Fabrication and Potential Applications of Highly Durable Superhydrophobic Polyethylene Terephthalate Fabrics Produced by In-Situ Zinc Oxide (ZnO) Nanowires Deposition and Polydimethylsiloxane (PDMS) Packaging
Abstract
1. Introduction
2. Experimental
2.1. Materials
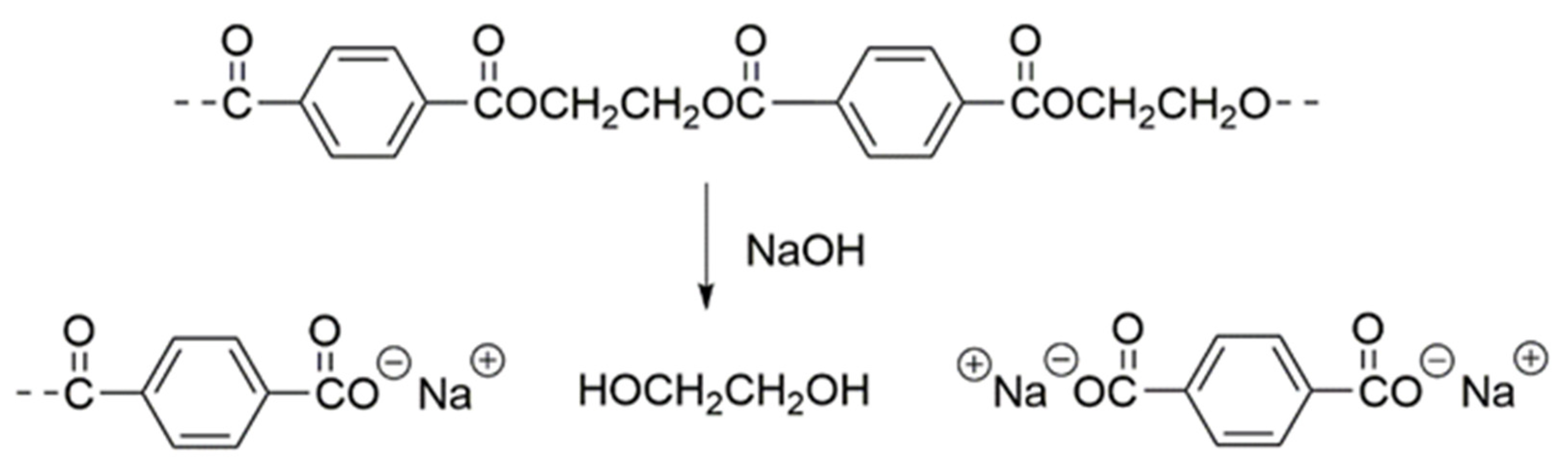
2.2. Preparation of PET-g-PMAPS
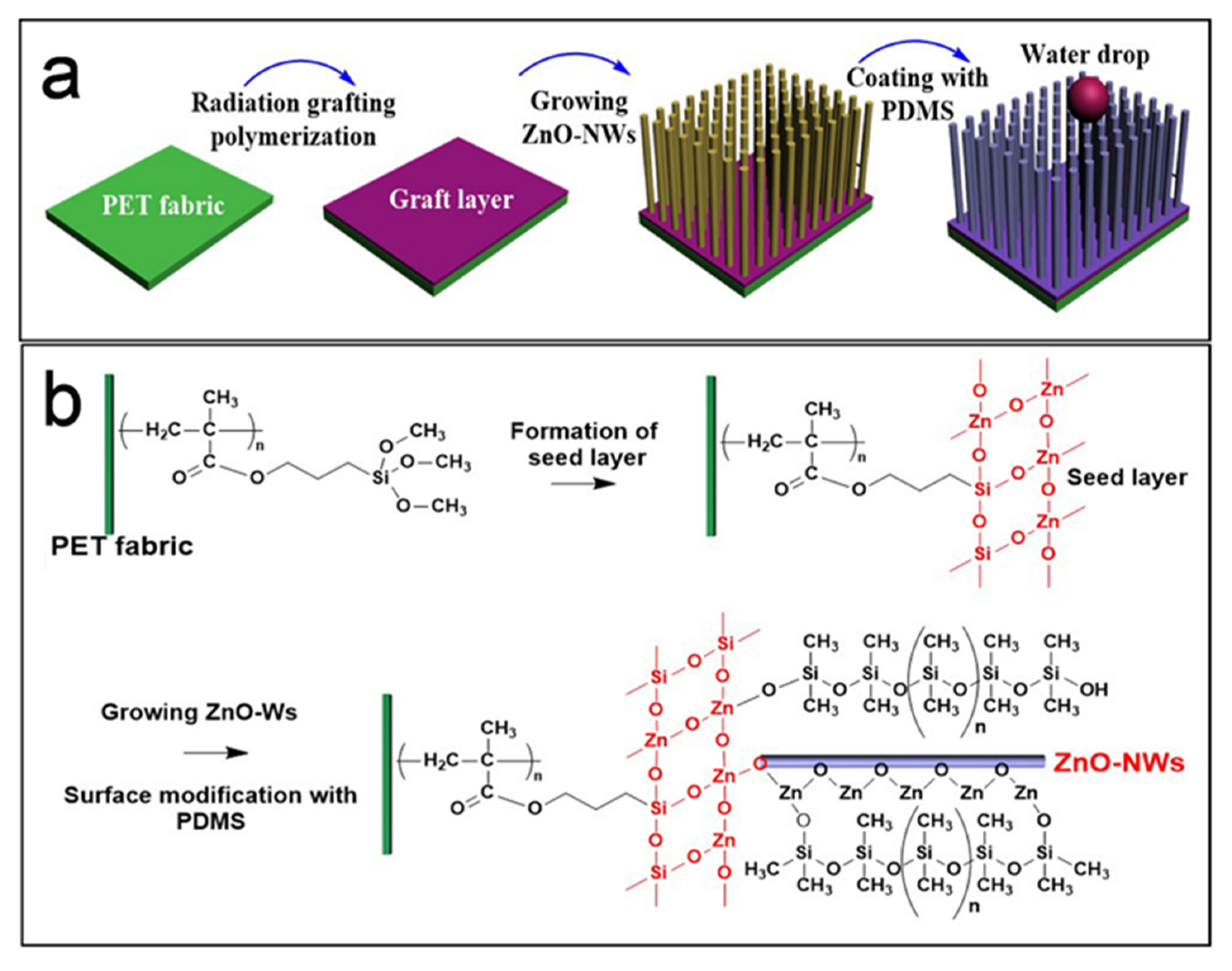
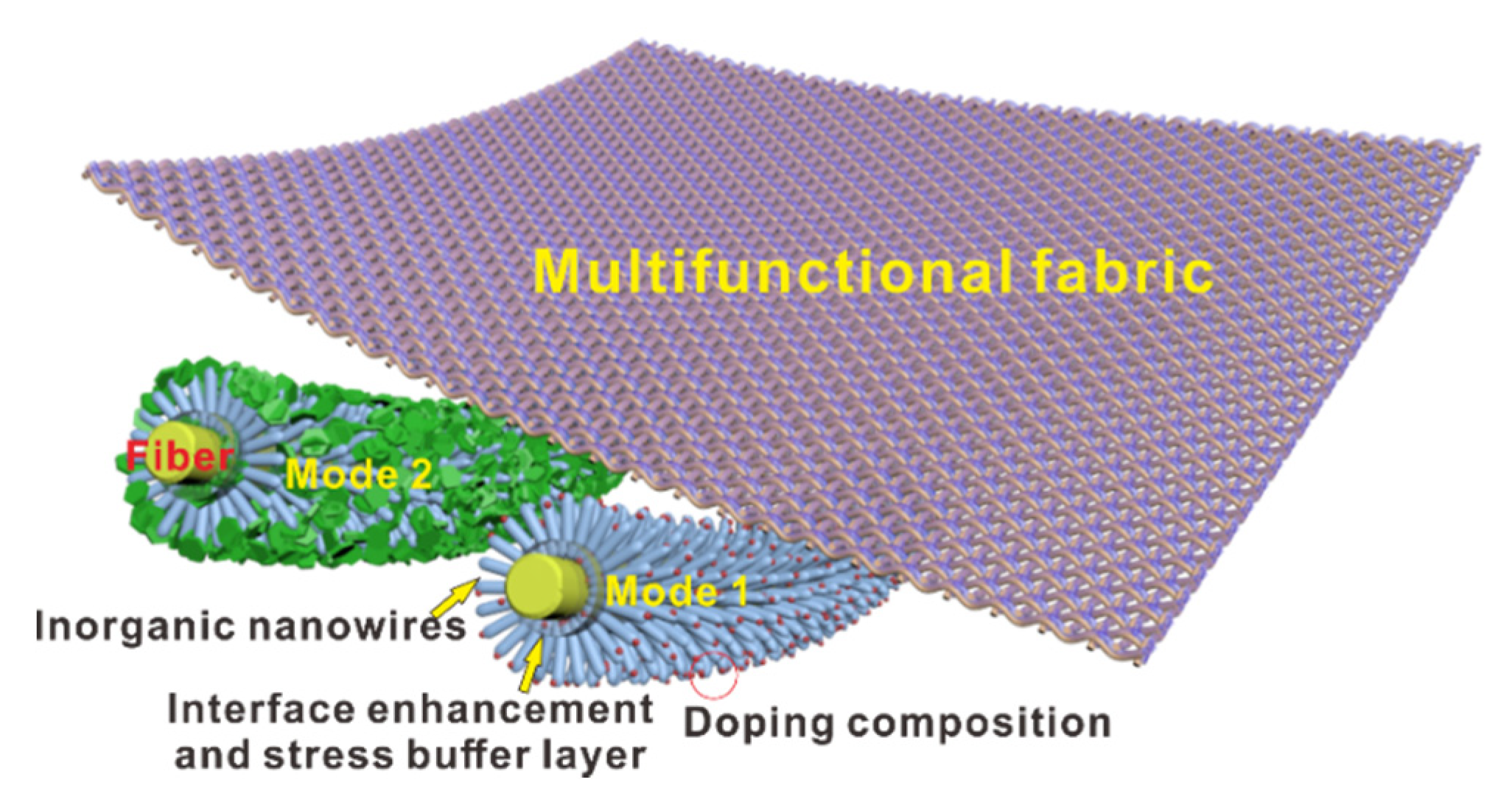
2.3. Preparation of PDMS@ZnO-NWs@PET
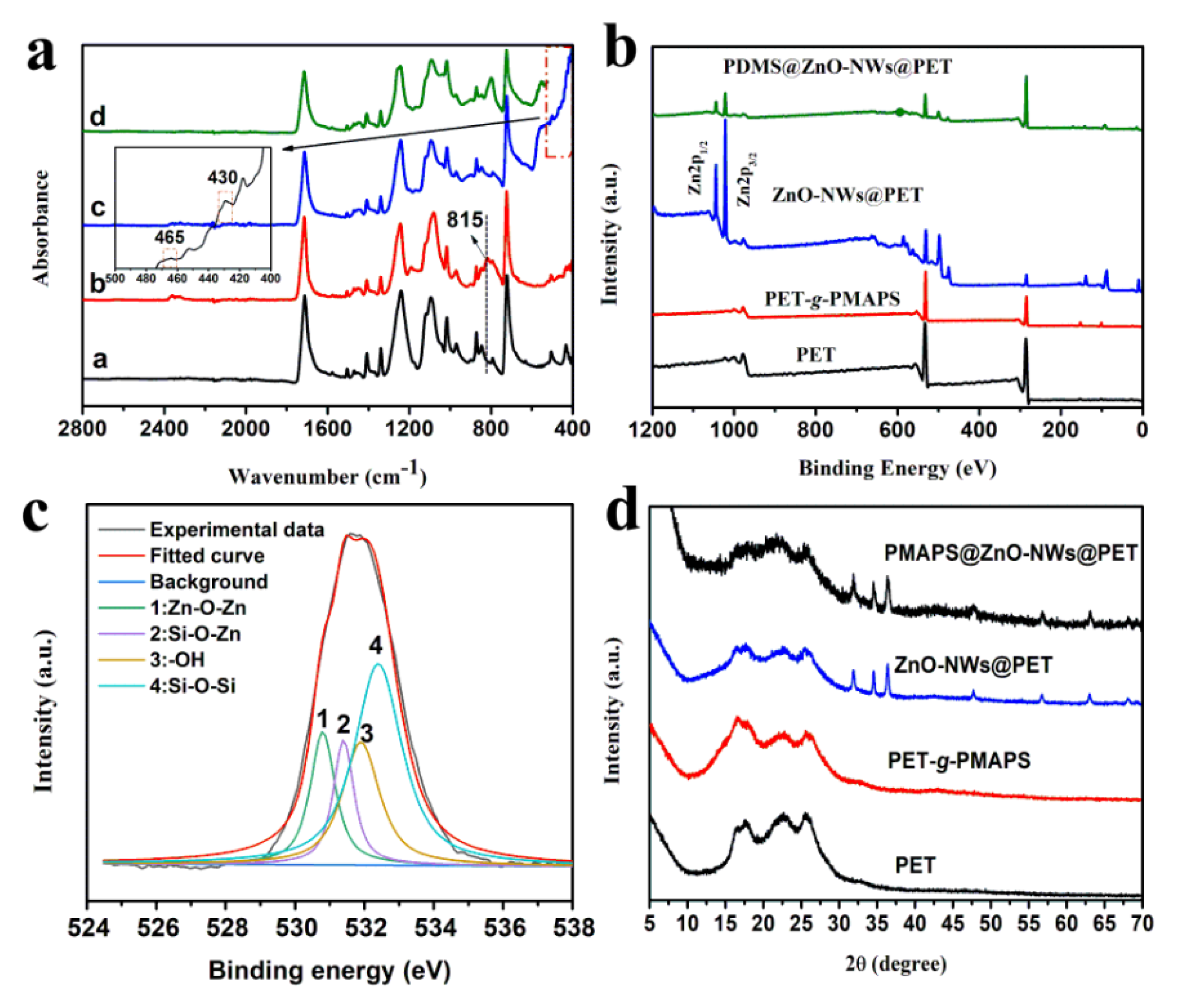
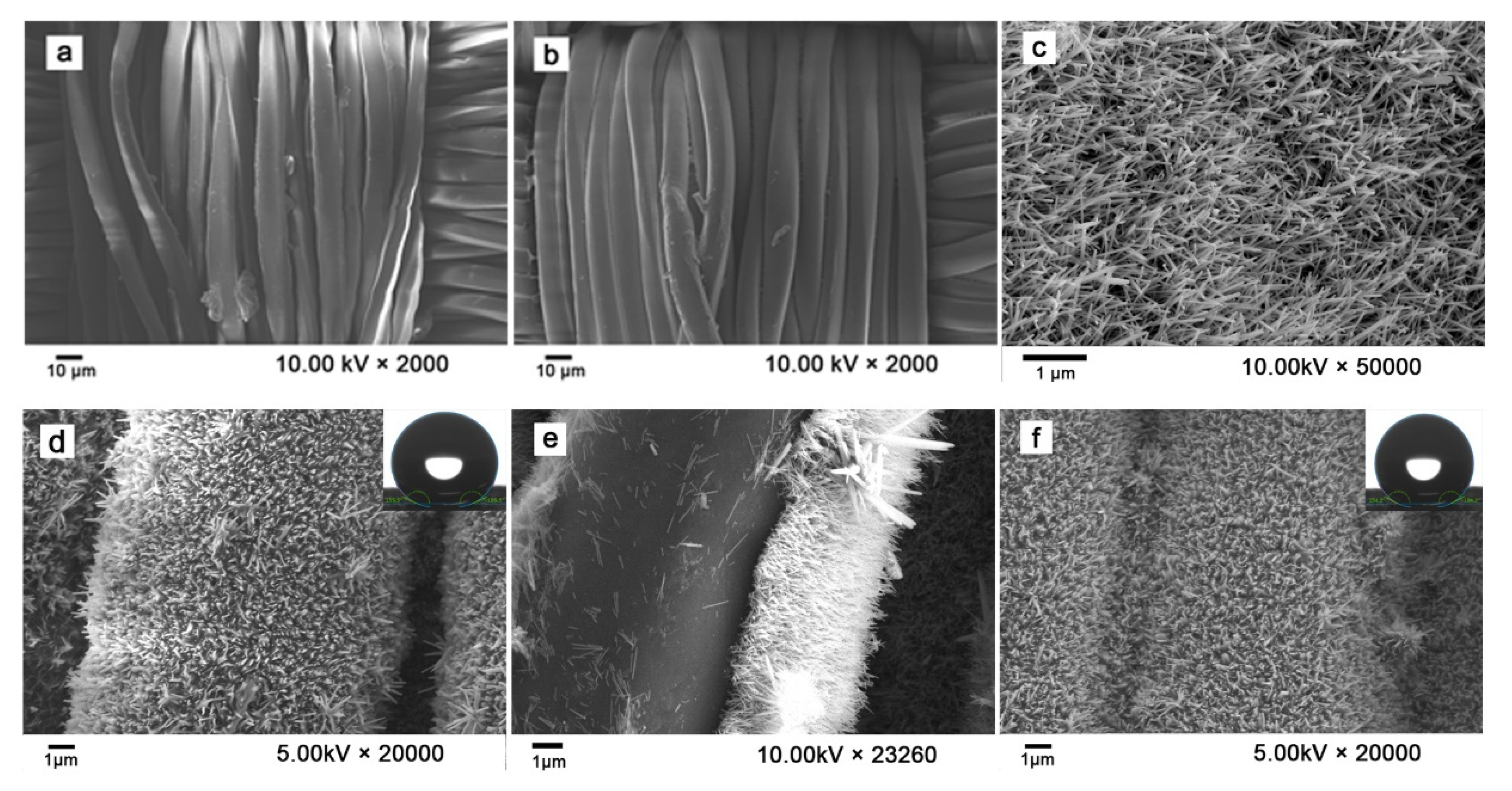
2.4. Characterization
3. Results and Discussion
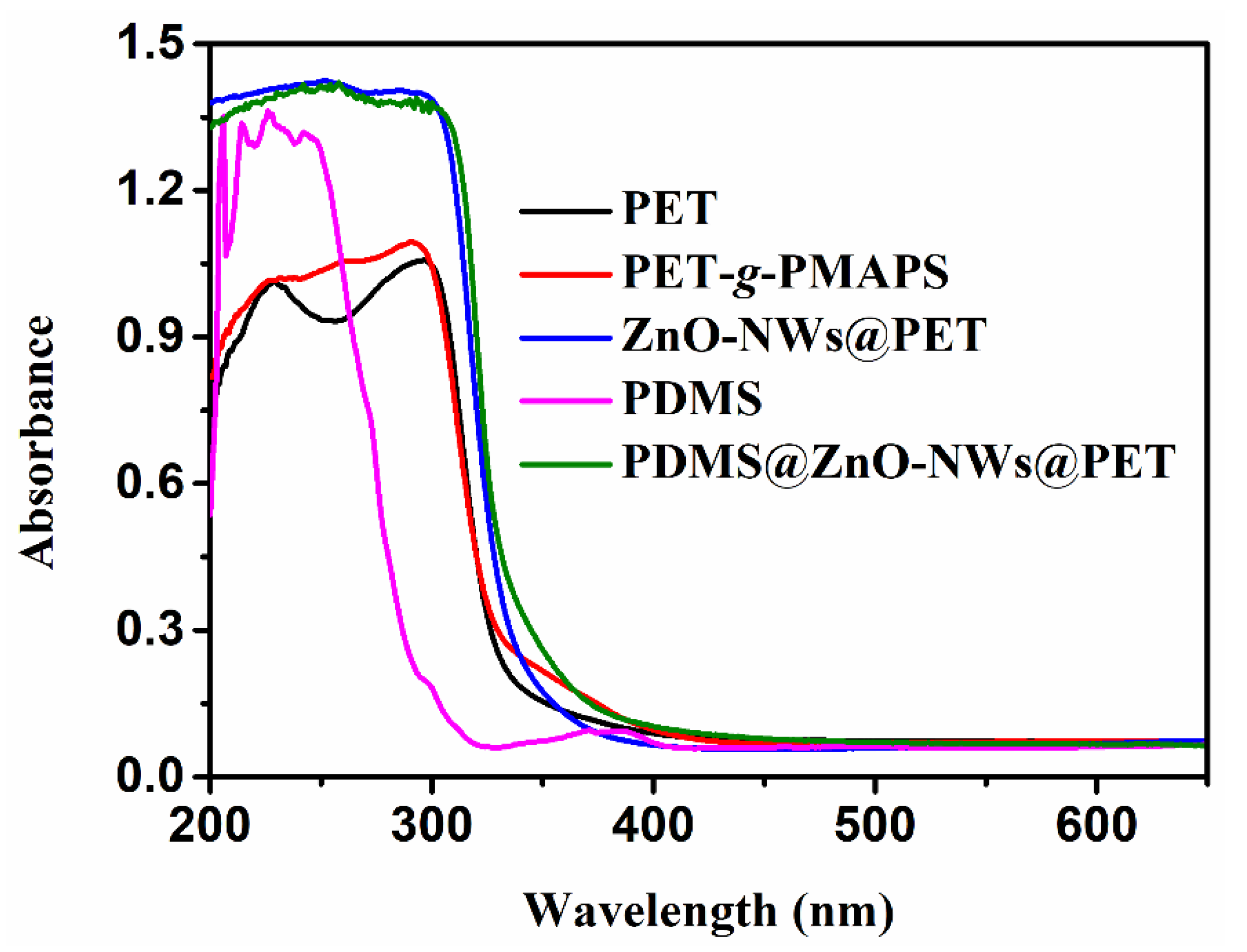
3.1. Design and Characterization of PDMS@ZnO-NWs@PET
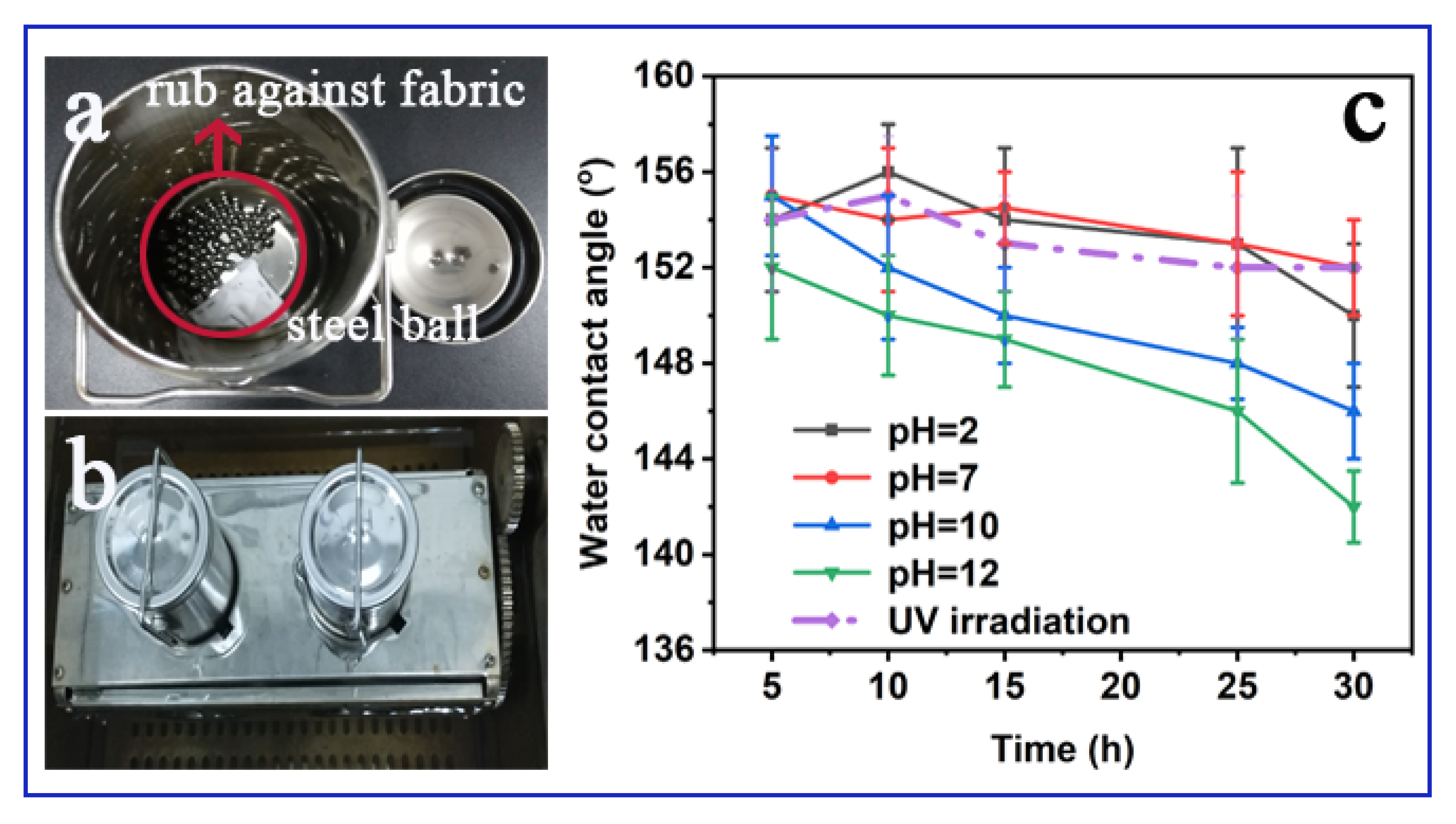
3.2. Oil–water Separation Behaviors of PDMS@ZnO-NWs@PET
3.3. Other Potential Applications of In-Situ Nano-Packaging Technology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, Q.; Xu, L.; Li, Q.; Song, Y.; Gao, X.; Quéré, D.; Jiang, L. Bioinspired ribbed nanoneedles with robust superhydrophobicity. Adv. Funct. Mater. 2010, 20, 656–662. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, M.; Ma, H.; Yu, C.; Li, K.; Yu, C.; Jiang, L. Morphology-control strategy of the superhydrophobic poly(methyl methacrylate) surface for efficient bubble adhesion and wastewater remediation. Adv. Funct. Mater. 