Investigation of the Effects of Adsorbed Water on Adhesion Energy and Nanostructure of Asphalt and Aggregate Surfaces Based on Molecular Dynamics Simulation
Abstract
1. Introduction
2. Models and Simulation Methods
2.1. Asphalt Binder Model
2.2. Aggregate Mineral Model
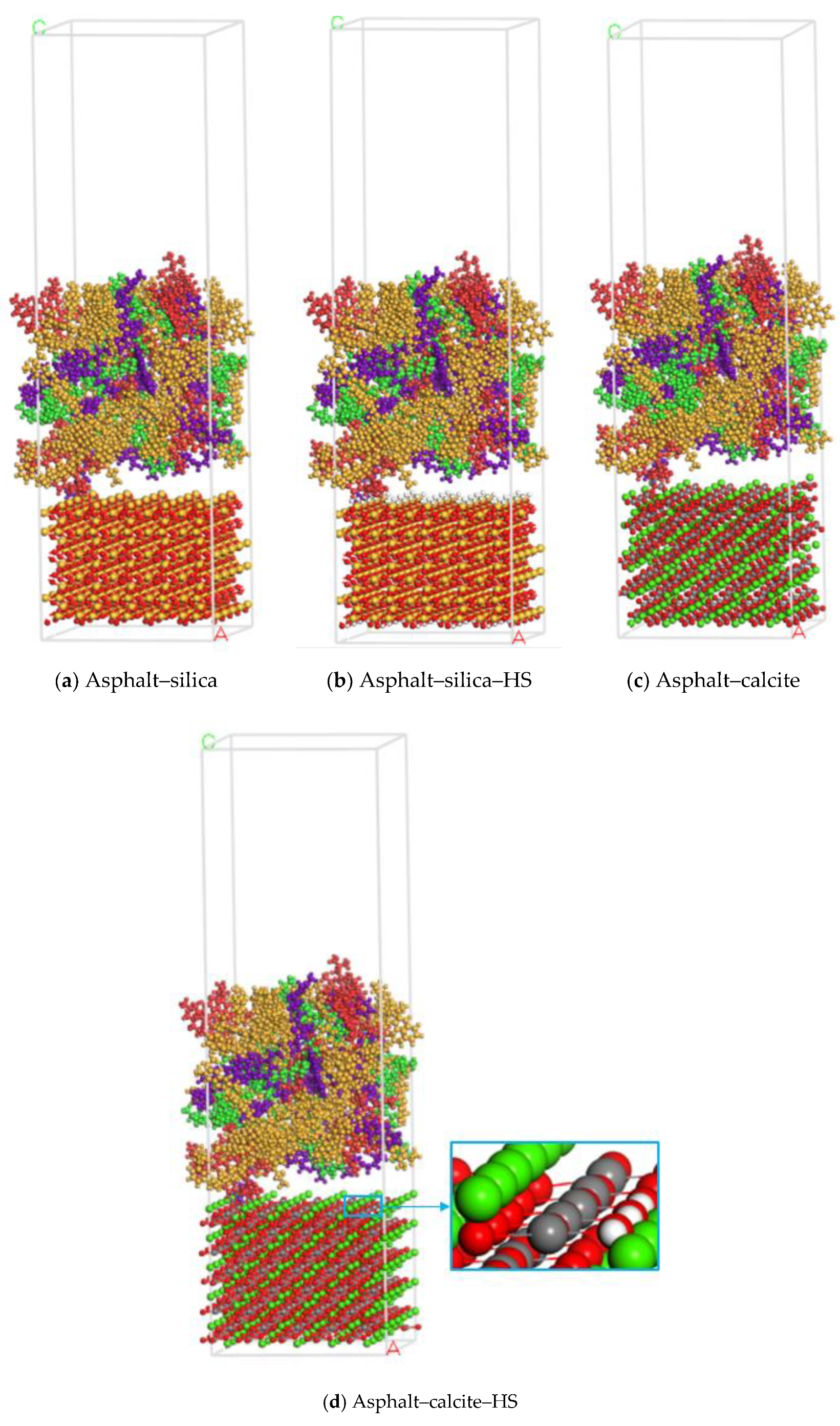
2.3. Asphalt–Aggregate Mineral System Model
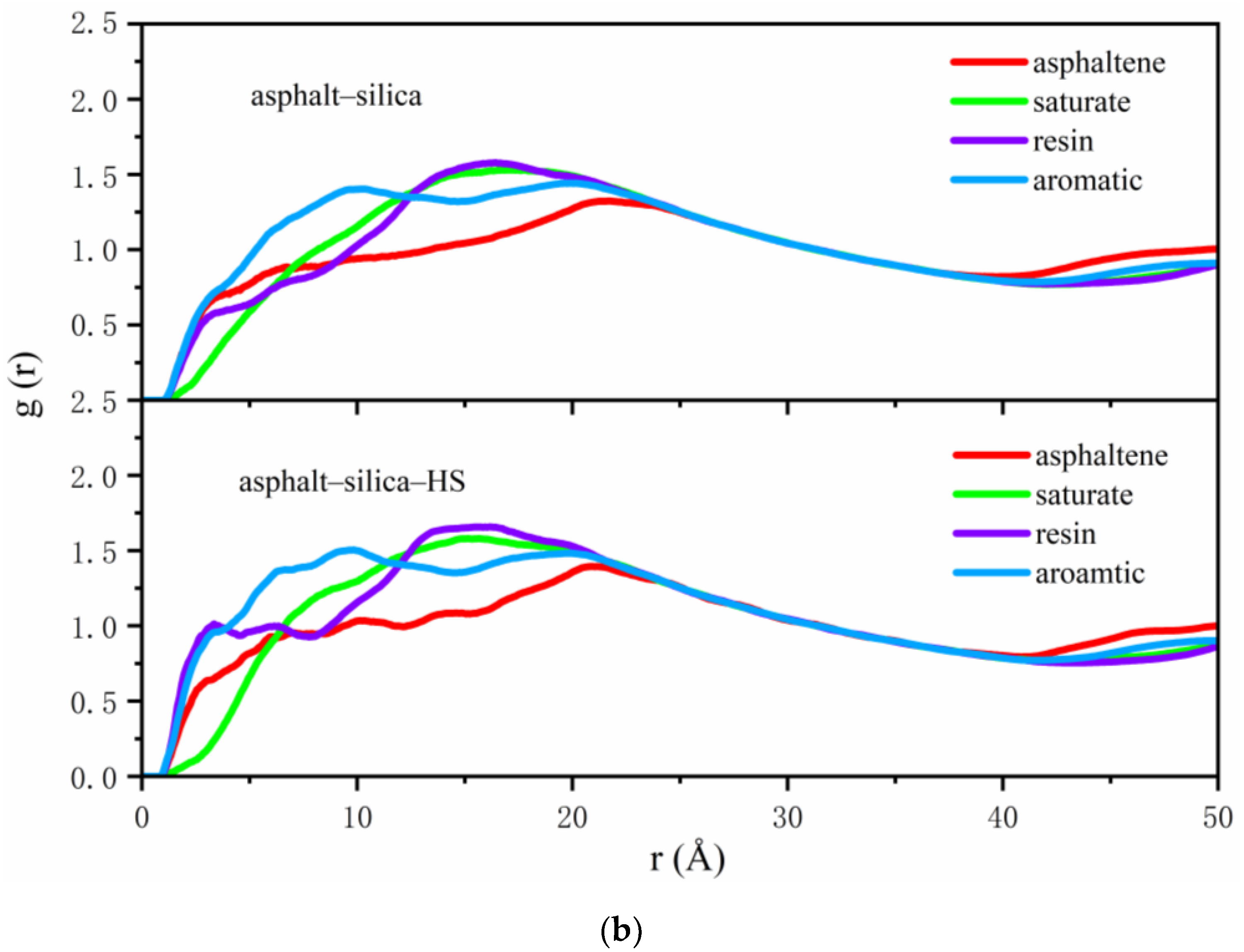
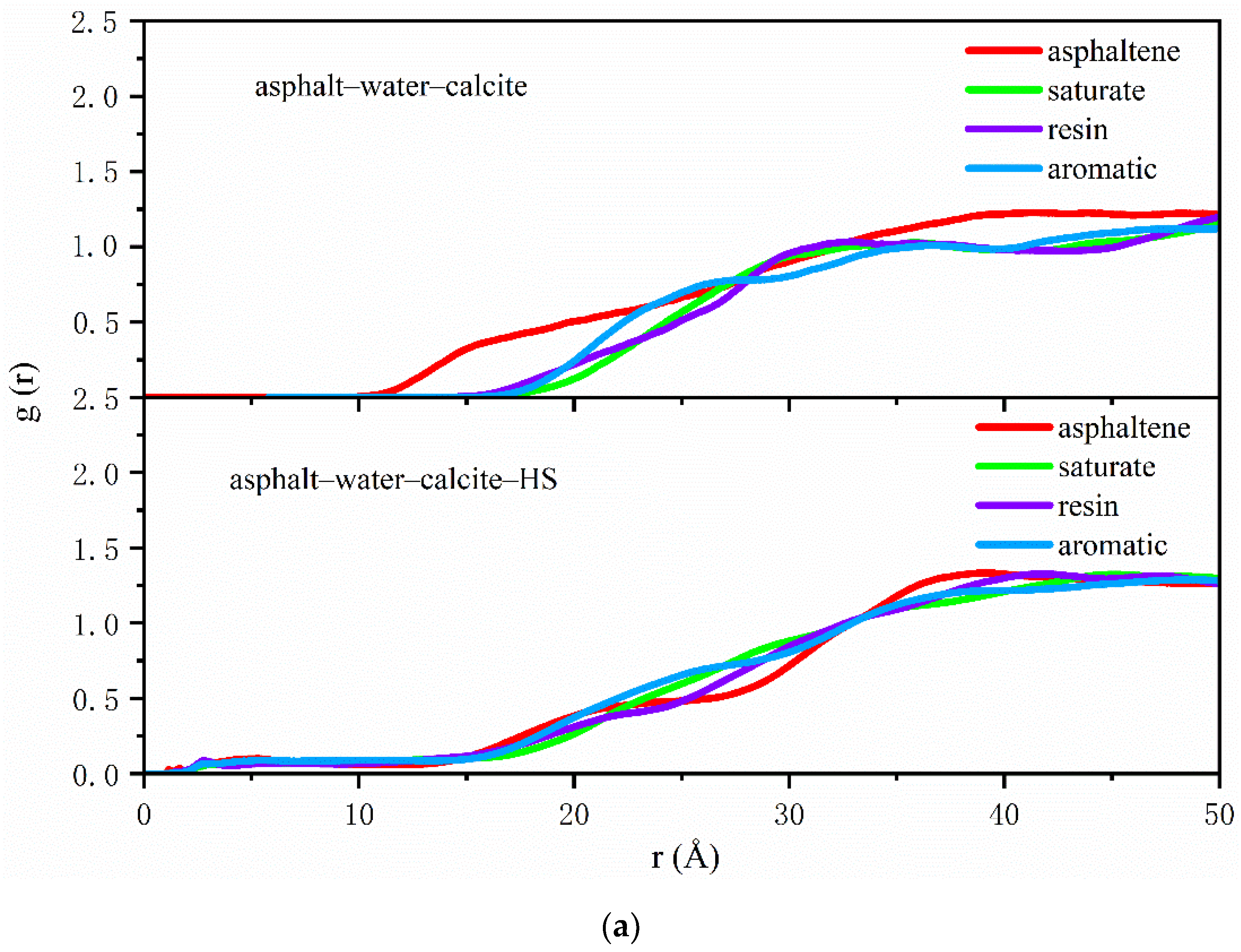
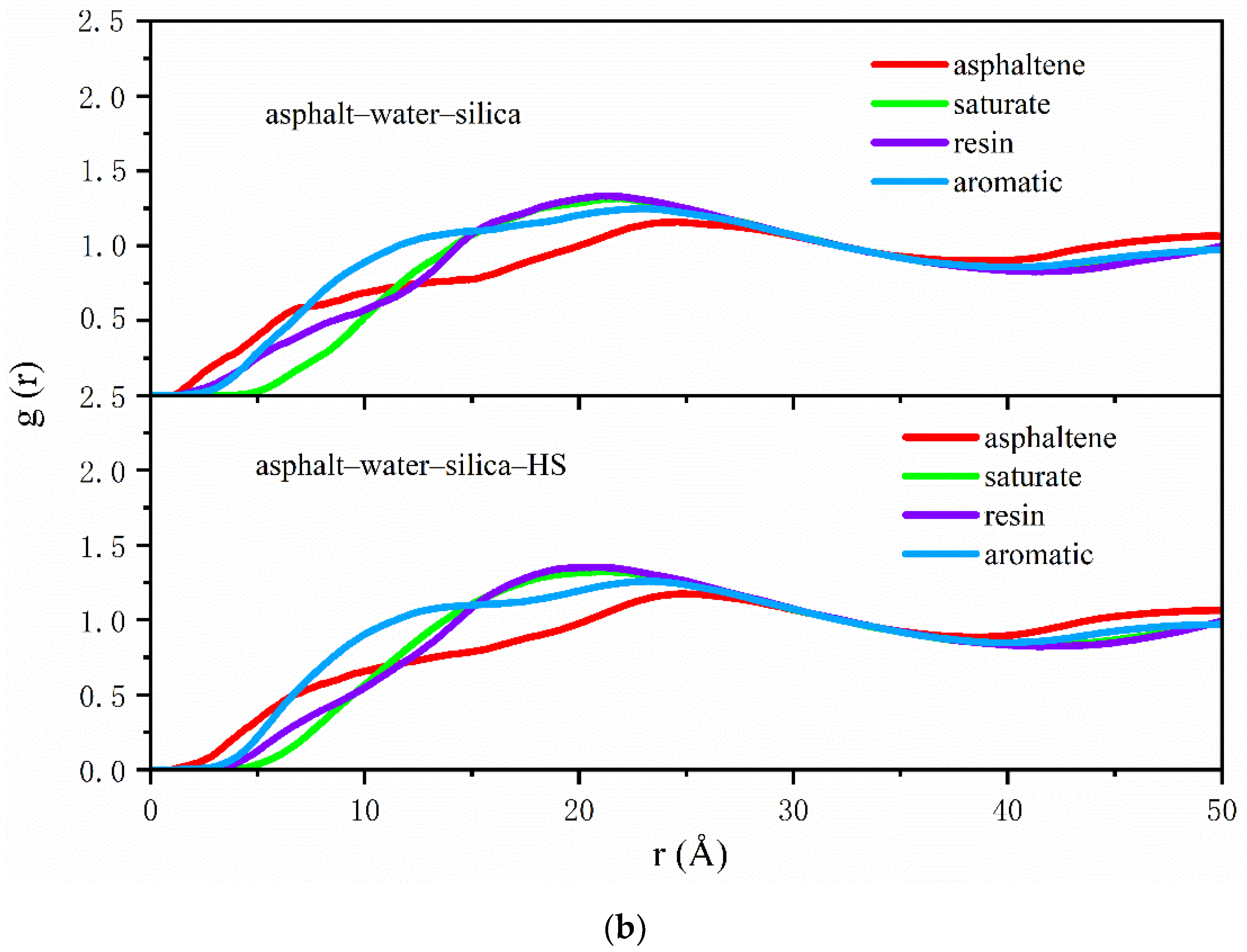
2.4. Radial Distribution Function
2.5. Interfacial Adhesion Energy and Debonding Energy
3. Results and Discussion
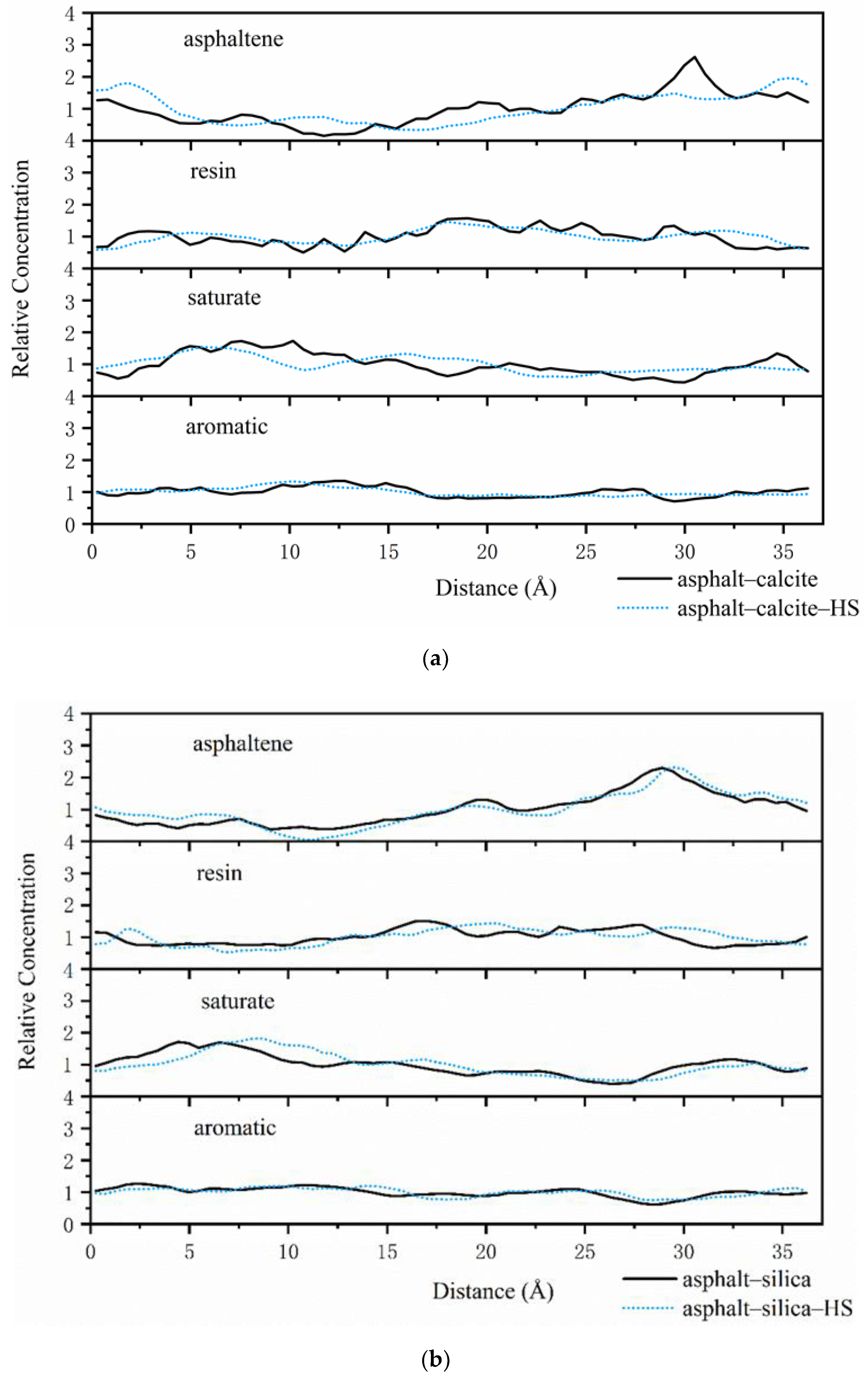
3.1. Distribution of Asphalt Compositions on Aggregates
3.2. Adhesion Energy Between the Aggregate and Asphalt
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aigner, E.; Lackner, R.; Pichler, C. Multiscale prediction of viscoelastic properties of asphalt concrete. J. Mater. Civ. Eng. 2009, 21, 771–780. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, F.; Pei, J.; Xu, S.; An, F. Viscosity-temperature characteristics of warm mix asphalt binder with Sasobit. Constr. Build. Mater. 2015, 78, 34–39. [Google Scholar] [CrossRef]
- Guo, F.; Pei, J.; Zhang, J. Study on the adhesion property between asphalt binder and aggregate: A state-of-the-art review. Constr. Build. Mater. 2020, 256, 119474. [Google Scholar] [CrossRef]
- Xu, T.; Wang, H.; Li, Z.D.; Zhao, Y.L. Evaluation of permanent deformation of asphalt mixtures using different laboratory performance tests. Constr. Build. Mater. 2014, 53, 561–567. [Google Scholar] [CrossRef]
