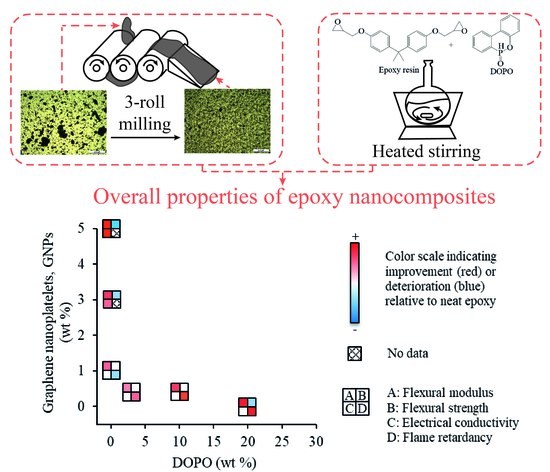
Effects of Combining Graphene Nanoplatelet and Phosphorous Flame Retardant as Additives on Mechanical Properties and Flame Retardancy of Epoxy Nanocomposite
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Incorporation of DOPO into Epoxy Resin
2.3. Dispersion of GNP in Epoxy Resin
2.4. Processing of Epoxy Resin
2.5. Characterization of GNP and EP/GNP/DOPO Composites and Measurement Procedures
3. Results and Discussion
3.1. Verification of the Incorporation of DOPO into Epoxy Resin using ATR-FTIR
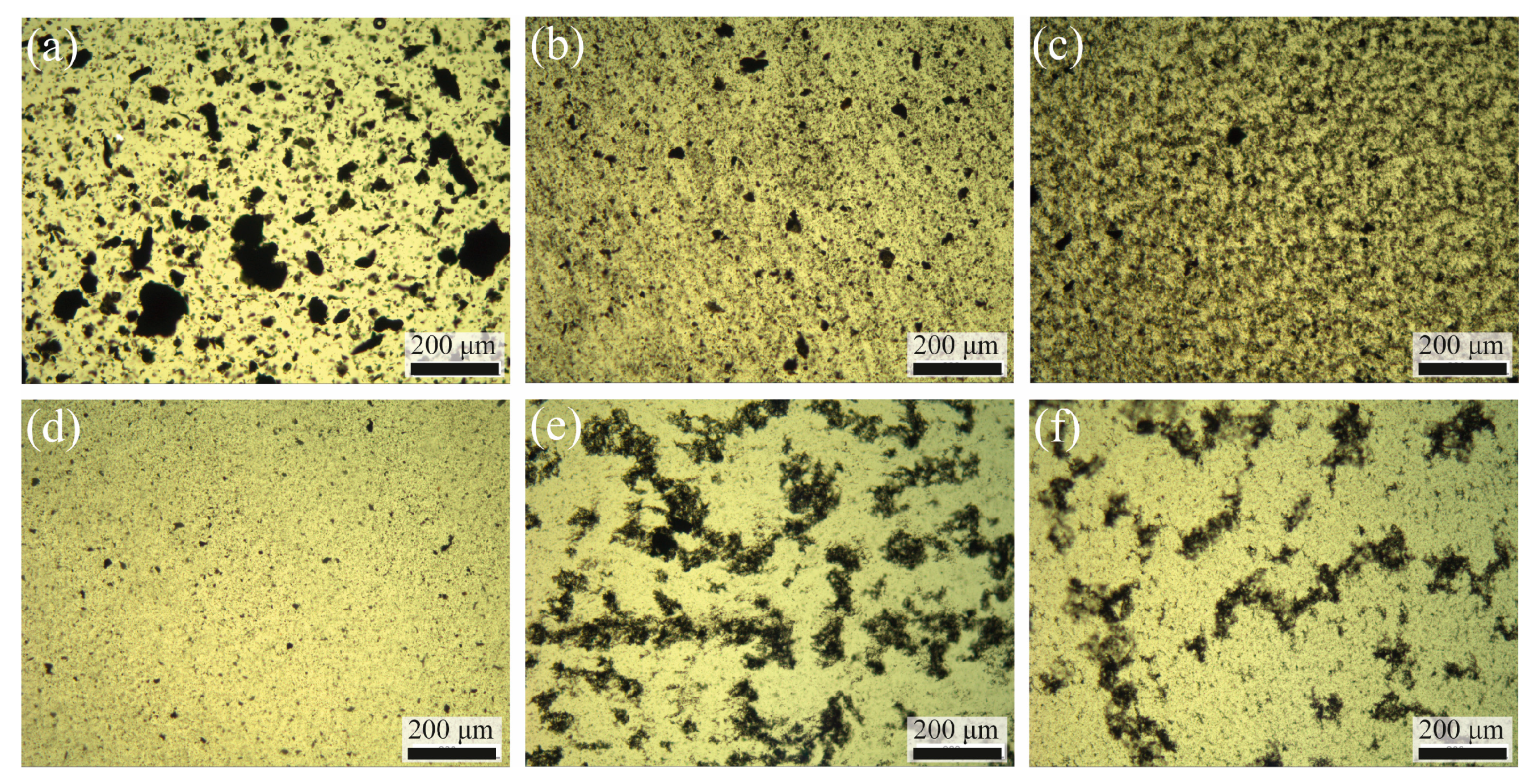
3.2. Dispersion of GNP in the Epoxy Resin
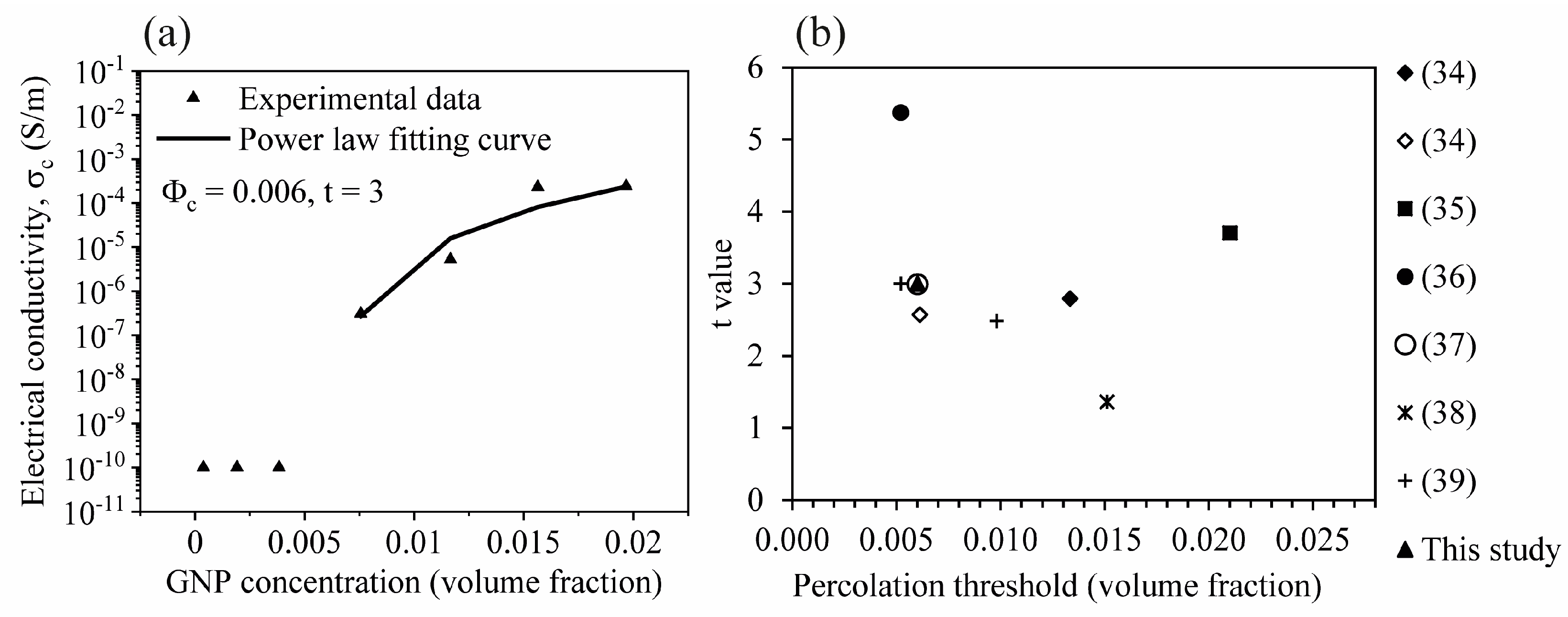
3.3. Electrical Property
3.4. Crosslinking Density of Epoxy Resins
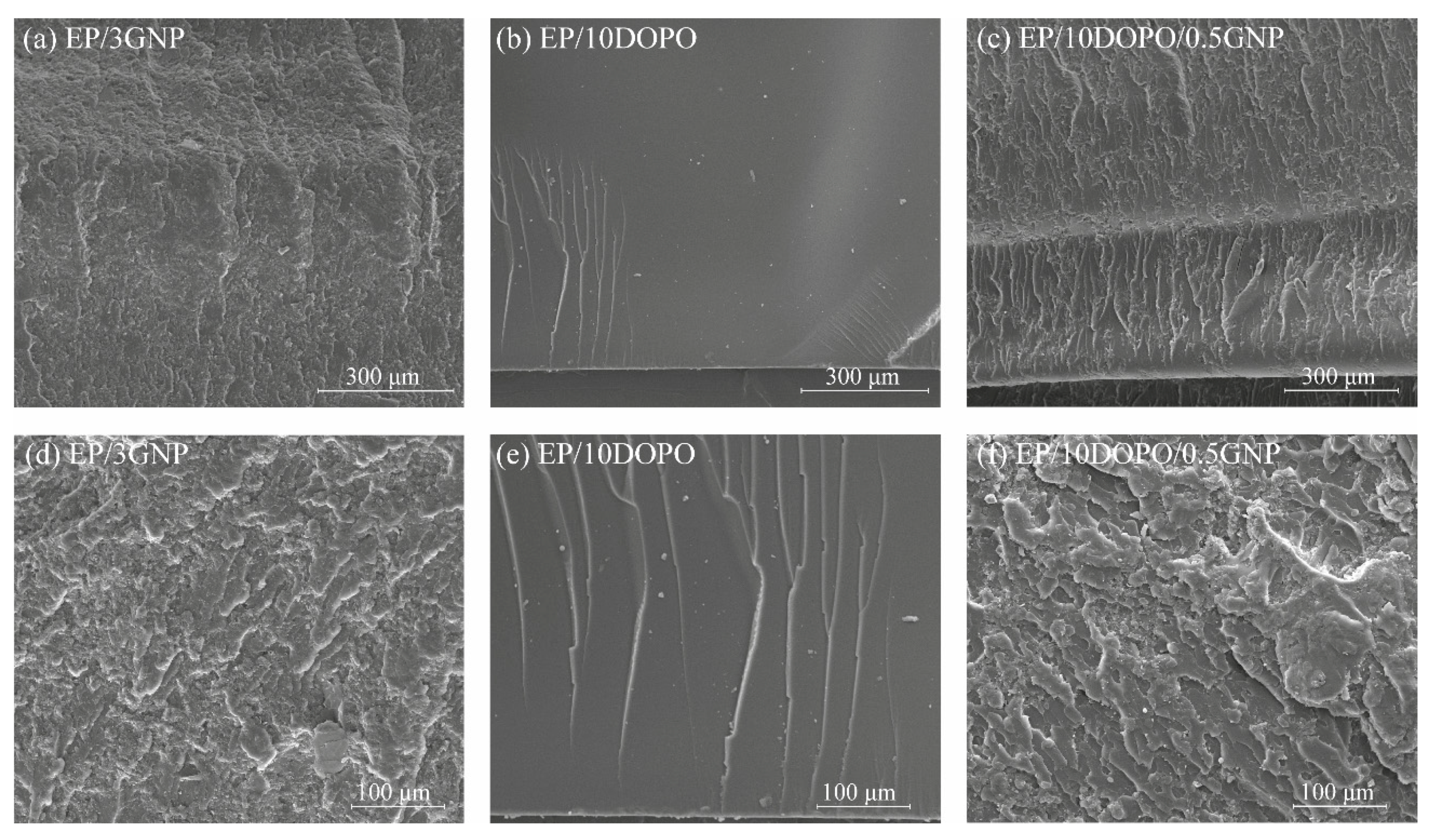
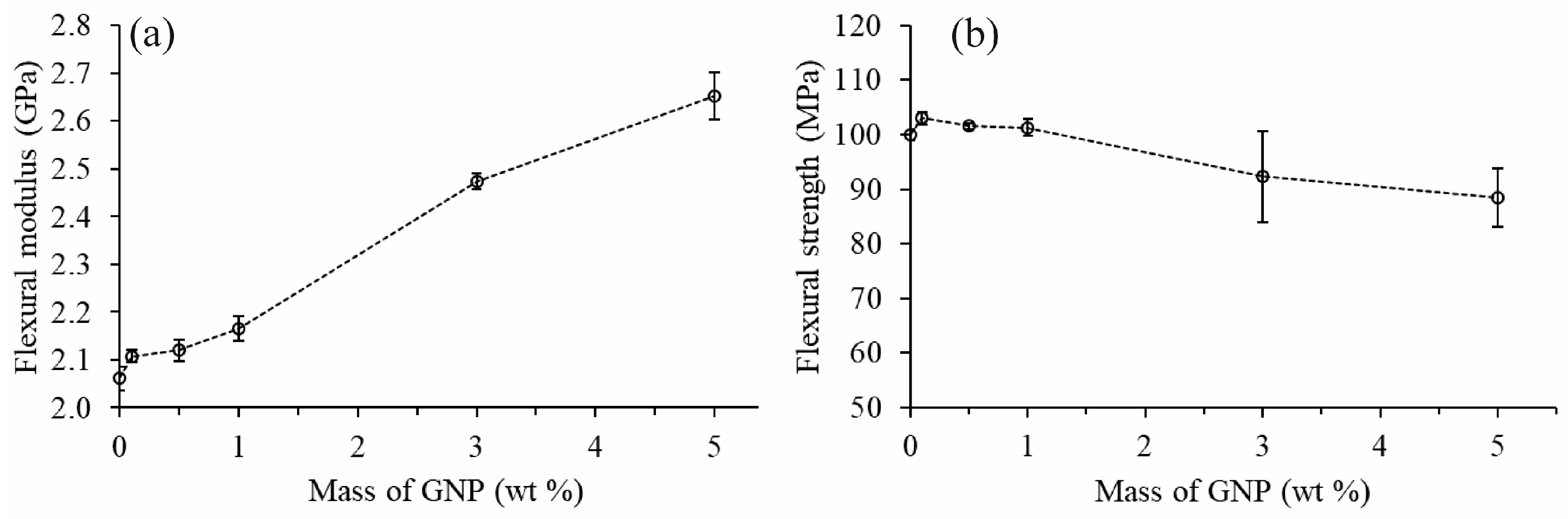
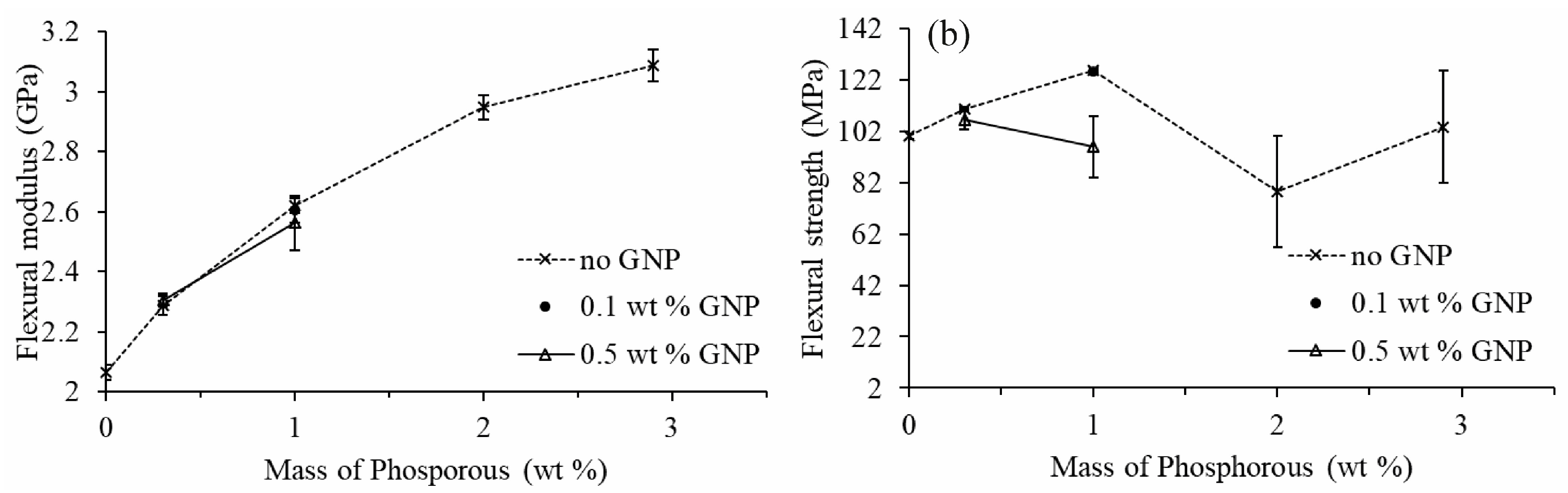
3.5. Mechanical Properties
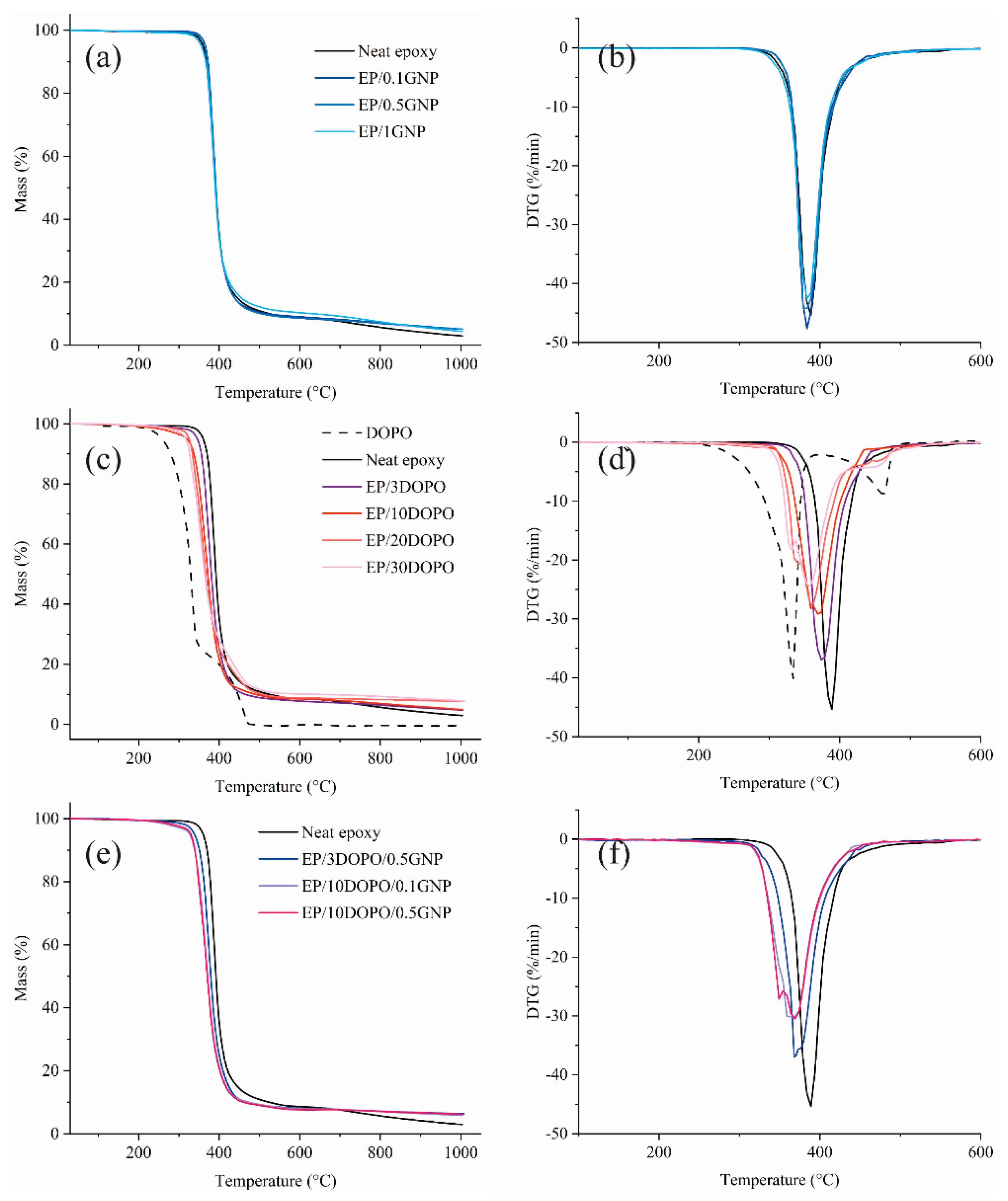
3.6. Thermal Properties
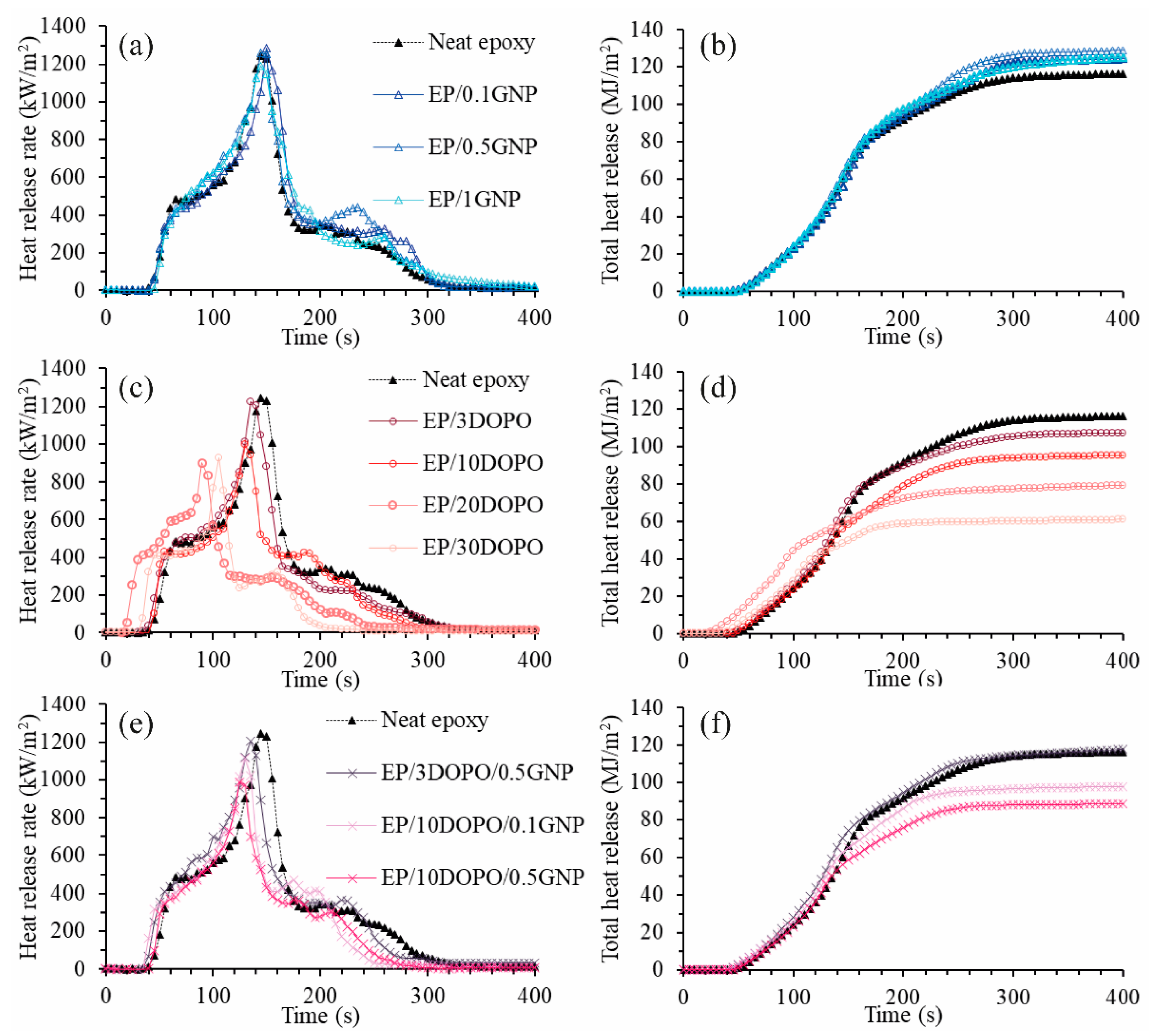
3.7. Flame Retardancy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascault, J.-P.; Williams, R.J.J. Epoxy Polymers: New Materials and Innovations, 1st ed.; Wiley: Weinheim, Germany, 2010; pp. 1–12. [Google Scholar]
- Saba, N.; Jawaid, M.; Paridah, M.T.; Al-othman, O.Y. A review on flammability of epoxy polymer, cellulosic and non-cellulosic fiber reinforced epoxy composites. Polym. Adv. Technol. 2016, 27, 577–590. [Google Scholar] [CrossRef]
- De Nograro, F.F.; Guerrero, P.; Corcuera, M.A.; Mondragon, I. Effects of chemical structure of hardener on curing evolution and on the dynamic mechanical behavior of epoxy resins. J. Appl. Polym. Sci. 1995, 56, 177–192. [Google Scholar] [CrossRef]
- Teh, P.L.; Mariatti, M.; Wagiman, A.N.R.; Beh, K.S. Effect of curing agent on the properties of mineral silica filled epoxy composites. Polym. Compos. 2008, 29, 27–36. [Google Scholar] [CrossRef]
