar] [CrossRef]
- Shi, X.; Ju, Y.; Zhang, M.; Wang, X. The intumescent flame-retardant biocomposites of poly(lactic acid) containing surface-coated ammonium polyphosphate and distiller’s dried grains with solubles (DDGS). Fire Mater. 2017, 42, 190–197. [Google Scholar] [CrossRef]
- Yan, Y.; Gu, X.; Li, L.; Li, H.; Sun, J.; Zhang, S. Preparation and characterization of intumescent flame retardant biodegradable poly(lactic acid) nanocomposites based on sulfamic acid intercalated layered double hydroxides. Fibers Polym. 2017, 18, 2060–2069. [Google Scholar] [CrossRef]
- Lu, L.; Guo, N.; Qian, X.; Yang, S.; Wang, X.; Jin, J.; Shao, G. Thermal degradation and combustion behavior of intumescent flame-retardant polypropylene with novel phosphorus-based flame retardants. J. Appl. Polym. Sci. 2017, 135, 45962. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Sun, N.; Xu, M.; Xu, G.; Xin, F.; Chen, Y. Pyrolysis route of a novel flame retardant constructed by phosphaphenanthrene and triazine-trione groups and its flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Qiu, Y.; Chen, Y. Flame-retardant behavior of bi-group molecule derived from phosphaphenanthrene and triazine groups on polylactic acid. Polym. Adv. Technol. 2015, 27, 781–788. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Functionalized MWCNTs modified flame retardant PLA nanocomposites and cold rolling process for improving mechanical properties. Compos. Sci. Technol. 2018, 161, 39–49. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Liu, Z.; Yao, Y.; Qian, L. Synthesis of a novel flame retardant containing phosphazene and triazine groups and its enhanced charring effect in poly(lactic acid) resin. J. Appl. Polym. Sci. 2016, 134, 44660–44668. [Google Scholar] [CrossRef]
- Tang, G.; Deng, D.; Chen, J.; Zhou, K.; Zhang, H.; Huang, X.; Zhou, Z. The influence of organo-modified sepiolite on the flame-retardant and thermal properties of intumescent flame-retardant polylactide composites. J. Therm. Anal. Calorim. 2017, 130, 763–772. [Google Scholar] [CrossRef]
- Pang, J.; Xianzhong, M.O.; Liu, Y. Preparation and characterization of flame retardant banana fiber reinforced PLA composites. Chem. Ind. Eng. Prog. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Shi, X.; Peng, X.; Zhu, J.; Lin, G.; Kuang, T. Synthesis of DOPO-HQ-functionalized graphene oxide as a novel and efficient flame retardant and its application on polylactic acid: Thermal property, flame retardancy, and mechanical performance. J. Colloid Interface Sci. 2018, 524, 267–278. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.; Zhang, J.; Chen, S.; Zhou, Y. Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stab. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Carriedo, G.A.; Fernández-Catuxo, L.; Alonso, F.J.G.; Gómez-Elipe, P.; González, P.A. Preparation of a new type of phosphazene high polymers containing 2,2’-dioxybiphenyl groups. Macromolecules 1996, 29, 5320–5325. [Google Scholar] [CrossRef]
- Jin, X.; Gu, X.; Chen, C.; Tang, W.; Li, H.; Liu, X.; Bourbigot, S.; Zhang, Z.; Sun, J.; Zhang, S. The fire performance of polylactic acid containing a novel intumescent flame retardant and intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 12235–12250. [Google Scholar] [CrossRef]
- Jin, X.; Sun, J.; Zhang, J.S.; Gu, X.; Bourbigot, S.; Li, H.; Tang, W.; Zhang, S. Preparation of a novel intumescent flame retardant based on supramolecular interactions and its application in polyamide 11. ACS Appl. Mater. Interfaces 2017, 9, 24964–24975. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.M.; Edalat, A.; Berahman, R.; Hosseini, F.S. Highly-Toughened Polylactide- (PLA-) Based Ternary Blends with Significantly Enhanced Glass Transition and Melt Strength: Tailoring the Interfacial Interactions, Phase Morphology, and Performance. Macromolecules 2018, 51, 4298–4314. [Google Scholar] [CrossRef]
