Synergistic Effect of Cellulose Nanofiber and Nanoclay as Distributed Phase in a Polypropylene Based Nanocomposite System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
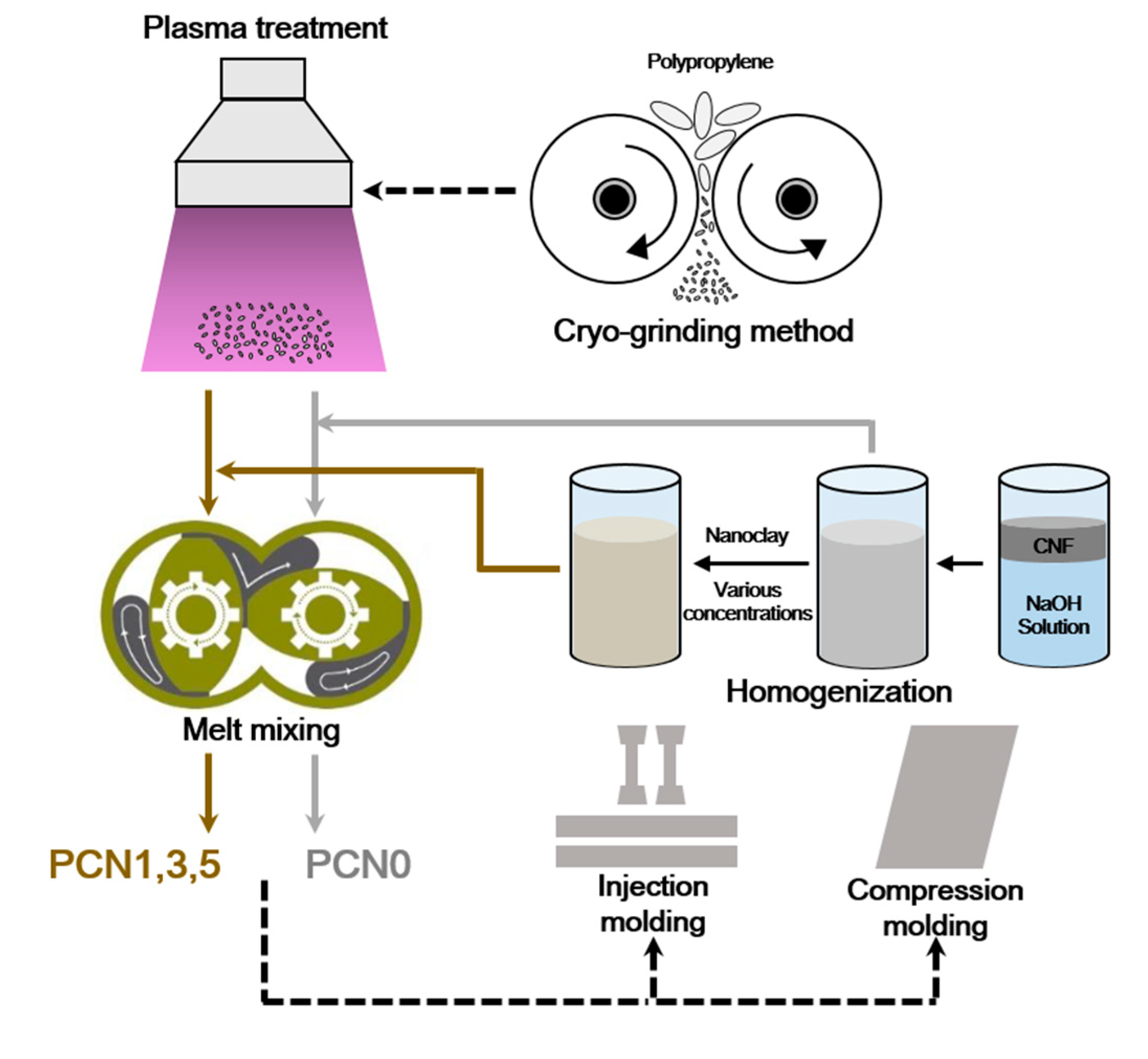
2.2. Preparation of PP/CNF and PP/CNF/Nanoclay Nanocomposites
2.3. Characterization
2.3.1. Sample Preparation
2.3.2. Plasma Treatment Efficiency Analysis
2.3.3. Morphological Analysis
2.3.4. X-ray Diffraction Analysis
2.3.5. Thermal Analysis
2.3.6. Mechanical Analysis
2.3.7. Barrier Analysis
2.3.8. Statistical Analysis
3. Results and Discussion
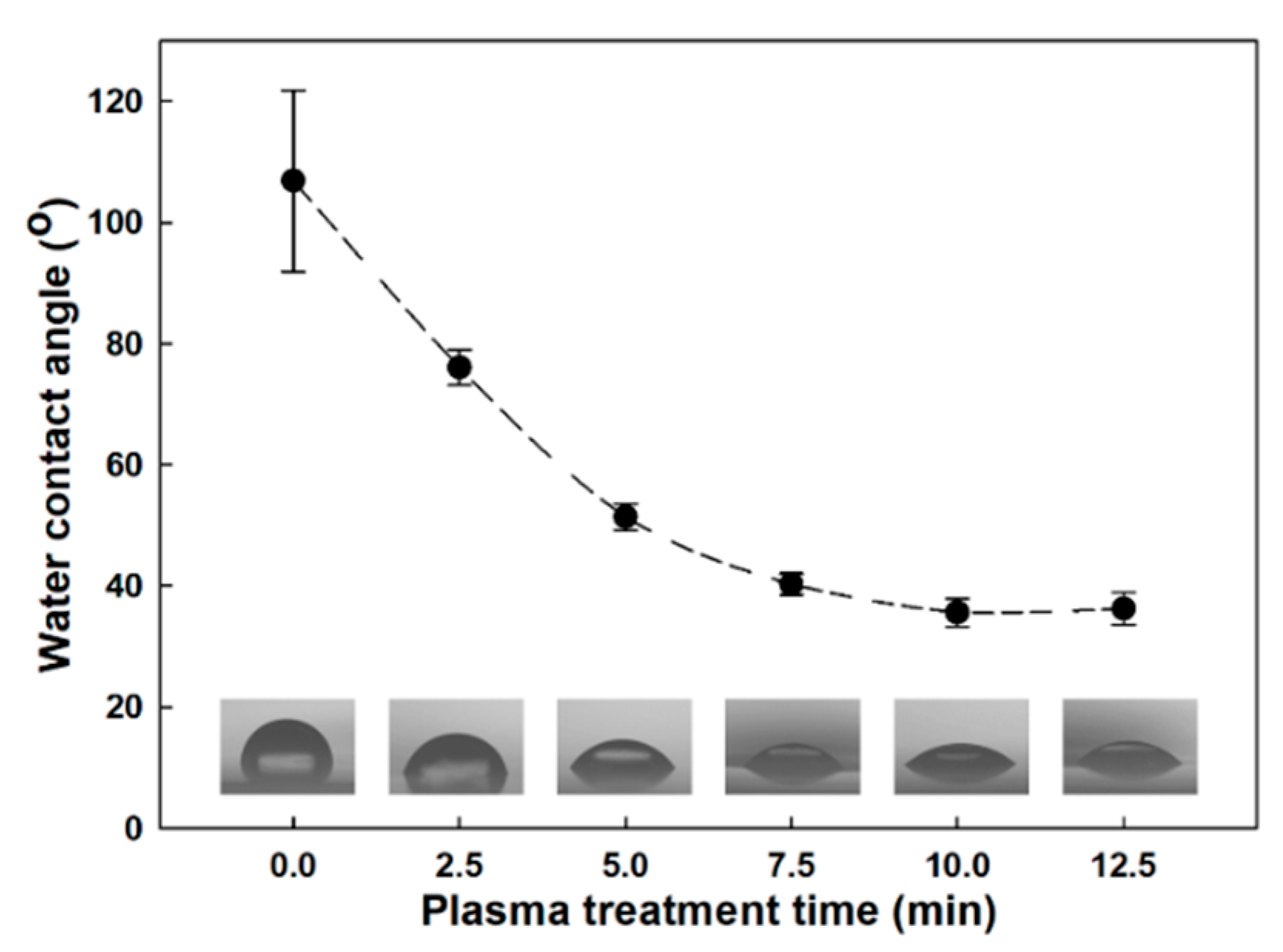
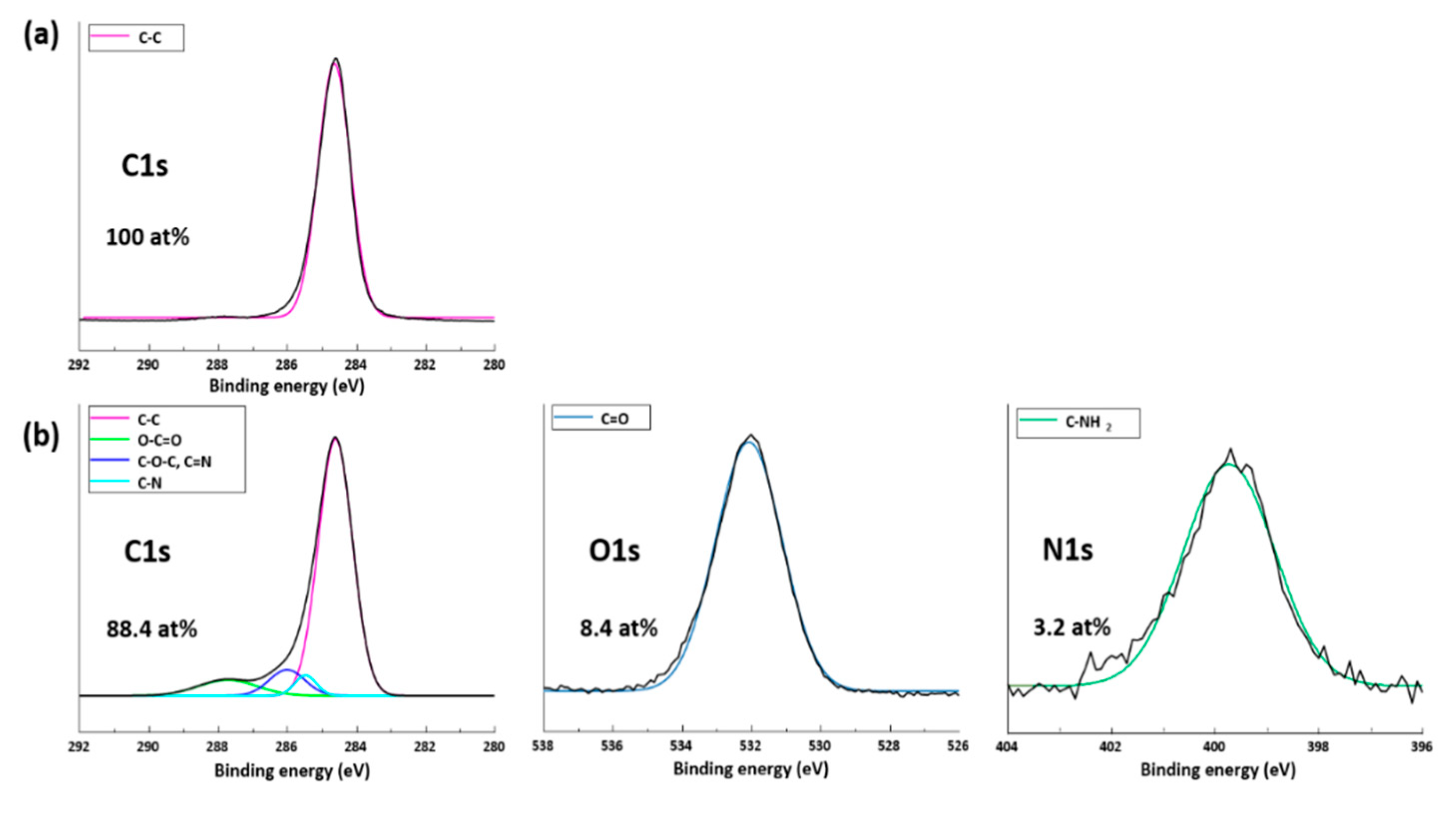
3.1. Plasma Treatment Efficiency Properties
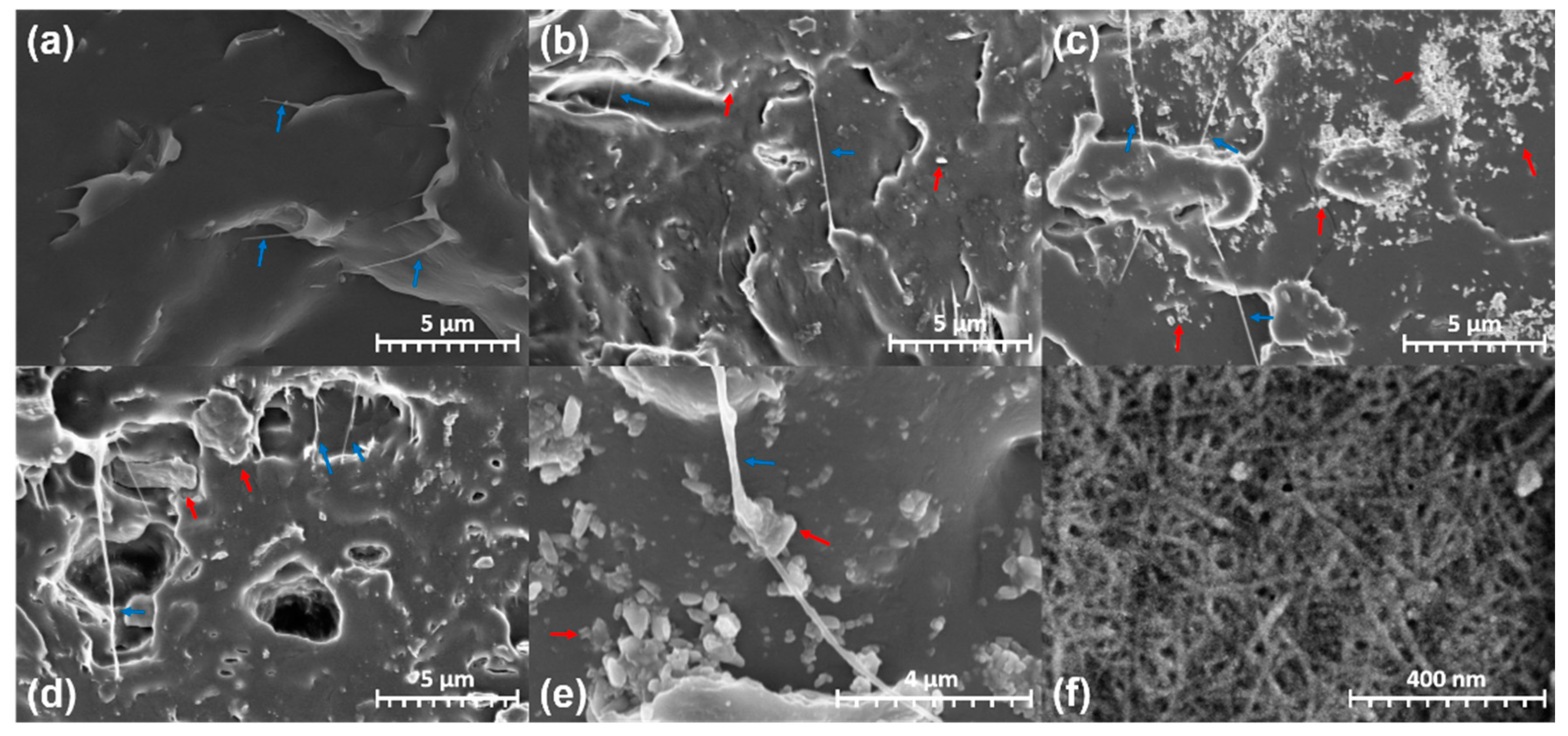
3.2. Morphological Properties
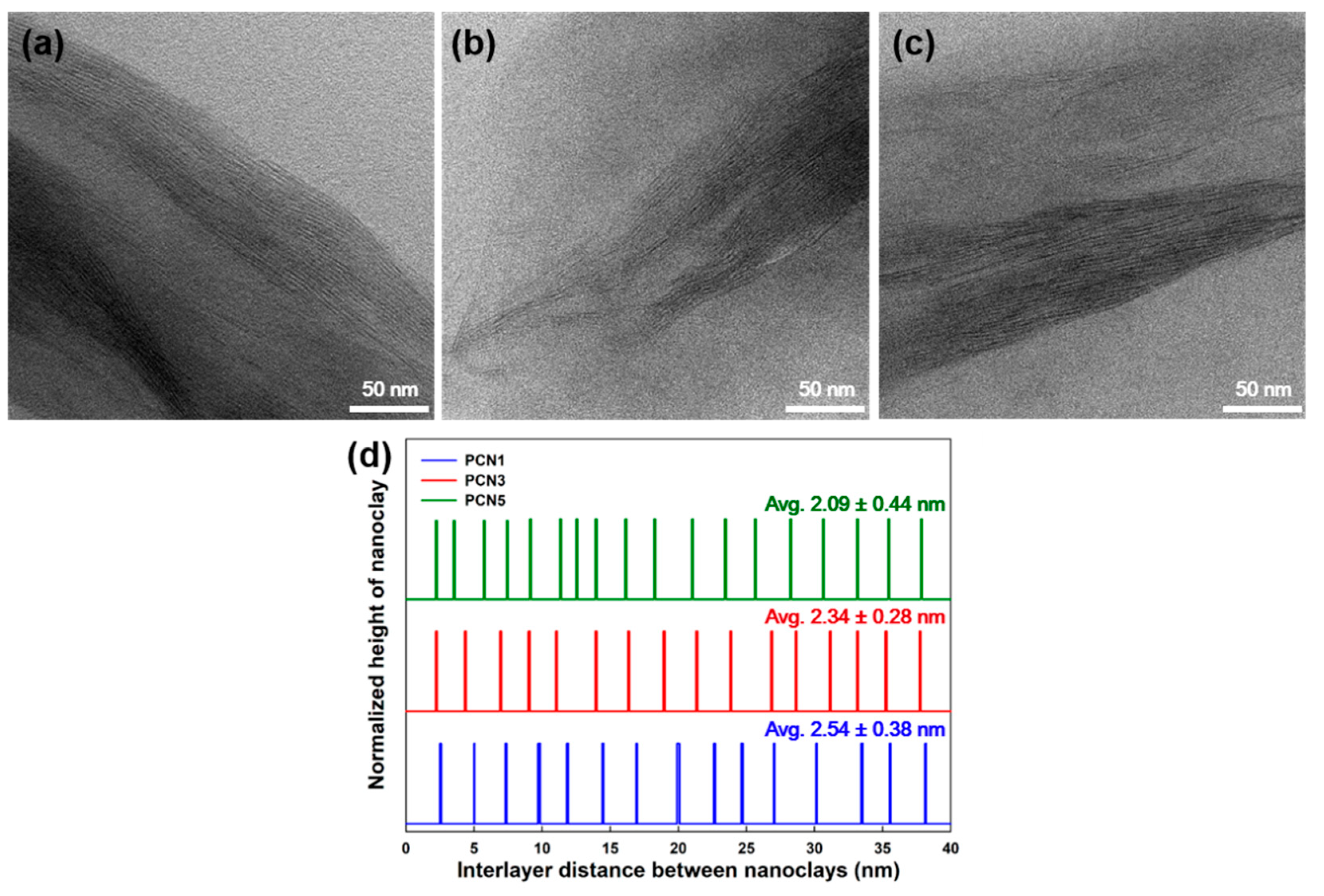
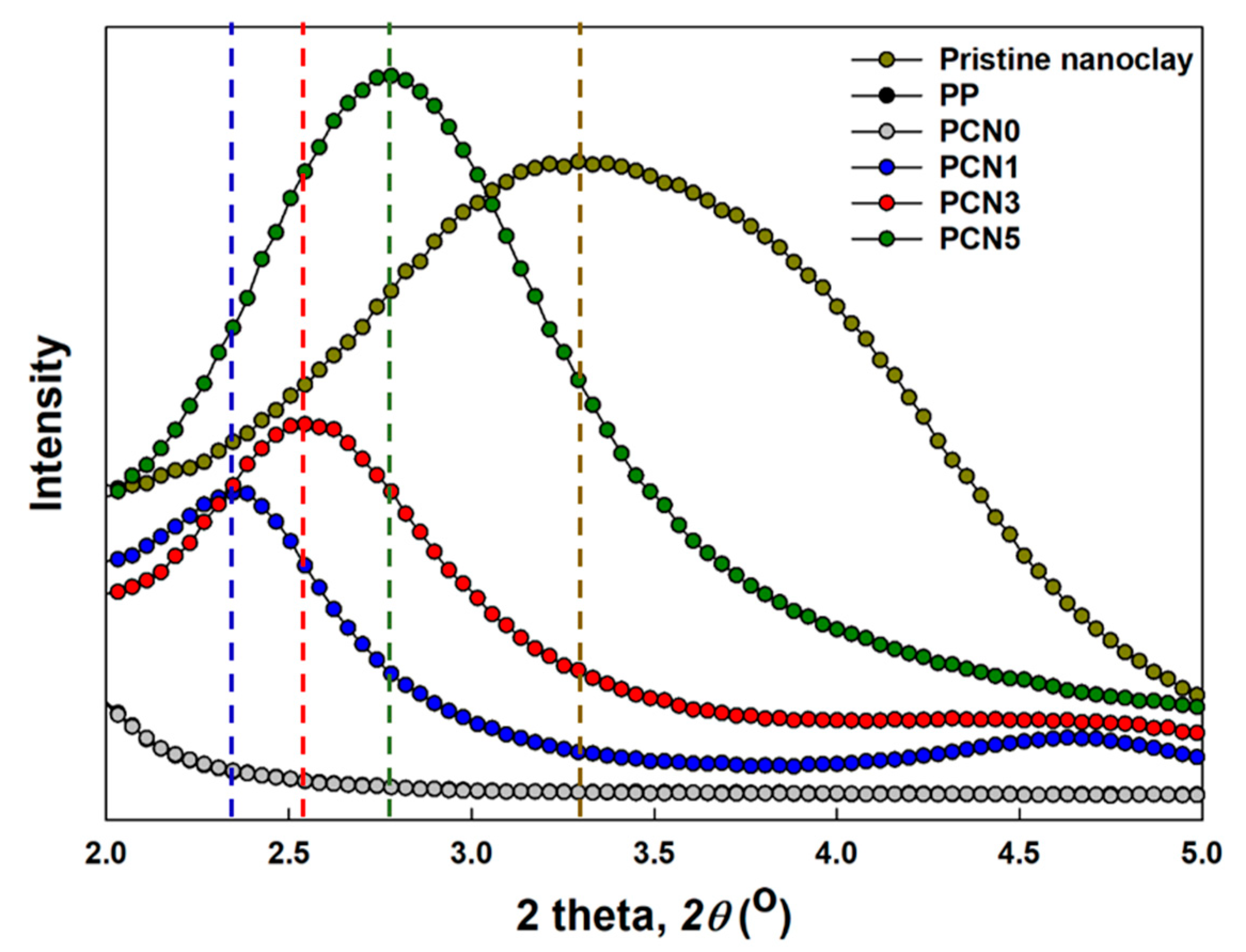
3.3. Nanoclay Dispersion Properties
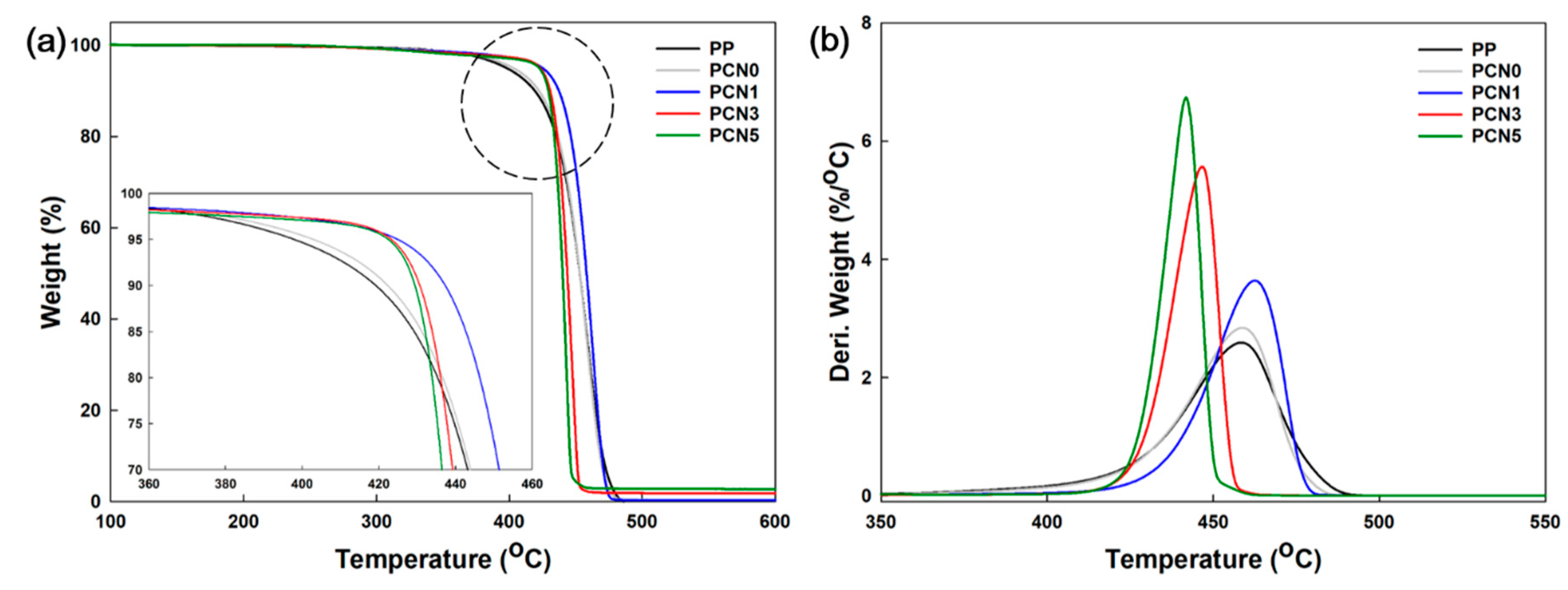
3.4. Thermal Stability Properties
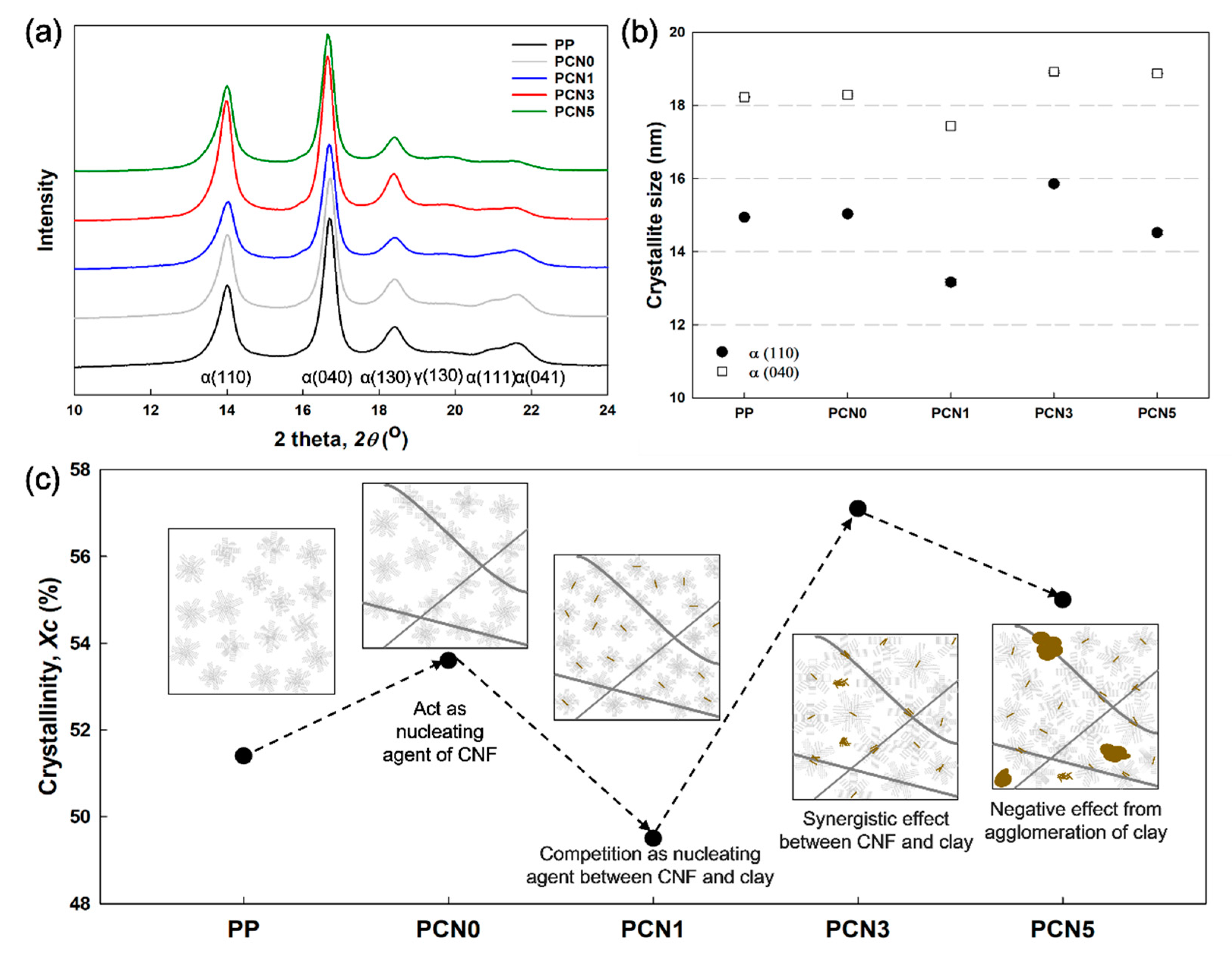
3.5. Crystallization Behavior Properties
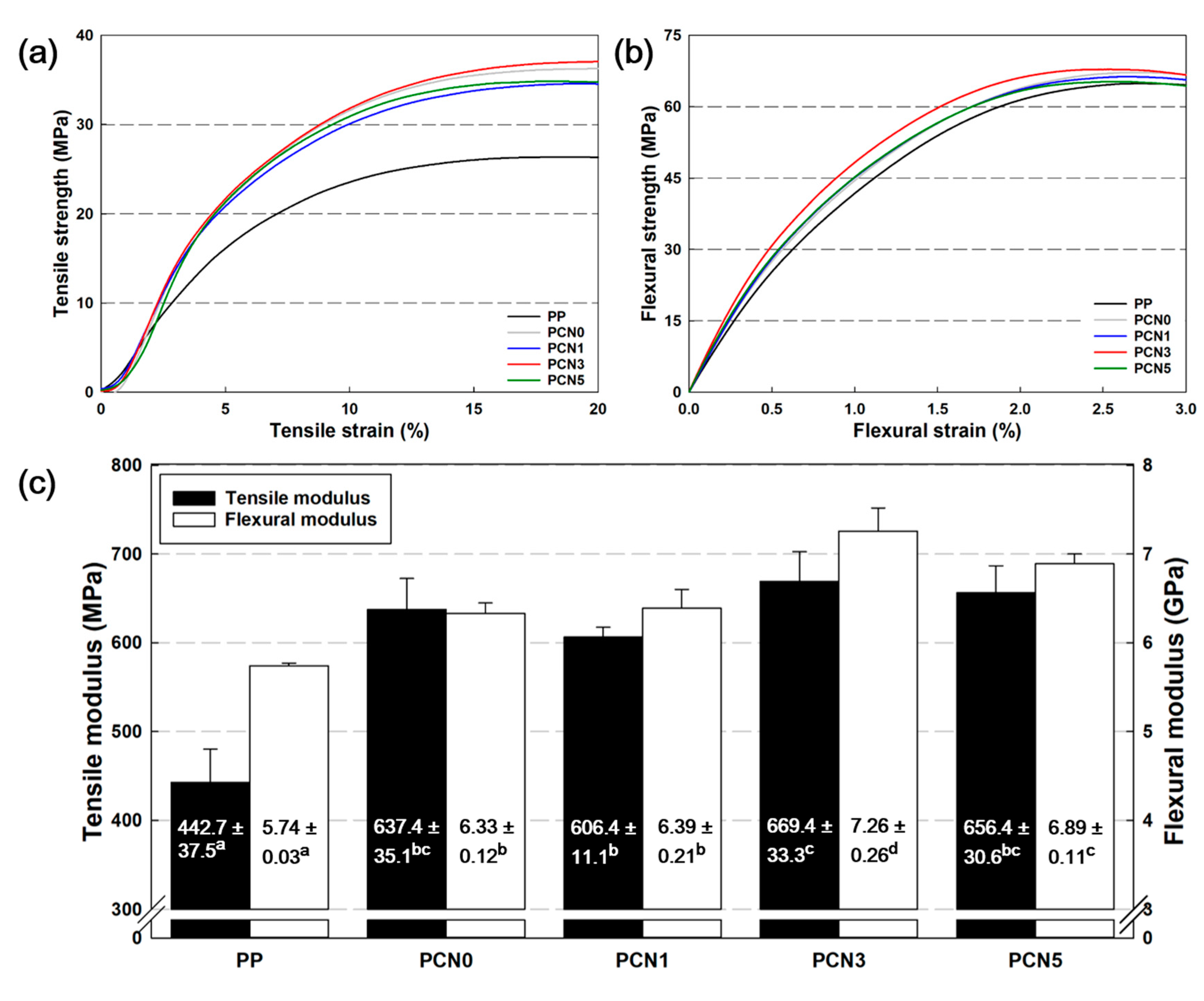
3.6. Mechanical Properties
3.7. Barrier Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoppe, M.; De Voogt, P.; Franz, R. Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates–A review. Trends Food Sci. Technol. 2016, 50, 118–130. [Google Scholar] [CrossRef]
- Edlund, U.; Lagerberg, T.; Ålander, E. Admicellar polymerization coating of CNF enhances integration in degradable nanocomposites. Biomacromolecules 2018, 20, 684–692. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef]
- Mirmehdi, S.; Hein, P.R.G.; de Luca Sarantópoulos, C.I.G.; Dias, M.V.; Tonoli, G.H.D. Cellulose nanofibrils/nanoclay hybrid composite as a paper coating: Effects of spray time, nanoclay content and corona discharge on barrier and mechanical properties of the coated papers. Food Packag. Shelf Life 2018, 15, 87–94. [Google Scholar] [CrossRef]
