Improving Fire Performances of PEAL: More Second-Life Options for Recycled Tetra Pak®
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Compounds
2.3. Characterizations
2.3.1. TGA
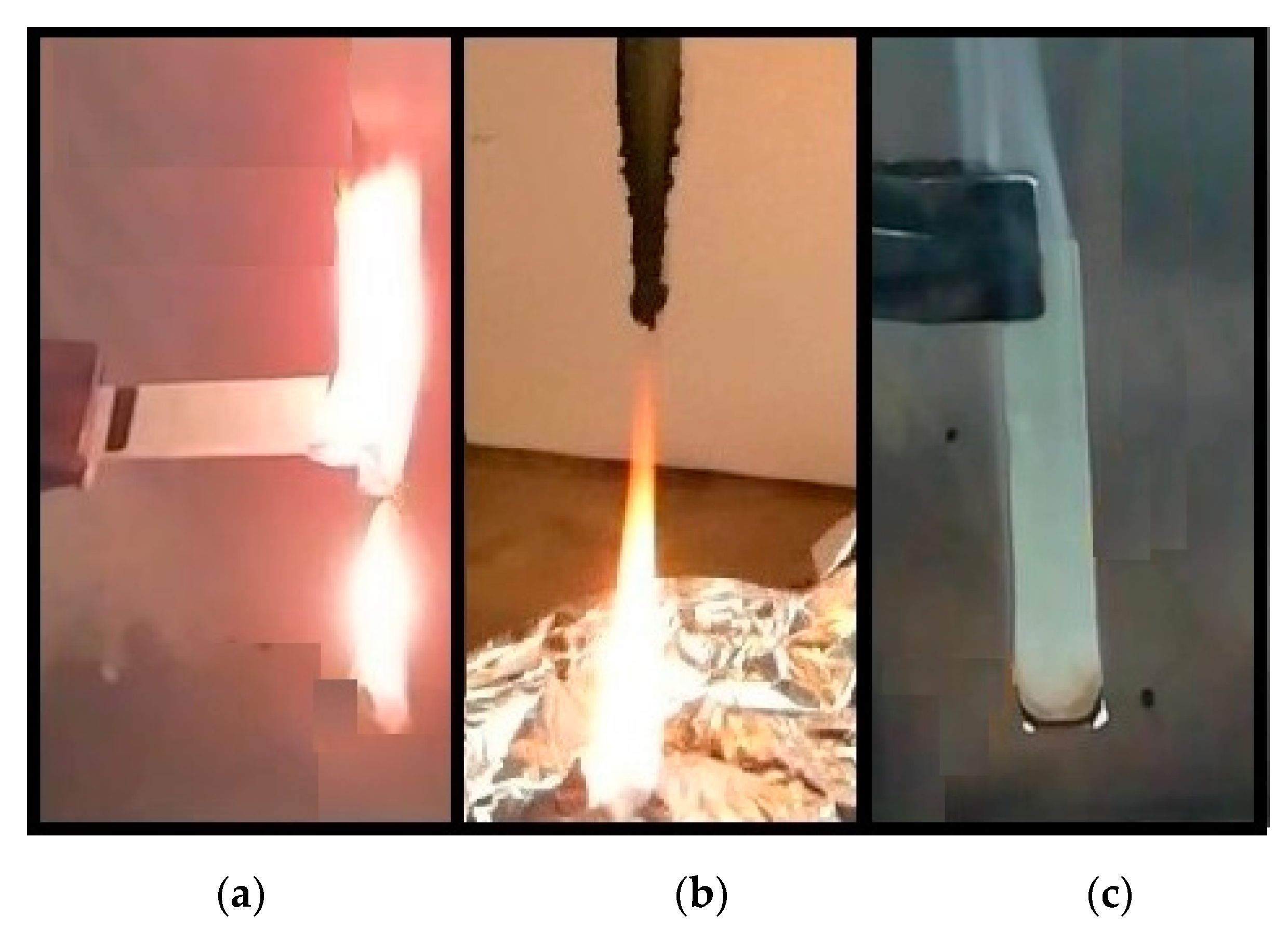
2.3.2. UL-94
2.3.3. Cone Calorimeter
2.3.4. SEM
3. Results and Discussion
3.1. PEAL
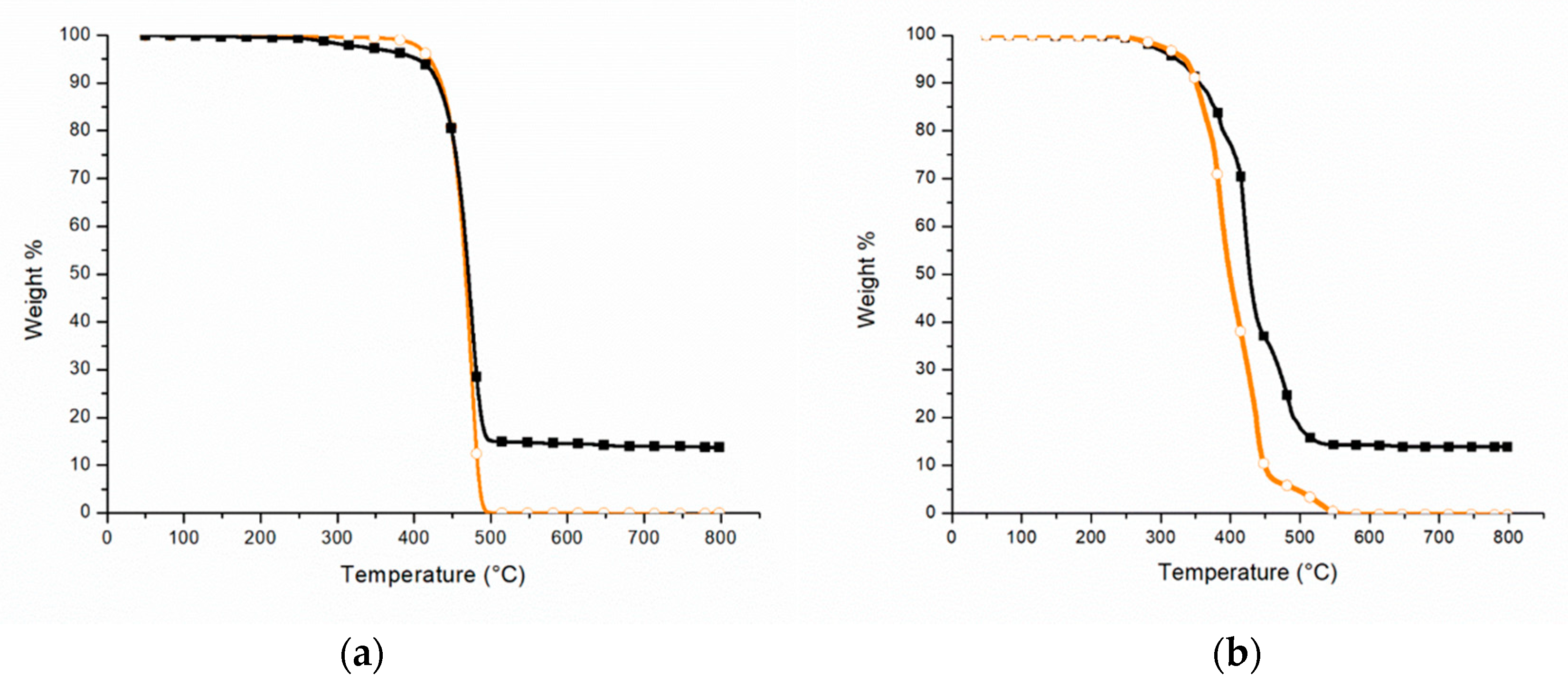
3.1.1. TGA
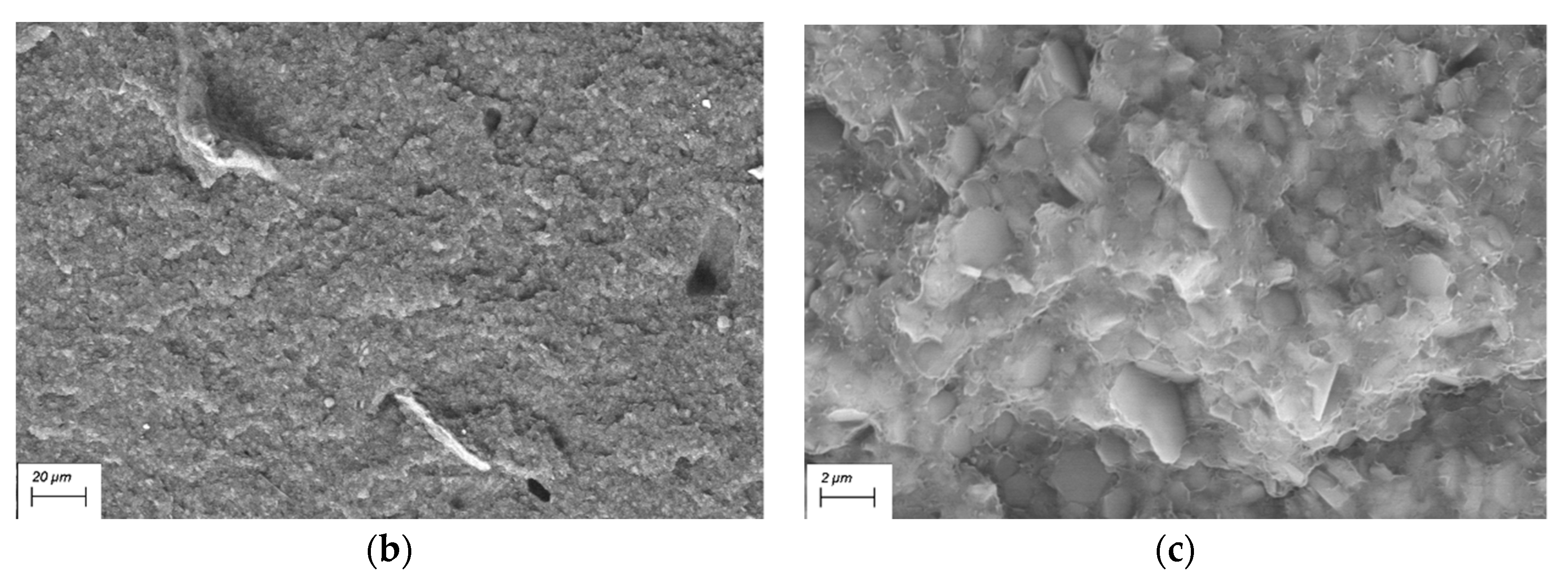
3.1.2. SEM
3.2. FR PEAL
3.2.1. TGA
3.2.2. UL-94
3.2.3. Cone Calorimeter
3.2.4. SEM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope. Available online: www.plasticseurope.org/it/resources/publications/1804-plastics-facts-2019 (accessed on 12 February 2020).
- Tetra Pak®. Available online: www.tetrapak.com/it/packaging/aseptic-solutions (accessed on 12 February 2020).
- Tetra Pak®. Available online: www.tetrapak.com/it (accessed on 12 February 2020).
- Associazione Comuni Virtuosi. Available online: Comunivirtuosi.org/imballaggi-poliaccoppiato-le-due-tre-cose-sapere-riciclo/ (accessed on 12 February 2020).
- Tetra Pak®. Available online: www.tetrapak.com/about/history (accessed on 12 February 2020).
- Comieco. Available online: www.comieco.org/ilnostro-ruolo/l-attivita-dei-convenzionati/news/protocollo-intesa-tetra-pak---comieco.aspx (accessed on 12 February 2020).
- Polimerica. Available online: www.polimerica.it/articolo.asp?id=20164 (accessed on 12 February 2020).
- Ecoplasteam S.p.a. Available online: www.ecoplasteam.com/ (accessed on 30 January 2020).
- Ecoplasteam S.p.a. Available online: www.ecoplasteam.com/ecoallene/applicazioni/ (accessed on 9 June 2020).