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- Gu, J.; Dang, J.; Wu, Y.; Xie, C.; Han, Y. Flame-Retardant, Thermal, Mechanical and Dielectric Properties of Structural Non-Halogenated Epoxy Resin Composites. Polym. Plast. Technol. Eng. 2012, 51, 1198–1203. [Google Scholar] [CrossRef]
- Schäfer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Döring, M. Synthesis and properties of flame-retardant epoxy resins based on DOPO and one of its analog DPPO. J. Appl. Polym. Sci. 2007, 105, 685–696. [Google Scholar] [CrossRef]
- Schartel, B.; Balabanovich, A.I.; Braun, U.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Pyrolysis of epoxy resins and fire behavior of epoxy resin composites flame-retarded with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide additives. J. Appl. Polym. Sci. 2007, 104, 2260–2269. [Google Scholar] [CrossRef]
- Hussain, M.; Varley, R.J.; Zenka, M.; Simon, G.P. Synthesis, Thermal Behavior, and Cone Calorimetry of Organophosphorus Epoxy Materials. J. Appl. Polym. Sci. 2003, 90, 3696–3707. [Google Scholar] [CrossRef]
- Ciesielski, M.; Burk, B.; Heinzmann, C.; Döring, M. Fire-retardant high-performance epoxy-based materials. In Novel Fire Retardant Polymers and Composite Materials, 1st ed.; Wang, D.-Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–51. [Google Scholar]
- Ciesielski, M.; Diederichs, J.; Döring, M.; Schäfer, A. Advanced Flame-Retardant Epoxy Resins for Composite Materials. In Fire and Polymers V: Materials and Concepts for Fire Retardancy, 1st ed.; Wilkie, C.A., Nelson, G.L., Eds.; American Chemical Society: Washington, DC, USA, 2009; Volume 1013, pp. 174–190. [Google Scholar]
- Wang, X.; Song, L.; Pornwannchai, W.; Hu, Y.; Kandola, B. The effect of graphene presence in flame retarded epoxy resin matrix on the mechanical and flammability properties of glass fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 88–96. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Seidel, C.; Schulte, K. Preparation and characterization of graphite nano-platelet (GNP)/epoxy nano-composite: Mechanical, electrical and thermal properties. Eur. Polym. J. 2013, 49, 3878–3888. [Google Scholar] [CrossRef]