- Dhieb, F.B.; Dil, E.J.; Tabatabaei, S.H.; Mighri, F.; Ajji, A. Effect of nanoclay orientation on oxygen barrier properties of LbL nanocomposite coated films. RSC ADV 2019, 9, 1632–1641. [Google Scholar] [CrossRef]
- Soltani, I.; Smith, S.D.; Spontak, R.J. Effect of polyelectrolyte on the barrier efficacy of layer-by-layer nanoclay coatings. J. Membr. Sci. 2017, 526, 172–180. [Google Scholar] [CrossRef]
- Nguyen, H.-L.; Hanif, Z.; Park, S.-A.; Choi, B.G.; Tran, T.H.; Hwang, D.S.; Park, J.; Hwang, S.Y.; Oh, D.X. Sustainable boron nitride nanosheet-reinforced cellulose nanofiber composite film with oxygen barrier without the cost of color and cytotoxicity. Polymers 2018, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Tan, W.; Duan, Q.; Jin, Y.; Pei, L.; Ren, P.; Yan, D. Ultra-low gas permeable cellulose nanofiber nanocomposite films filled with highly oriented graphene oxide nanosheets induced by shear field. Carbohydr. Polym. 2019, 209, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Majeed, K.; Jawaid, M.; Hassan, A.; Bakar, A.A.; Khalil, H.A.; Salema, A.; Inuwaa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef]
- Majeed, K.; Hassan, A.; Bakar, A.A. Influence of maleic anhydride-grafted polyethylene compatibiliser on the tensile, oxygen barrier and thermal properties of rice husk and nanoclay-filled low-density polyethylene composite films. J. Plast. Film Sheeting 2014, 30, 120–140. [Google Scholar] [CrossRef]
- Ou, C.F.; Ho, M.T.; Lin, J.R. The nucleating effect of montmorillonite on crystallization of PET/montmorillonite nanocomposite. J. Polym. Res. 2003, 10, 127–132. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef]