- Hidalgo-Salazar, M.A.; Muñoz, M.F.; Mina, J.H. Influence of Incorporation of Natural Fibers on the Physical, Mechanical, and Thermal Properties of Composites LDPE-Al Reinforced with Fique Fibers. Int. J. Polym. Sci. 2015, 2015, 386325. [Google Scholar] [CrossRef]
- Lopes, C.M.A.; Felisberti, M.I. Composite of Low-Density Polyethylene and Aluminum Obtained from the Recycling of Postconsumer Aseptic Packaging. J. Appl. Polym. Sci. 2006, 101, 3183–3191. [Google Scholar] [CrossRef]
- Lopes, C.M.A.; Felisberti, M.I. Thermal conductivity of PET/(LDPE/AI) composites determined by MDSC. Polym. Test. 2004, 23, 637–643. [Google Scholar] [CrossRef]
- Muñoz-Vélez, M.F.; Hidalgo-Salazar, M.A.; Mina-Hernández, J.H. Effect of Content and Surface Modification of Fique Fibers on the Properties of a Low-Density Polyethylene (LDPE)-Al/Fique Composite. Polymers 2018, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Ayrilmis, N.; Kaymakci, A.; Akbulut, T.; Mertoglu Elmas, G. Mechanical performance of composites based on wastes of polyethylene aluminum and lignocellulosics. Compos. Part B 2013, 47, 150–154. [Google Scholar] [CrossRef]
- Xu, C.; Jian, W.; Xing, C.; Zhou, H.; Zhao, Y.; Pan, H.; Xiong, X. Flame Retardancy and Mechanical Properties of Thermal Plastic Composite Panels Made from Tetra Pak Waste and High-Density Polyethylene. Polym. Compos. 2016, 37, 1797–1804. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Li, M.; Song, L. Effects of Charring Agents on the Thermal and Flammability Properties of Intumescent Flame-Retardant HDPEbased Clay Nanocomposites. Polym. Plast. Technol. Eng. 2008, 47, 152–156. [Google Scholar] [CrossRef]
- Han, Z.; Dong, L.; Li, Y.; Zhao, H. A Comparative Study on the Synergistic Effect of Expandable Graphite with APP and IFR in Polyethylene. J. Fire Sci. 2007, 25, 79–91. [Google Scholar] [CrossRef]
- Khanal, S.; Zhanga, W.; Ahmeda, S.; Alia, M.; Xu, S. Effects of intumescent flame retardant system consisting of tris (2-hydroxyethyl) isocyanurate and ammonium polyphosphate on the flame retardant properties of high-density polyethylene composites. Compos. Part A 2018, 112, 444–451. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Shu, W. Effect of Ultrafine Zinc Borate on the Smoke Suppression and Toxicity Reduction of a Low-Density Polyethylene/Intumescent Flame-Retardant System. J. Appl. Polym. Sci. 2010, 117, 443–449. [Google Scholar] [CrossRef]
- Lenza, J.; Merkel, K.; Rydarowski, H. Comparison of the effect of montmorillonite, magnesium hydroxide and a mixture of both on the flammability properties and mechanism of char formation of HDPE composites. Polym. Degrad. Stab. 2012, 97, 2581–2593. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, B.; Fan, W.; Huang, P. Combustion Characteristics of Halogen-Free FlameRetarded Polyethylene Containing Magnesium Hydroxide and Some Synergists. J. Appl. Polym. Sci. 2001, 81, 206–214. [Google Scholar] [CrossRef]
- Liu, S. Flame retardant and mechanical properties of polyethylene/magnesium hydroxide/montmorillonite nanocomposites. J. Ind. Eng. Chem. 2014, 20, 2401–2408. [Google Scholar] [CrossRef]
- Zhang, J.; Hereid, J.; Hagen, M.; Bakirtzis, D.; Delichatsios, M.A.; Fina, A.; Castrovinci, A.; Camino, G.; Samyn, F.; Bourbigot, S. Effects of nanoclay and fire retardants on fire retardancy of a polymer blend of EVA and LDPE. Fire Saf. J. 2009, 44, 504–513. [Google Scholar] [CrossRef]
- Hornsby, P.R. Fire retardant fillers for polymers. Int. Mater. Rev. 2001, 46, 199–210. [Google Scholar] [CrossRef]