- Azarhoosh, A.R.; Moghadas, F.; Khodaii, A. The influence of cohesion and adhesion parameters on the fatigue life of hot mix asphalt. J. Adhes. 2017, 93, 1048–1067. [Google Scholar] [CrossRef]
- Wang, L.; Ren, M.; Xing, Y.; Chen, G. Study on affecting factors of interface crack for asphalt mixture based on microstructure. Constr. Build. Mater. 2017, 156, 1053–1062. [Google Scholar] [CrossRef]
- Chen, X.W.; Huang, B.S. Evaluation of moisture damage in hot mix asphalt using simple performance and superpave indirect tensile tests. Constr. Build. Mater. 2008, 22, 1950–1962. [Google Scholar] [CrossRef]
- Moraes, R.; Velasquez, R.; Bahia, H. Measuring the effect of moisture on asphalt-aggregate bond with the bitumen bond strength test. Transp. Res. Rec. 2011, 2209, 70–81. [Google Scholar] [CrossRef]
- Canestrari, F.; Cardone, F.; Graziani, A.; Santagata, F.A.; Bahia, H.U. Adhesive and cohesive properties of asphalt-aggregate systems subjected to moisture damage. Road Mater. Pavement Des. 2010, 11, 11–32. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Jia, M. Effect of short-term aging on interface-cracking behaviors of warm mix asphalt under dry and wet conditions. Constr. Build. Mater. 2020, 261, 119885. [Google Scholar]
- Jorge, L.O.; Lucas, J. Moisture-induced damage resistance, stiffness and fatigue life of asphalt mixtures with different aggregate-binder adhesion properties. Constr. Build. Mater. 2019, 216, 166–175. [Google Scholar]