- Rahman, M.R.; Hamdan, S.; Hashim, D.M.A.; Islam, M.S.; Takagi, H. Bamboo fiber polypropylene composites: Effect of fiber treatment and nano clay on mechanical and thermal properties. J. Vinyl. Addit. Technol. 2015, 21, 253–258. [Google Scholar] [CrossRef]
- Hashemi, S.; Esfandeh, M.; Mohammadi, J. Mechanical, thermal and rheological properties of polypropylene/wheat straw composites and study of the effect of nanoclay on their mechanical properties. Polym. Compos. 2010, 18, 67–73. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Pirayesh, H.; Salari, A. Long term hygroscopic characteristics of polypropylene based hybrid composites with and without organo-modified clay. Eur. J. Wood Wood Prod. 2013, 71, 211–218. [Google Scholar] [CrossRef]
- Pratheep Kumar, A.; Pal Singh, R. Novel hybrid of clay, cellulose, and thermoplastics. I. Preparation and characterization of composites of ethylene–propylene copolymer. J. Appl. Polym. Sci. 2007, 104, 2672–2682. [Google Scholar] [CrossRef]
- Pandey, J.K.; Lee, S.; Kim, H.J.; Takagi, H.; Lee, C.; Ahn, S. Preparation and properties of cellulose-based nano composites of clay and polypropylene. J. Appl. Polym. Sci. 2012, 125, E651–E660. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Sillard, C.; Szabo, P.; Bras, J.; Daugaard, A.E. Hybrid poly (lactic acid)/nanocellulose/nanoclay composites with synergistically enhanced barrier properties and improved thermomechanical resistance. Polym. Int. 2016, 65, 988–995. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Sillard, C.; Hassager, O.; Daugaard, A.E.; Bras, J.; Szabo, P. A comparison of partially acetylated nanocellulose, nanocrystalline cellulose, and nanoclay as fillers for high-performance polylactide nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43257. [Google Scholar] [CrossRef]