- ASTM D635-18. Standard Test Method for Rate of Burning and/or Extent and Time of Burning of Plastics in a Horizontal Position; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM D3801-20a. Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ISO 5660-2:2002(E). Reaction-To-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Gallina, G.; Bravin, E.; Badalucco, C.; Audisio, G.; Armanini, M.; De Chirico, A.; Provasoli, F. Application of Cone Calorimeter for the Assessment of Class of Flame Retardants for Polypropylene. Fire Mater. 1998, 22, 15–18. [Google Scholar] [CrossRef]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame Retardancy Index for Thermoplastic Composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, S.; Lecce, R. Method for Recycling Composite Material. Patent EP2296858B1, 3 July 2013. [Google Scholar]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the Mechanism of Intumescence in Fire Retardant Polymers: Part l—Thermal Degradation of Ammonium Polyphosphate-Pentaerythritol Mixtures. Polym. Degrad. Stab. 1984, 6, 243–252. [Google Scholar] [CrossRef]
- Costa, F.R.; Wagenknecht, U.; Heinrich, G. LDPE/MgeAl layered double hydroxide nanocomposite: Thermal and flammability properties. Polym. Degrad. Stab. 2007, 92, 1813–1823. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |




| Name | Composition |
|---|---|
| PEALAPP30 | PEAL: 70%; APP 30% |
| PEALIFR3031 | PEAL: 70%; IFR: 30% |
| PEALIFR4031 | PEAL: 60%; IFR: 40% |
| PEAL50MH | PEAL: 50%; MH: 50% |
| PEAL60MH | PEAL: 40%; MH: 60% |
| Atmosphere | Material | T2% [°C] | Tmax [°C] | Residue at 800 °C [%] |
|---|---|---|---|---|
| Inert | LDPE | 402 | 474 | 0 |
| PEAL | 312 | 474 | 14 | |
| Oxidative | LDPE | 294 | 385 | 0 |
| PEAL | 283 | 421 | 14 |
| Material | T2% [°C] | Tmax [°C] | Residue at 800 °C [%] |
|---|---|---|---|
| PEAL | 283 | 421 | 14 |
| PEALAPP30 | 295 | 479 | 21 |
| PEALIFR3031 | 213 | 484 | 23 |
| PEALIFR4031 | 211 | 481 | 25 |
| PEAL50MH | 192 | 467 | 38 |
| PEAL60MH | 351 | 462 | 46 |
| Material | TTI [s] (±Std. Dev.) (Δ%PEAL) | Time of Peak HRR [s] (±Std. Dev.) (Δ%PEAL) | Peak HRR [kW/m2] (±Std. Dev.) (Δ%PEAL) | FPI [s * m2/kW] (±Std. Dev.) (Δ%PEAL) | THR [MJ/m2] (±Std. Dev.) (Δ%PEAL) | TSR [m2/m2] (±Std. Dev.) (Δ% PEAL) | FRI (±Std. Dev.) | Residue % (±Std. Dev.) (Δ% PEAL) |
|---|---|---|---|---|---|---|---|---|
| PEAL | 58 (±3) | 183 (±13) | 856 (±25) | 0.07 (±0.001) | 97 (±1) | 1100 (±28) | - | 14 (±0) |
| PEALIFR4031 | 50 (±1) (−14) | 93 (±4) (−49) | 343 (±18) (−60) | 0.15 (±0.006) (+114) | 79 (±5) (−19) | 1200 (±141) (+9) | 2.6 (±0.3) | 33 (±1) (+136) |
| PEAL60MH | 144 (±10) (+148) | 480 (±52) (+162) | 269 (±45) (−69) | 0.55 (±0) (+686) | 84 (±22) (−13) | 212 (±41) (−81) | 10.0 (±4) | 48 (±0) (+242) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cravero, F.; Frache, A. Improving Fire Performances of PEAL: More Second-Life Options for Recycled Tetra Pak®. Polymers 2020, 12, 2357. https://doi.org/10.3390/polym12102357
Cravero F, Frache A. Improving Fire Performances of PEAL: More Second-Life Options for Recycled Tetra Pak®. Polymers. 2020; 12(10):2357. https://doi.org/10.3390/polym12102357
Chicago/Turabian StyleCravero, Fulvia, and Alberto Frache. 2020. "Improving Fire Performances of PEAL: More Second-Life Options for Recycled Tetra Pak®" Polymers 12, no. 10: 2357. https://doi.org/10.3390/polym12102357
APA StyleCravero, F., & Frache, A. (2020). Improving Fire Performances of PEAL: More Second-Life Options for Recycled Tetra Pak®. Polymers, 12(10), 2357. https://doi.org/10.3390/polym12102357