- Fauzia, S.; Mujib, R.; Denis, C. Asphalt surface damage due to combined action of water and dynamic loading. Constr. Build. Mater. 2019, 196, 530–538. [Google Scholar]
- Teh, S.Y.; Meor, O.H. Asphalt mixture workability and effects of long-term conditioning methods on moisture damage susceptibility and performance of warm mix asphalt. Constr. Build. Mater. 2019, 207, 316–328. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Chemo-physical analysis and molecular dynamics (MD) simulation of moisture susceptibility of nano hydrated lime modified asphalt mixtures. Constr. Build. Mater. 2015, 101, 536–547. [Google Scholar]
- Xu, G.; Wang, H. Study of cohesion and adhesion properties of asphalt concrete with molecular dynamics simulation. Comput. Mater. Sci. 2016, 112, 161–169. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H. Moisture effect on nanostructure and adhesion energy of asphalt on aggregate surface: A molecular dynamics study. Appl. Surf. Sci. 2020, 510, 145435. [Google Scholar] [CrossRef]
- Luo, L.; Chu, L.; Fwa, T.F. Molecular dynamics analysis of moisture effect on asphalt-aggregate adhesion considering anisotropic mineral surfaces. Appl. Surf. Sci. 2020, 527, 146830. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Gu, F. Impact of minerals and water on bitumen-mineral adhesion and debonding behaviours using molecular dynamics simulations. Constr. Build. Mater. 2018, 171, 214–222. [Google Scholar] [CrossRef]