2017, 27, 1702020. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Hu, H.; Liu, G. MRabnawaz, Hydrophilically patterned superhydrophobic cotton fabrics and their use in ink printing. J. Mater. Chem. A 2014, 2, 8094–8102. [Google Scholar] [CrossRef]
- Zou, H.; Lin, S.; Tu, Y.; Liu, G.; Hu, J.; Li, F.; Miao, L.; Zhang, G.; Luo, H.; Liu, F.; et al. Simple approach towards fabrication of highly durable and robust superhydrophobic cotton fabric from functional diblock copolymer. J. Mater. Chem. A 2013, 1, 11246–11260. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 7208–7214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Xu, W.; Carmalt, C.J.; Parkin, I.P. Water droplets bouncing on superhydrophobic soft porous materials. J. Mater. Chem. A 2014, 2, 12177–12184. [Google Scholar] [CrossRef]
- Chen, C.; Liu, M.; Hou, Y.; Zhang, L.; Li, M.; Wang, D.; Fu, S. Microstructure-controllable nanocomplexes bulk possessed with durable superhydrophobicity. Chem. Eng. J. 2020, 389, 124420. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Guo, Z. Tomato-lotus inspired edible superhydrophobic artificial lotus leaf. Chem. Eng. J. 2020, 400, 125883. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sundaramurthy, J.; Mangalaraj, D.; Nataraj, D.; Rajarathnam, D.; Srinivasan, M.P. Enhanced super-hydrophobic and switching behavior of ZnO nanostructured surfaces prepared by simple solution–immersion successive ionic layer adsorption and reaction process. J. Colloid Interface Sci. 2011, 363, 51–58. [Google Scholar]
- Van, T.N.; Lee, Y.K.; Lee, J.; Park, J.Y. Tuning hydrophobicity of TiO2 layers with silanization and self-assembled nanopatterning. Langmuir 2013, 29, 3054–3060. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sun, Y.; Zhang, W.; Gao, F.; Li, P.; Jiang, D.; Chen, Y. Fabrication of hierarchical structures with ZnO nanowires on micropillars by UV soft imprinting and hydrothermal growth for a controlled morphology and wettability. Appl. Surf. Sci. 2014, 317, 545–551. [Google Scholar] [CrossRef]
- Piret, G.; Drobecq, H.; Coffinier, Y.; Melnyk, O.; Boukherroub, R. Matrix-free laser desorption/ionization mass spectrometry on silicon nanowire arrays prepared by chemical etching of crystalline silicon. Langmuir 2010, 26, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.-H.; Chen, J.; Yin, W.; Jia, S.-T.; Ma, J.-Z. Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Appl. Surf. Sci. 2012, 258, 2468–2472. [Google Scholar] [CrossRef]