- Zhang, J.; De Juan, S.; Esteban-Cubillo, A.; Santarén, J.; Wang, D.-Y. Effect of Organo-Modified Nanosepiolite on Fire Behaviors and Mechanical Performance of Polypropylene Composites. Chin. J. Chem. 2015, 33, 285–291. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Wang, J.; Xi, W. High-performance flame retardancy by char-cage hindering and free radical quenching effects in epoxy thermosets. Polymer 2015, 68, 262–269. [Google Scholar] [CrossRef]
- You, G.; Cheng, Z.; Peng, H.; He, H.-W. Synthesis and performance of a novel nitrogen-containing cyclic phosphate for intumescent flame retardant and its application in epoxy resin. J. Appl. Polym. Sci. 2014, 132, 6257–6267. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, K.-Y.; Dong, Z.-M.; Dong, L.-S.; Li, Y.-S. Study of hydrogen-bonded blend of polylactide with biodegradable hyperbranched poly(ester amide). Macromolecules 2007, 40, 6257–6267. [Google Scholar] [CrossRef]
- Zhang, N.; Lu, X. Mechanical, thermal and combustion properties of intumescent flame retardant biodegradable poly (lactic acid) composites. Plast. Rubber Compos. 2018, 47, 458–467. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, W.; Jia, Y.; Lu, Y.; Zhang, G. A concise water-solvent synthesis of highly effective, durable, and eco-friendly flame-retardant coating on cotton fabrics. Carbohydr. Polym. 2018, 199, 256–265. [Google Scholar] [CrossRef]
- Si, M.M.; Ding, S.; Hao, J.W.; Xu, L.S.; Du, J.X. Synergistic effect of NaNOSb2O3 and aluminium phosphinate on flame retardancy of PET. Acta Polym. Sin. 2013, 13, 1483–1491. [Google Scholar] [CrossRef]





| LOI (%) | UL-94 | Dripping | Ignition | |
|---|---|---|---|---|
| PLA | 18.5 | NR | Y | Y |
| PLA/15 wt% HABP-DOPO | 22.1 | V-1 | Y | Y |
| PLA/20 wt% HABP-DOPO | 25.1 | V-1 | Y | Y |
| PLA/25 wt% HABP-DOPO | 28.5 | V-0 | N | N |
| T5% (°C) | Tmax (°C) | T25% (°C) | Residue (wt%) at 800 °C | |
|---|---|---|---|---|
| PLA | 326.7 | 357.2 | 348.2 | 0 |
| HABP-DOPO | 359.4 | 476.8 | 470.3 | 47.7 |
| PLA/15 wt% HABP-DOPO | 301.4 | 344.5 | 330.3 | 3.50 |
| PLA/20 wt% HABP-DOPO | 297.5 | 352.3 | 332.0 | 8.68 |
| PLA/25 wt% HABP-DOPO | 307.6 | 339.2 | 337.9 | 14.43 |
| Sample | Tg(°C) | Tcc(°C) | ΔHc(J/g) | Tm1(°C) | Tm2(°C) | ΔHm1(J/g) | Xc(%) |
|---|---|---|---|---|---|---|---|
| PLA | 59.9 | 107.7 | 35.46 | 150.1 | 168.2 | 49.91 | 53.3 |
| PLA/FR15 | 62.6 | 109.8 | 1.583 | 163.2 | 168.7 | 29.34 | 36.8 |
| PLA/FR20 | 60.9 | 109.5 | 8.907 | 162.7 | 167.2 | 35.49 | 47.3 |
| PLA/FR25 | 62.6 | - | 1.668 | 165.6 | 170.6 | 26.17 | 37.2 |
| Sample | TTI (s) | Pk-HRR (kW/m2) | Av-HRR (kW/m2) | THR (MJ/m2) | TSR (m2/m2) | Av-MLR g/(s·m²) | EHC (MJ/kg) |
|---|---|---|---|---|---|---|---|
| PLA | 53 | 336.86 | 263.42 | 72.15 | 22.2 | 20.58 | 74.47 |
| PLA/15 wt% HABP-DOPO | 39 | 322.9 | 241.35 | 63.7 | 860.6 | 12.64 | 70.14 |
| PLA/20 wt% HABP-DOPO | 36 | 303.2 | 231.7 | 53.8 | 909.9 | 18.78 | 73.36 |
| PLA/25 wt% HABP-DOPO | 32 | 271.38 | 212.54 | 42.9 | 970.2 | 25.87 | 79.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, M.; Zhang, Z.; Wei, Z.; Wang, W. Effect of a Novel Flame Retardant on the Mechanical, Thermal and Combustion Properties of Poly(Lactic Acid). Polymers 2020, 12, 2407. https://doi.org/10.3390/polym12102407
Niu M, Zhang Z, Wei Z, Wang W. Effect of a Novel Flame Retardant on the Mechanical, Thermal and Combustion Properties of Poly(Lactic Acid). Polymers. 2020; 12(10):2407. https://doi.org/10.3390/polym12102407
Chicago/Turabian StyleNiu, Mingjun, Zhongzhou Zhang, Zizhen Wei, and Wanjie Wang. 2020. "Effect of a Novel Flame Retardant on the Mechanical, Thermal and Combustion Properties of Poly(Lactic Acid)" Polymers 12, no. 10: 2407. https://doi.org/10.3390/polym12102407
APA StyleNiu, M., Zhang, Z., Wei, Z., & Wang, W. (2020). Effect of a Novel Flame Retardant on the Mechanical, Thermal and Combustion Properties of Poly(Lactic Acid). Polymers, 12(10), 2407. https://doi.org/10.3390/polym12102407