- Zou, J.; Reza, R.; Yuan, Y. Distribution of adsorbed water in shale: An experimental study on isolated kerogen and bulk shale samples. J. Petrol. Sci. Eng. 2020, 187, 106858. [Google Scholar] [CrossRef]
- Bojana, D.; Borut, M. Determining the thickness of adsorbed water layers on the external surfaces of clay minerals based on the engineering properties of soils. Appl. Clay Sci. 2016, 123, 279–284. [Google Scholar]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J. Advances in asphaltene science and the Yen-Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Claire, A.L.; Michael, L.G.; Jeppe, C.D.; Hansen, J.S. ROSE bitumen: Mesoscopic model of bitumen and bituminous mixtures. J. Chem. Phys. 2018, 149, 214901. [Google Scholar]
- Yao, H.; Dai, Q.; You, Z. Molecular dynamics simulation of physicochemical properties of the asphalt model. Fuel 2016, 164, 83–93. [Google Scholar] [CrossRef]
- Hansen, J.S.; Lemarchand, C.A.; Nielsen, E.; Dyre, J.C.; Schrøder, T. Four-component united-atom model of bitumen. J. Chem. Phys. 2013, 138, 094508. [Google Scholar] [CrossRef] [PubMed]
- Corbett, L.W. Composition of asphalt based on generic fractionation using solvent deasphaltening elution-adsorption chromatography and densimetric characterization. Anal. Chem. 1969, 41, 576. [Google Scholar] [CrossRef]