- Wu, T.; Pan, Y.; Li, L. Fabrication of superhydrophobic hybrids from multiwalled carbon nanotubes and poly(vinylidene fluoride). Colloids Surf. A 2011, 384, 47–52. [Google Scholar] [CrossRef]
- Tao, S.; Yang, M.; Chen, H.; Zhao, S.; Chen, G. Continuous Synthesis of Ag/AgCl/ZnO composites using flow chemistry and photocatalytic application. Ind. Eng. Chem. Res. 2018, 57, 3263–3273. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Hou, F.; Li, H.; Yang, Y.; Zhang, X.; Yang, Y.; Wang, Y. Effects of Ag loading on structural and photocatalytic properties of flower-like ZnO microspheres. Appl. Surf. Sci. 2017, 391, 476–483. [Google Scholar] [CrossRef]
- Kwon, D.-K.; Porte, Y.; Myoung, J.M. Fabrication of ZnO nanorods p–n homojunction light-emitting diodes using Ag film as self-doping source for p-type ZnO nanorods. J. Phys. Chem. C 2018, 122, 11993–12001. [Google Scholar] [CrossRef]
- Rajabi, M.; Ghorbani, M. Performance evaluation of nanogenerators based on Ag doped ZnO nanorods. Sens. Actuators A 2017, 266, 338–344. [Google Scholar] [CrossRef]
- Liao, H.; Miao, X.; Ye, J.; Wu, T.; Deng, Z.; Li, C.; Jia, J.; Cheng, X.; Wang, X. Falling leaves inspired ZnO nanorods–nanoslices hierarchical structure for implant surface modification with two stage releasing features. ACS Appl. Mater. Interfaces 2017, 9, 13009–13015. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, M.; Ning, F.; Xu, S.; Li, Z.; Wei, M.; Evans, D.G.; Duan, X. Au nanoparticles sensitized ZnO nanorod@nanoplatelet core–shell arrays for enhanced photoelectrochemical water splitting. Nano Energy 2015, 12, 231–239. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Ibrahim, A.A.; Al-Heniti, S.H.; Kumar, R.; Baskoutas, S.; Raffah, B.M. Synthesis, characterization and acetone gas sensing applications of Ag-doped ZnO nanoneedles. Ceram. Int. 2017, 43, 6765–6770. [Google Scholar] [CrossRef]
- Taemin, L.; Wonoh, L.; Woo, K.S.; Joon, K.J.; Su, K.B. Flexible textile strain wireless sensor functionalized with hybrid carbon nanomaterials supported ZnO nanowires with controlled aspect ratio. Adv. Funct. Mater. 2016, 26, 6206–6214. [Google Scholar]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc oxide nanostructure-modified textile and its application to biosensing, photocatalysis, and as antibacterial material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Yu, H.; Lee, S.H.; Wang, L.; Zheng, J.; Wang, T.; Yun, Y.; Lee, J.K. ZnO nanorod array modified PVDF membrane with superhydrophobic surface for vacuum membrane distillation application. ACS Appl. Mater. Interfaces 2018, 10, 13452–13461. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, H.; Yuan, Z. Superhydrophobic brocades modified with aligned ZnO nanorods. Appl. Surf. Sci. 2011, 258, 662–667. [Google Scholar] [CrossRef]
- Gao, X.; Wen, G.; Guo, Z. Durable superhydrophobic and underwater superoleophobic cotton fabrics growing zinc oxide nanoarrays for application in separation of heavy/light oil and water mixtures as need. Colloids Surf. A 2018, 559, 115–126. [Google Scholar] [CrossRef]
- Ajmal, H.M.S.; Khan, F.; Huda, N.U.; Lee, S.; Nam, K.; Kim, H.Y.; Eom, T.H.; Kim, S.D. High-Performance Flexible Ultraviolet Photodetectors with Ni/Cu-Codoped ZnO Nanorods Grown on PET Substrates. Nanomaterials 2019, 9, 1067. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, Y.; Cai, Z. Low-temperature growth of ZnO nanorods on PET fabrics with two-step hydrothermal method. Appl. Surf. Sci. 2010, 256, 4724–4728. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Pang, L.; Yang, C.; Zhang, Y.; Hu, J.; Wu, G. Fabrication of highly durable polysiloxane-zinc oxide (ZnO) coated polyethylene terephthalate (PET) fabric with improved ultraviolet resistance, hydrophobicity, and thermal resistance. J. Colloid Interface Sci. 2019, 537, 91–100. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir ACS J. Surf. Colloids 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gao, Q.; Xu, L.; Zhang, M.; Xing, Z.; Guo, X.; Zhang, K.; Wu, G. Significant improvement in thermal and UV resistances of UHMWPE fabric through in situ formation of polysiloxane–TiO2 hybrid layers. ACS Appl. Mater. Interfaces 2016, 8, 23311–23320. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, D.B.; Rao, A.V.; Rao, A.P.; Wagh, P.B.; Ingale, S.V.; Gupta, S.C. Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. J. Colloid Interface Sci. 2011, 356, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Lihitkar, P.B.; Violet, S.; Shirolkar, M.; Singh, J.; Srivastava, O.N.; Naik, R.H.; Kulkarni, S.K. Confinement of zinc oxide nanoparticles in ordered mesoporous silica MCM-41. Mater. Chem. Phys. 2012, 133, 850–856. [Google Scholar] [CrossRef]
- Servinis, L.; Henderson, L.C.; Gengenbach, T.R.; Kafi, A.A.; Huson, M.G.; Fox, B.L. Surface functionalization of unsized carbon fiber using nitrenes derived from organic azides. Carbon 2013, 54, 378–388. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Zhang, M.; Aizezi, M.; Zhang, Y.; Hu, J.; Wu, G. In-situ mineralized robust polysiloxane-Ag@ZnO on cotton for enhanced photocatalytic and antibacterial activities. Carbohyd. Polym. 