- Chatterjee, S.; Wang, J.W.; Kuo, W.S.; Tai, N.H.; Salzmann, C.; Li, W.L.; Hollertz, R.; Nüesch, F.A.; Chu, B.T.T. Mechanical reinforcement and thermal conductivity in expanded graphene nanoplatelets reinforced epoxy composites. Chem. Phys. Lett. 2012, 531, 6–10. [Google Scholar] [CrossRef]
- Wajid, A.S.; Ahmed, H.S.T.; Das, S.; Irin, F.; Jankowski, A.F.; Green, M.J. High-Performance Pristine Graphene/Epoxy Composites With Enhanced Mechanical and Electrical Properties. Macromol. Mater. Eng. 2013, 298, 339–347. [Google Scholar] [CrossRef]
- Ma, J.; Meng, Q.; Zaman, I.; Zhu, S.; Michelmore, A.; Kawashima, N.; Wang, C.H.; Kuan, H.-C. Development of polymer composites using modified, high-structural integrity graphene platelets. Compos. Sci. Technol. 2014, 91, 82–90. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Mechanical properties and thermal conductivity of graphene nanoplatelet/epoxy composites. J. Mater. Sci. 2015, 50, 1082–1093. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Dai, W.; Song, Y.; Wang, D.; Zeng, L.; Jiang, N. Enhanced thermal and electrical properties of epoxy composites reinforced with graphene nanoplatelets. Polym. Compos. 2015, 36, 556–565. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.C.; Bailey, C.G.; Yuen, R.K.K.; Parkin, J.; Yang, W.; Valles, C. Quantifying effects of graphene nanoplatelets on slowing down combustion of epoxy composites. Compos. Part B Eng. 2018, 146, 76–87. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Flame retardancy through carbon nanomaterials: Carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym. Degrad. Stab. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Liu, S.; Yan, H.; Fang, Z.; Wang, H. Effect of graphene nanosheets on morphology, thermal stability and flame retardancy of epoxy resin. Compos. Sci. Technol. 2014, 90, 40–47. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.; Li, X.; Yu, L.; Zhang, Z. Graphene-based flame retardants: A review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Wang, H. Superior flame retardancy of epoxy resin by the combined addition of graphene nanosheets and DOPO. RSC Adv. 2016, 6, 5288–5295. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Wang, C.S.; Lin, C.H. Synthesis and Properties of Phosphorus-Containing Epoxy. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3903–3909. [Google Scholar] [CrossRef]
- Lin, C.H.; Wang, C.S. Novel phosphorus-containing epoxy resins Part I. Synthesis and properties. Polymer 2001, 42, 1869–1878. [Google Scholar] [CrossRef]
- Schlagenhauf, L.; Chu, B.T.T.; Buha, J.; Nüesch, F.; Wang, J. Release of Carbon Nanotubes from an Epoxy-Based Nanocomposite during an Abrasion Process. Environ. Sci. Technol. 2012, 46, 7366–7372. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). ISO 178: 2001 Plastics—Determination of flexural properties. In ISO Standards; ISO Central Secretariat: Geneva, Switzerland, 2003. [Google Scholar]
- Tang, L.C.; Wan, Y.J.; Yan, D.; Pei, Y.B.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Hollertz, R.; Chatterjee, S.; Gutmann, H.; Geiger, T.; Nüesch, F.A.; Chu, B.T.T. Improvement of toughness and electrical properties of epoxy composites with carbon nanotubes prepared by industrially relevant processes. Nanotechnology 2011, 22, 125702. [Google Scholar] [CrossRef]
- Ma, P.C.; Mo, S.Y.; Tang, B.Z.; Kim, J.K. Dispersion, interfacial interaction and re-agglomeration of functionalized carbon nanotubes in epoxy composites. Carbon 2010, 48, 1824–1834. [Google Scholar] [CrossRef]