- Trifol, J.; van Drongelen, M.; Clegg, F.; Plackett, D.; Szabo, P.; Daugaard, A. Impact of thermal processing or solvent casting upon crystallization of PLA nanocellulose and/or nanoclay composites. J. Appl. Polym. Sci. 2019, 136, 47486. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and characterization of modified cellulose nanofibers reinforced polylactic acid nanocomposite. Polym. Test 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Jung, B.N.; Kang, D.; Cheon, S.; Shim, J.K.; Hwang, S.W. The addition effect of hollow glass microsphere on the dispersion behavior and physical properties of polypropylene/clay nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47476. [Google Scholar] [CrossRef]
- Jung, B.N.; Jung, H.W.; Kang, D.; Kim, G.H.; Lee, M.; Hwang, S.W.; Shim, J. The fabrication of affinity improved nanocomposites with plasma treated polypropylene (PP) and alkaline cellulose nanofiber (CNF) suspension. Polym. Test 2020, 85, 106352. [Google Scholar] [CrossRef]
- You, J.; Choi, H.-H.; Cho, J.; Son, J.G.; Park, M.; Lee, S.-S.; Park, J.H. Highly thermally conductive and mechanically robust polyamide/graphite nanoplatelet composites via mechanochemical bonding techniques with plasma treatment. Compos. Sci. Technol. 2018, 160, 245–254. [Google Scholar] [CrossRef]
- Lee, Y.M.; You, J.; Kim, M.; Kim, T.A.; Lee, S.-S.; Bang, J.; Park, J.H. Highly improved interfacial affinity in carbon fiber-reinforced polymer composites via oxygen and nitrogen plasma-assisted mechanochemistry. Compos. Part B Eng. 2019, 165, 725–732. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer−clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Li, S.-M.; Jia, N.; Zhu, J.-F.; Ma, M.-G.; Sun, R.-C. Synthesis of cellulose–calcium silicate nanocomposites in ethanol/water mixed solvents and their characterization. Carbohydr. Polym. 2010, 80, 270–275. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Nanoreinforced bacterial cellulose–montmorillonite composites for biomedical applications. Carbohydr. Polym. 2012, 89, 1189–1197. [Google Scholar] [CrossRef]