- Chu, L.; Luo, L.; Fwa, T.F. Effects of aggregate mineral surface anisotropy on asphalt-aggregate interfacial bonding using molecular dynamics (MD) simulation. Constr. Build. Mater. 2019, 225, 1–12. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Zhang, X.F.; Lu, G.W.; Wen, X.M.; Yang, H. Molecular dynamics investigation into the adsorption of oil-water-surfactant mixture on quartz. Appl. Surf. Sci. 2009, 255, 6493–6498. [Google Scholar]
- Fardin, K.; Rajesh, K. Glass Transition and Molecular Mobility in Styrene−Butadiene Rubber Modified Asphalt. J. Phys. Chem. B 2015, 119, 14261–14269. [Google Scholar]
- Fardin, K.; Rajesh, K. Molecular simulations of asphalt rheology: Application of time–temperature superposition principle. J. Rheol. 2018, 62, 941. [Google Scholar]
- Tabatabaee, H.A.; Velasquez, R.; Bahia, H.U. Modeling thermal stress in asphalt mixtures undergoing glass transition and physical hardening. Transp. Res. Rec. 2012, 2296, 106–114. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Molecular dynamics study of oxidative aging effect on asphalt binder properties. Fuel 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Z.; Tan, Y.; Liu, Z. Investigating the interactions of the saturate, aromatic, resin, and asphaltene four fractions in asphalt binders by molecular simulations. Energy Fuels 2015, 29, 112–121. [Google Scholar]
- Kawasaki, M.; Onuma, K.; Sunagawa, I. Morphological instabilities during growth of a rough interface: AFM observations of cobbles on the (0 0 0 1) face of synthetic quartz crystals. J. Cryst. Growth 2003, 258, 188–196. [Google Scholar] [CrossRef]