- International Electrotechnical Commission (IEC). IEC 61340-5-1:2016 Electrostatics—Part 5-1: Protection of electronic devices from electrostatic phenomena—General requirements. In IEC standards; IEC Central Office: Geneva, Switzerland, 2016. [Google Scholar]
- Zaman, I.; Kuan, H.-C.; Dai, J.; Kawashima, N.; Michelmore, A.; Sovi, A.; Dong, S.; Luong, L.; Ma, J. From carbon nanotubes and silicate layers to graphene platelets for polymer nanocomposites. Nanoscale 2012, 4, 4578–4586. [Google Scholar] [CrossRef]
- Matzui, L.Y.; Vovchenko, L.L.; Perets, Y.S.; Lazarenko, O.A. Electrical conductivity of epoxy resin filled with graphite nanoplatelets and boron nitride. Materwiss. Werksttech. 2013, 44, 254–258. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Huang, Y.; Ma, Y.; Liu, Z.; Cai, J.; Zhang, C.; Gao, H.; Chen, Y. Electromagnetic interference shielding of graphene/epoxy composites. Carbon 2009, 47, 922–925. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, H.; Zhao, Z.; Araby, S.; Lu, S.; Ma, J. Free-standing, flexible, electrically conductive epoxy/graphene composite films. Compos. Part A Appl. Sci. Manuf. 2017, 92, 42–50. [Google Scholar] [CrossRef]
- Min, C.; Yu, D.; Cao, J.; Wang, G.; Feng, L. A graphite nanoplatelet/epoxy composite with high dielectric constant and high thermal conductivity. Carbon 2013, 55, 116–125. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Porwal, H.; Huang, Z.; Bilotti, E.; Peijs, T. Mechanical, electrical and thermal properties of in-situ exfoliated graphene/epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 95, 229–236. [Google Scholar] [CrossRef]
- Chen, G.; Weng, W.; Wu, D.; Wu, C. PMMA/graphite nanosheets composite and its conducting properties. Eur. Polym. J. 2003, 39, 2329–2335. [Google Scholar] [CrossRef]