- Arora, A.; Choudhary, V.; Sharma, D. Effect of clay content and clay/surfactant on the mechanical, thermal and barrier properties of polystyrene/organoclay nanocomposites. J. Polym. Res. 2011, 18, 843–857. [Google Scholar] [CrossRef]
- Decker, J.J.; Chvalun, S.N.; Nazarenko, S. Intercalation behavior of hydroxylated dendritic polyesters in polymer clay nanocomposites prepared from aqueous solution. Polymer 2011, 52, 3943–3955. [Google Scholar] [CrossRef]
- Lee, D.; Char, K.; Lee, S.W.; Park, Y.W. Structural changes of polyaniline/montmorillonite nanocomposites and their effects on physical properties. J. Mater. Chem. 2003, 13, 2942–2947. [Google Scholar] [CrossRef]
- Fitaroni, L.B.; de Lima, J.A.; Cruz, S.A.; Waldman, W.R. Thermal stability of polypropylene–montmorillonite clay nanocomposites: Limitation of the thermogravimetric analysis. Polym. Degrad. Stabil. 2015, 111, 102–108. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer–clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Zehetmeyer, G.; Soares, R.M.; Brandelli, A.; Mauler, R.S.; Oliveira, R.V. Evaluation of polypropylene/montmorillonite nanocomposites as food packaging material. Polym. Bull. 2012, 68, 2199–2217. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Chu, P.-H. Characterization of solution-mixed polypropylene/clay nanocomposites without compatibilizers. J. Polym. Res. 2006, 13, 73–78. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Zhong, G.-J.; Wang, Y.; Li, Z.-M.; Li, L. Unusual tuning of mechanical properties of isotactic polypropylene using counteraction of shear flow and β-nucleating agent on β-form nucleation. Macromolecules 2009, 42, 4343–4348. [Google Scholar] [CrossRef]