- Murgich, J.; Abanero, J.A.; Strausz, O.P. Molecular recognition in aggregates formed by asphaltene and resin molecules from the Athabasca oil sand. Energy Fuels 1999, 13, 278–286. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Lee, Y. Behavior and characteristics of amorphous calcium carbonate and calcite using CaCO3 film synthesis. Mater. Des. 2016, 112, 367–373. [Google Scholar] [CrossRef]
- Sulimai, N.H.; Rozina, A.R. Facile synthesis of CaCO3 and investigation on structural and optical properties of high purity crystalline calcite. Mater. Sci. Eng. B 2019, 243, 78–85. [Google Scholar] [CrossRef]
- Alghamdi, A.O.; Alotaibi, M.B.; Yousef, A.A. Atomistic simulation of calcite interaction with ionic species and oil components in water-flooding. Colloids Surf. A 2017, 529, 760–764. [Google Scholar] [CrossRef]
- Amir, H.; Mohammad, H. Impact of ionic composition on modulating wetting preference of calcite surface: Implication for chemically tuned water flooding. Colloids Surf. A 2019, 568, 470–480. [Google Scholar]
- Cui, B.; Gu, X.; Hu, D.; Dong, Q. A multiphysics evaluation of the rejuvenator effects on aged asphalt using molecular dynamics simulations. J. Clean. Prod. 2020, 259, 120629. [Google Scholar] [CrossRef]
- Long, Z.W.; You, L.Y. Analysis of interfacial adhesion properties of nano-silica modified asphalt mixtures using molecular dynamics simulation. Constr. Build. Mater. 2020, 255, 119354. [Google Scholar] [CrossRef]