- Li, J.; Kim, J.K. Percolation threshold of conducting polymer composites containing 3D randomly distributed graphite nanoplatelets. Compos. Sci. Technol. 2007, 67, 2114–2120. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. ‘Cure Index’ for thermoset composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Ureña, A. Advantages and disadvantages of the addition of graphene nanoplatelets to epoxy resins. Eur. Polym. J. 2014, 61, 206–214. [Google Scholar] [CrossRef]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites—A Review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Garcia, F.G.; Soares, B.G.; Pita, V.J.R.R.; Sánchez, R.; Rieumont, J. Mechanical properties of epoxy networks based on DGEBA and aliphatic amines. J. Appl. Polym. Sci. 2007, 106, 2047–2055. [Google Scholar] [CrossRef]
- Matsumoto, A.; Hasegawa, K.; Fukuda, A.; Pae, J.-S. Properties of epoxy resin cured by phenol novolac/4-hydroxyphenylmaleimide polymer blend hardeners. Polym. Int. 1993, 31, 275–282. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Wang, J.; Zhang, B. Synergistic flame-retardant effect of expandable graphite and phosphorus-containing compounds for epoxy resin: Strong bonding of different carbon residues. Polym. Degrad. Stab. 2016, 128, 89–98. [Google Scholar] [CrossRef]
- Layek, R.K.; Samanta, S.; Chatterjee, D.P.; Nandi, A.K. Physical and mechanical properties of poly(methyl methacrylate) -functionalized graphene/poly(vinylidine fluoride) nanocomposites: Piezoelectric β polymorph formation. Polymer 2010, 51, 5846–5856. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Rittigstein, P.; Priestley, R.D.; Broadbelt, L.J.; Torkelson, J.M. Model polymer nanocomposites provide an understanding of confinement effects in real nanocomposites. Nat. Mater. 2007, 6, 278–282. [Google Scholar] [CrossRef]
- Vahabi, H.; Kandola, B.; Saeb, M. Flame Retardancy Index for Thermoplastic Composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef]