- Medellín-Rodríguez, F.; Mata-Padilla, J.; Hsiao, B.; Waldo-Mendoza, M.; Ramírez-Vargas, E.; Sánchez-Valdes, S. The effect of nanoclays on the nucleation, crystallization, and melting mechanisms of isotactic polypropylene. Polym. Eng. Sci. 2007, 47, 1889–1897. [Google Scholar] [CrossRef]
- Wang, X.C.; Jing, X.; Peng, Y.Y.; Ma, Z.K.; Liu, C.T.; Turng, L.S.; Shen, C.-Y. The effect of nanoclay on the crystallization behavior, microcellular structure, and mechanical properties of thermoplastic polyurethane nanocomposite foams. Polym. Eng. Sci. 2016, 56, 319–327. [Google Scholar] [CrossRef]
- Al-Mulla, A.; Mathew, J.; Yeh, S.-K.; Gupta, R. Nonisothermal crystallization kinetics of PBT nanocomposites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 204–217. [Google Scholar] [CrossRef]
- Preschilla, N.; Rasheed, A.A.; Sahadevan, S.; Biswas, A.; Bellare, J.R.; Shyamroy, S. Study of layered silicate clays as synergistic nucleating agent for polypropylene. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1786–1794. [Google Scholar] [CrossRef]
- Tarapow, J.; Bernal, C.R.; Alvarez, V.A. Mechanical properties of polypropylene/clay nanocomposites: Effect of clay content, polymer/clay compatibility, and processing conditions. J. Appl. Polym. Sci. 2009, 111, 768–778. [Google Scholar] [CrossRef]
- Mohan, T.; Kanny, K. Influence of nanoclay on rheological and mechanical properties of short glass fiber-reinforced polypropylene composites. J. Reinf. Plast. Compos. 2011, 30, 152–160. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the crystallinity and mechanical properties of PLA by nucleation and annealing. Polym. Degrad. Stabil. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Nascimento, E.M.; Eiras, D.; Pessan, L.A. Effect of thermal treatment on impact resistance and mechanical properties of polypropylene/calcium carbonate nanocomposites. Compos. Part B Eng. 2016, 91, 228–234. [Google Scholar] [CrossRef]
- Friedrich, J.; Unger, W.; Lippitz, A.; Koprinarov, I.; Ghode, A.; Geng, S.; Kühn, G. Plasma-based introduction of monosort functional groups of different type and density onto polymer surfaces. Part 1: Behaviour of polymers exposed to oxygen plasma. Compos. Interfaces 2003, 10, 139–171. [Google Scholar] [CrossRef]
| Sample Code | Suspensions (CNF, g/Nanoclay, g) | Final Composition of Nanocomposites (PP, wt %/CNF, wt %/Nanoclay wt %) |
|---|---|---|
| PCN0 | CNF suspension (0.4/0) | 99/1/0 |
| PCN1 | CNF/nanoclay suspension (0.4/0.4) | 98/1/1 |
| PCN3 | CNF/nanoclay suspension (0.4/1.2) | 96/1/3 |
| PCN5 | CNF/nanoclay suspension (0.4/2.0) | 94/1/5 |
| Sample Code | 2θ Peak (°) | d-Spacing of Nanoclay, d001 (nm) | FWHM |
|---|---|---|---|
| Pristine nanoclay | 3.27 | 2.70 | 2.23 |
| PCN1 | 2.35 | 3.76 | 0.83 |
| PCN3 | 2.54 | 3.47 | 0.97 |
| PCN5 | 2.78 | 3.18 | 1.21 |
| Sample Code | T−5% (°C) | T−10% (°C) | Tonset (°C) | Residue Amounts (%) |
|---|---|---|---|---|
| PP | 401.5 ± 5.1 a | 420.9 ± 2.6 a | 434.6 ± 1.1 ab | 0.1 ± 0.0 a |
| PCN0 | 408.1 ± 5.2 a | 425.3 ± 3.0 ab | 437.2 ± 1.4 b | 0.1 ± 0.1 a |
| PCN1 | 425.4 ± 0.9 b | 436.5 ± 0.9 c | 443.7 ± 1.2 c | 0.9 ± 0.1 b |
| PCN3 | 422.1 ± 4.3 b | 430.1 ± 2.2 b | 435.1 ± 0.9 ab | 2.5 ± 0.2 c |
| PCN5 | 422.2 ± 0.0 b | 429.4 ± 0.0 b | 433.7 ± 0.0 a | 3.9 ± 0.1 d |
| Sample Code | Tensile Stress at Yield Stress (MPa) | Elongation at Break (%) | Flexural Strength (MPa) | Flexural Strain at Flexural Strength (%) |
|---|---|---|---|---|