- Liu, J.Z.; Yu, B.; Hong, Q.Z. Molecular dynamics simulation of distribution and adhesion of asphalt components on steel slag. Constr. Build. Mater. 2020, 255, 119332. [Google Scholar] [CrossRef]
- Luo, R.; Tu, C. Actual diffusivities and diffusion paths of water vapor in asphalt mixtures. Constr. Build. Mater. 2019, 207, 145–157. [Google Scholar] [CrossRef]
- Mostafa, V.; Mahmoud, A. Experimental investigation of effect of PP/SBR polymer blends on the moisture resistance and rutting performance of asphalt mixtures. Constr. Build. Mater. 2020, 253, 119197. [Google Scholar]
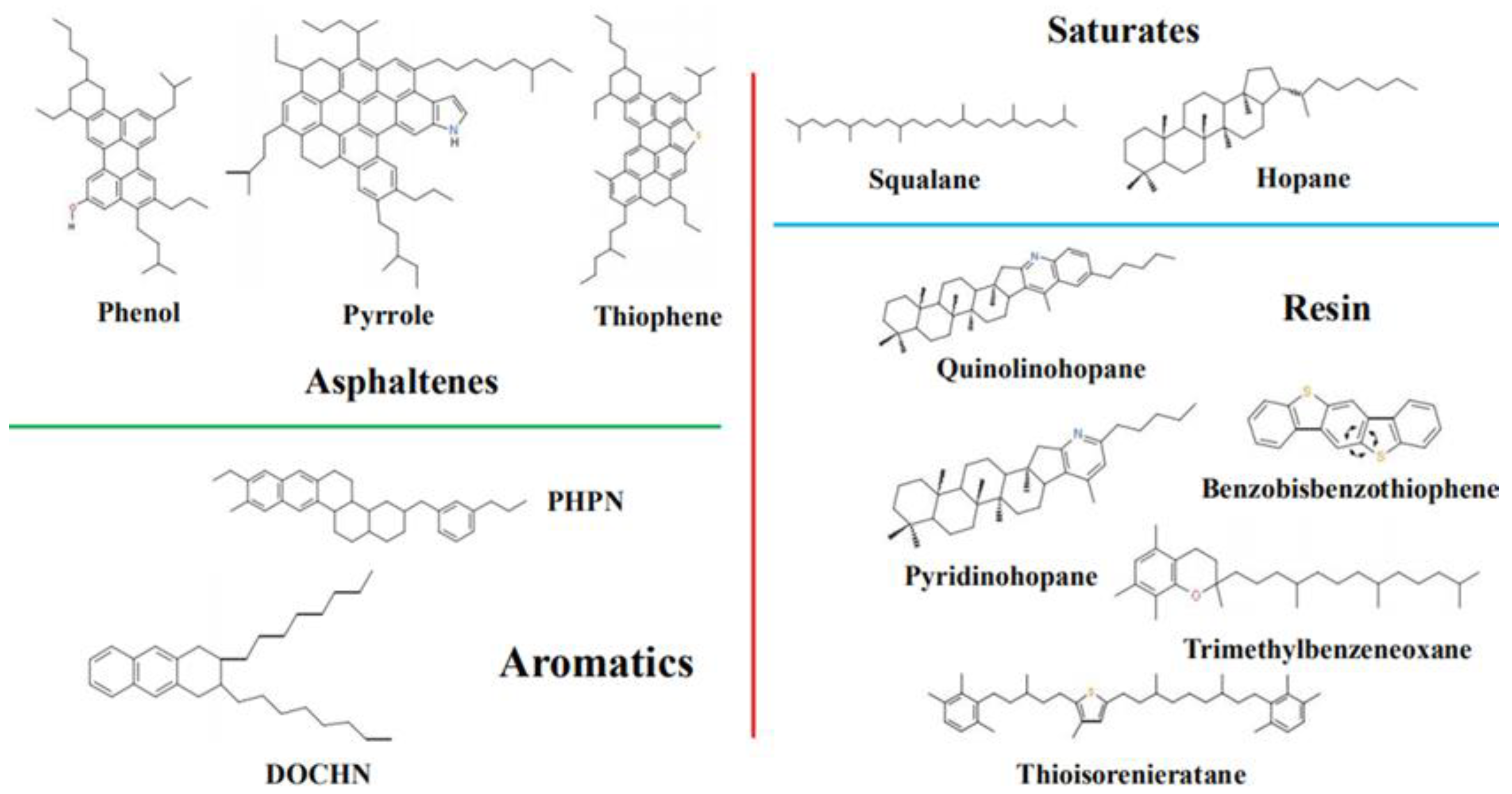





| Chemical Fractions | Molecules | Formula | Number | Mass Fraction (%) |
|---|---|---|---|---|
| Asphaltene | Phenol | C42H54O | 3 | 17.1 |
| Pyrrole | C66H81N | 2 | ||
| Thiophene | C51H62S | 2 | ||
| Saturate | Squalane | C30H62 | 4 | 16.0 |
| Hopane | C35H62 | 6 | ||
| Aromatic | PHPN | C35H44 | 13 | 43.6 |
| DOCHN | C30H46 | 16 | ||
| Resin | Quinolinohopane | C40H59N | 2 | 23.3 |
| Thioisorenieratane | C40H60S | 2 | ||
| Benzobisbenzothiophene | C18H10S2 | 9 | ||
| Pyridinohopane | C36H57N | 2 | ||
| Trimethylbenzeneoxane | C29H50O | 2 |
| Thermodynamic Properties | Simulation Calculation | Experimental Measurements [35] |
|---|---|---|
| Density (298.15 K, g/cm3) | 1.006 | 1.01–1.04 |
| Cohesive energy density (108 J/m3) | 3.19 | 3.19–3.32 |
| ((J/m3)1/2) | 17.84 | 13.30–22.50 |
| ((J/m3)1/2) | 17.321 | N/A |
| ((J/m3)1/2) | 1.496 | N/A |
| Models | (kcal/mol) | (kcal/mol) | (kcal/mol) | (kcal/mol) |
|---|---|---|---|---|