- Laachachi, A.; Burger, N.; Apaydin, K.; Sonnier, R.; Ferriol, M. Is expanded graphite acting as flame retardant in epoxy resin? Polym. Degrad. Stab. 2015, 117, 22–29. [Google Scholar] [CrossRef]
- Genovese, A.; Shanks, R.A. Fire performance of poly(dimethyl siloxane) composites evaluated by cone calorimetry. Compos. Part A Appl. Sci. Manuf. 2008, 39, 398–405. [Google Scholar] [CrossRef]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Flame Retardant Epoxy Composites on the Road of Innovation: An Analysis with Flame Retardancy Index for Future Development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

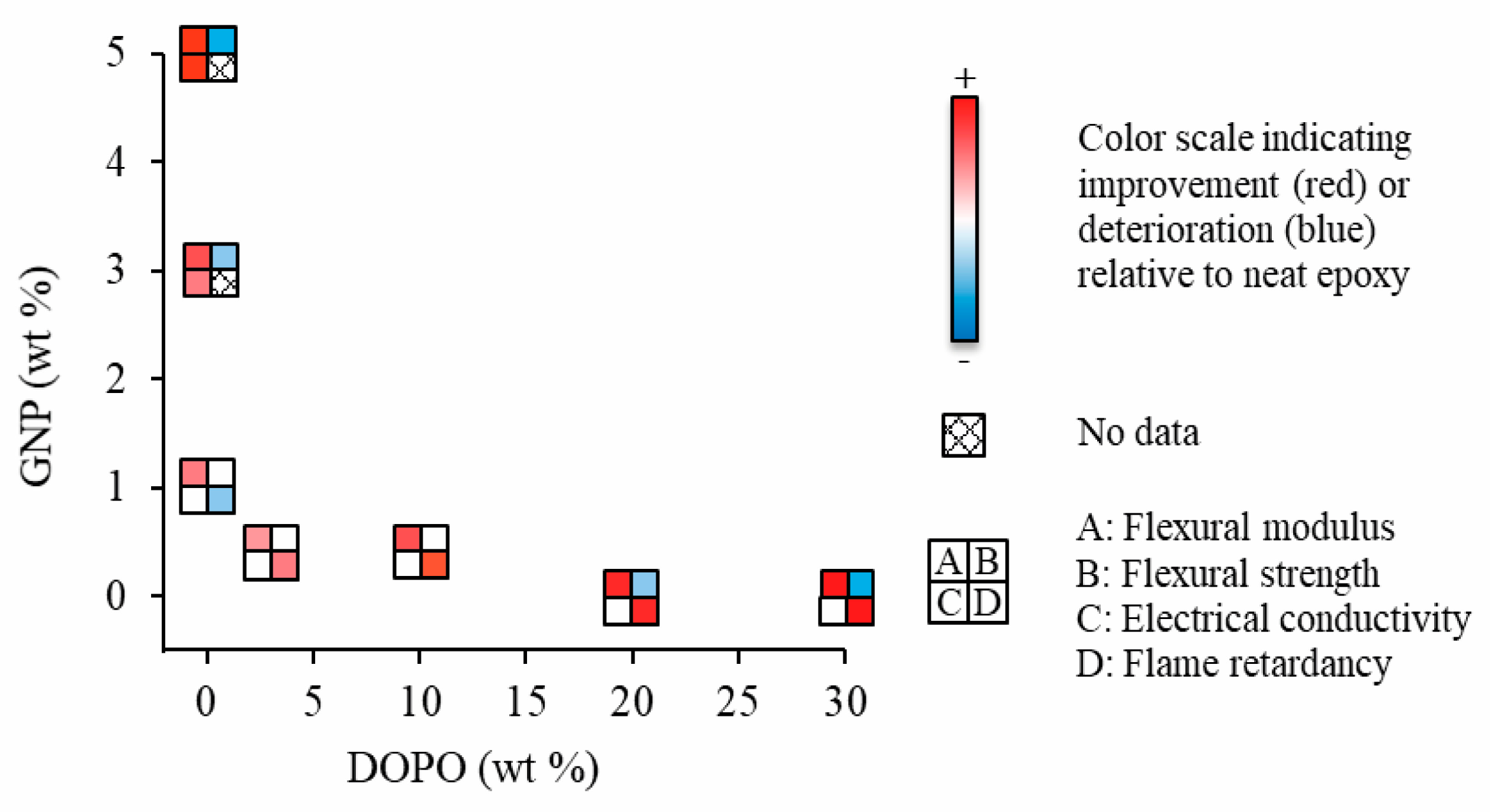
| Samples | E’ at Peak Temperature of tan δ + 40 °C (MPa) | ρ (mol·m−3) |
|---|---|---|
| Neat epoxy | 7.67 | 751.5 |
| EP/10DOPO | 6.36 | 623.2 |
| Samples | Tg (°C) | Td (°C) | T−50% (°C) | Tmax (°C) | % Char |
|---|---|---|---|---|---|
| DOPO | N.A. | 279 | 329 | 333 | 0 |
| Neat epoxy | 98.0 | 369 | 389 | 387 | 2.88 |
| EP/0.1GNP | 91.8 | 370 | 389 | 386 | 5.07 |
| EP/0.5GNP | 95.1 | 368 | 389 | 381 | 5.06 |
| EP/1GNP | 95.8 | 366 | 389 | 385 | 4.31 |
| EP/3DOPO | 94.3 | 355 | 379 | 374 | 4.76 |
| EP/10DOPO | 87.4 | 339 | 369 | 370 | 4.92 |
| EP/20DOPO | 83.3 | 325 | 366 | 360 | 7.69 |
| EP/30DOPO | 72.8 | 319 | 363 | 357 | 7.80 |
| EP/3DOPO/0.5GNP | 94.1 | 353 | 378 | 369 | 6.32 |
| EP/10DOPO/0.1GNP | 88.1 | 340 | 369 | 362 | 5.81 |
| EP/10DOPO/0.5GNP | 84.9 | 338 | 369 | 368 | 6.14 |
| Samples | TTI (s) | pHRR (kW·m−2) | Ave-HRR (300s) (kW·m−2) | Ave-EHC (300 s) (MJ·kg) | THR (MJ·m−²) | TSP (m2) | Ave- CO yield (kg·kg−1) | Ave- CO2 yield (kg·kg−1) | FRI (-) |
|---|---|---|---|---|---|---|---|---|---|
| Neat epoxy | 34 | 1246 | 384 | 21.3 | 117 | 30.3 | 0.05 | 1.6 | - |
| EP/0.1GNP | 40 | 1285 | 411 | 23.9 | 125 | 34.0 | 0.06 | 1.7 | 1.1 |
| EP/0.5GNP | 38 | 1263 | 425 | 24.8 | 130 | 33.7 | 0.06 | 1.8 | 1.0 |
| EP/1GNP | 42 | 1189 | 410 | 24.0 | 126 | 33.4 | 0.06 | 1.7 | 1.2 |
| EP/3DOPO | 28 | 1226 | 355 | 22.0 | 108 | 37.1 | 0.08 | 1.5 | 0.9 |