| PP | 26.69 ± 0.77 a | 935.24 ± 38.58 d | 64.58 ± 0.42 a | 2.66 ± 0.08 c |
| PCN0 | 36.44 ± 0.18 c | 416.56 ± 82.83 b | 66.68 ± 0.69 bc | 2.58 ± 0.02 bc |
| PCN1 | 34.73 ± 0.56 b | 605.95 ± 13.84 c | 65.65 ± 0.90 ab | 2.56 ± 0.05 ab |
| PCN3 | 36.61 ± 0.55 c | 289.79 ± 65.85 a | 67.44 ± 0.55 c | 2.47 ± 0.05 a |
| PCN5 | 34.62 ± 0.63 b | 499.06 ± 66.34 bc | 65.53 ± 0.39 ab | 2.47 ± 0.05 ab |
| Sample Code | Oxygen Permeability (cc-mm/m2-Day-atm) | Water Vapor Permeability (g-mm/m2-Day-atm) |
|---|---|---|
| PP | 132.66 ± 0.79 c | 0.38 ± 0.02 a |
| PCN0 | 107.02 ± 6.78 b | 0.37 ± 0.01 a |
| PCN1 | 106.71 ± 2.94 b | 0.39 ± 0.05 a |
| PCN3 | 95.77 ± 0.97 a | 0.39 ± 0.03 a |
| PCN5 | 92.95 ± 1.58 a | 0.37 ± 0.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, B.N.; Jung, H.W.; Kang, D.; Kim, G.H.; Shim, J.K. Synergistic Effect of Cellulose Nanofiber and Nanoclay as Distributed Phase in a Polypropylene Based Nanocomposite System. Polymers 2020, 12, 2399. https://doi.org/10.3390/polym12102399
Jung BN, Jung HW, Kang D, Kim GH, Shim JK. Synergistic Effect of Cellulose Nanofiber and Nanoclay as Distributed Phase in a Polypropylene Based Nanocomposite System. Polymers. 2020; 12(10):2399. https://doi.org/10.3390/polym12102399
Chicago/Turabian StyleJung, Bich Nam, Hyun Wook Jung, DongHo Kang, Gi Hong Kim, and Jin Kie Shim. 2020. "Synergistic Effect of Cellulose Nanofiber and Nanoclay as Distributed Phase in a Polypropylene Based Nanocomposite System" Polymers 12, no. 10: 2399. https://doi.org/10.3390/polym12102399
APA StyleJung, B. N., Jung, H. W., Kang, D., Kim, G. H., & Shim, J. K. (2020). Synergistic Effect of Cellulose Nanofiber and Nanoclay as Distributed Phase in a Polypropylene Based Nanocomposite System. Polymers, 12(10), 2399. https://doi.org/10.3390/polym12102399