| Asphalt–Calcite | 7531.17 | −184,317.15 | −170,136.62 | −6649.36 |
| Asphalt–Calcite–HS | 7825.16 | −169,761.52 | −156,962.02 | −4974.34 |
| Asphalt–Silica | 6188.74 | −83,958.59 | −77,719.60 | −50.25 |
| Asphalt–Silica–HS | 6265.63 | −72,830.21 | −66,522.45 | −42.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Huang, W.; Hu, B.; Xie, J.; Xiao, Z.; Cai, X.; Wu, K. Investigation of the Effects of Adsorbed Water on Adhesion Energy and Nanostructure of Asphalt and Aggregate Surfaces Based on Molecular Dynamics Simulation. Polymers 2020, 12, 2339. https://doi.org/10.3390/polym12102339
Cui W, Huang W, Hu B, Xie J, Xiao Z, Cai X, Wu K. Investigation of the Effects of Adsorbed Water on Adhesion Energy and Nanostructure of Asphalt and Aggregate Surfaces Based on Molecular Dynamics Simulation. Polymers. 2020; 12(10):2339. https://doi.org/10.3390/polym12102339
Chicago/Turabian StyleCui, Wentian, Wenke Huang, Bei Hu, Jiawen Xie, Zhicheng Xiao, Xu Cai, and Kuanghuai Wu. 2020. "Investigation of the Effects of Adsorbed Water on Adhesion Energy and Nanostructure of Asphalt and Aggregate Surfaces Based on Molecular Dynamics Simulation" Polymers 12, no. 10: 2339. https://doi.org/10.3390/polym12102339
APA StyleCui, W., Huang, W., Hu, B., Xie, J., Xiao, Z., Cai, X., & Wu, K. (2020). Investigation of the Effects of Adsorbed Water on Adhesion Energy and Nanostructure of Asphalt and Aggregate Surfaces Based on Molecular Dynamics Simulation. Polymers, 12(10), 2339. https://doi.org/10.3390/polym12102339