| EP/10DOPO | 37 | 996 | 315 | 19.5 | 96 | 36.4 | 0.11 | 1.3 | 1.7 |
| EP/20DOPO | 18 | 902 | 259 | 13.6 | 81 | 39.2 | 0.10 | 0.7 | 1.1 |
| EP/30DOPO | 29 | 927 | 201.1 | 13.9 | 60.1 | 35.7 | 0.10 | 0.8 | 2.3 |
| EP/3DOPO/0.5GNP | 34 | 1208 | 385.4 | 23.2 | 118 | 49.4 | 0.12 | 1.7 | 1.0 |
| EP/10DOPO/0.1GNP | 31 | 1116 | 323.7 | 20.9 | 98.4 | 38.5 | 0.12 | 1.3 | 1.2 |
| EP/10DOPO/0.5GNP | 38 | 989 | 294.2 | 19.1 | 89.3 | 35.5 | 0.11 | 1.3 | 1.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netkueakul, W.; Fischer, B.; Walder, C.; Nüesch, F.; Rees, M.; Jovic, M.; Gaan, S.; Jacob, P.; Wang, J. Effects of Combining Graphene Nanoplatelet and Phosphorous Flame Retardant as Additives on Mechanical Properties and Flame Retardancy of Epoxy Nanocomposite. Polymers 2020, 12, 2349. https://doi.org/10.3390/polym12102349
Netkueakul W, Fischer B, Walder C, Nüesch F, Rees M, Jovic M, Gaan S, Jacob P, Wang J. Effects of Combining Graphene Nanoplatelet and Phosphorous Flame Retardant as Additives on Mechanical Properties and Flame Retardancy of Epoxy Nanocomposite. Polymers. 2020; 12(10):2349. https://doi.org/10.3390/polym12102349
Chicago/Turabian StyleNetkueakul, Woranan, Beatrice Fischer, Christian Walder, Frank Nüesch, Marcel Rees, Milijana Jovic, Sabyasachi Gaan, Peter Jacob, and Jing Wang. 2020. "Effects of Combining Graphene Nanoplatelet and Phosphorous Flame Retardant as Additives on Mechanical Properties and Flame Retardancy of Epoxy Nanocomposite" Polymers 12, no. 10: 2349. https://doi.org/10.3390/polym12102349
APA StyleNetkueakul, W., Fischer, B., Walder, C., Nüesch, F., Rees, M., Jovic, M., Gaan, S., Jacob, P., & Wang, J. (2020). Effects of Combining Graphene Nanoplatelet and Phosphorous Flame Retardant as Additives on Mechanical Properties and Flame Retardancy of Epoxy Nanocomposite. Polymers, 12(10), 2349. https://doi.org/10.3390/polym12102349