2019, 217, 15–25. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. ZnO nano-mushrooms for photocatalytic degradation of methyl orange. Mater. Lett. 2013, 97, 100–103. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Z.J.; Chang, Z.J.; Liu, J.J.; Ren, Y.X.; Sun, H.Y. Synthesis and properties of Ag-doped ZnO films with room temperature ferromagnetism. Chem. Phys. Lett. 2016, 666, 28–32. [Google Scholar] [CrossRef]
- Kumar, S.; Guria, C. Alkaline Hydrolysis of Waste Poly (Ethylene Terephthalate): A Modified Shrinking Core Model. J. Macromol. Sci. A 2005, 42, 237–251. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Q.; Xu, L.; Wang, M.; Zhang, M.; Zhang, K.; Liu, W.; Wu, G. Functionalization of cotton fabrics with highly durable polysiloxane-TiO2 hybrid layers: Potential applications for photo-induced water-oil separation, UV shielding, and self-cleaning. J. Mater. Chem. A 2018, 6, 6085–6095. [Google Scholar] [CrossRef]
- Zhu, X.L.; Yuan, L.; Liang, G.Z.; Gu, A.J. Unique surface modified aramid fibers with improved flame retardancy, tensile properties, surface activity and UV-resistance through in situ formation of hyperbranched polysiloxane-Ce0.8Ca0.2O1.8 hybrids. J. Mater. Chem. A 2015, 3, 12515–12529. [Google Scholar] [CrossRef]
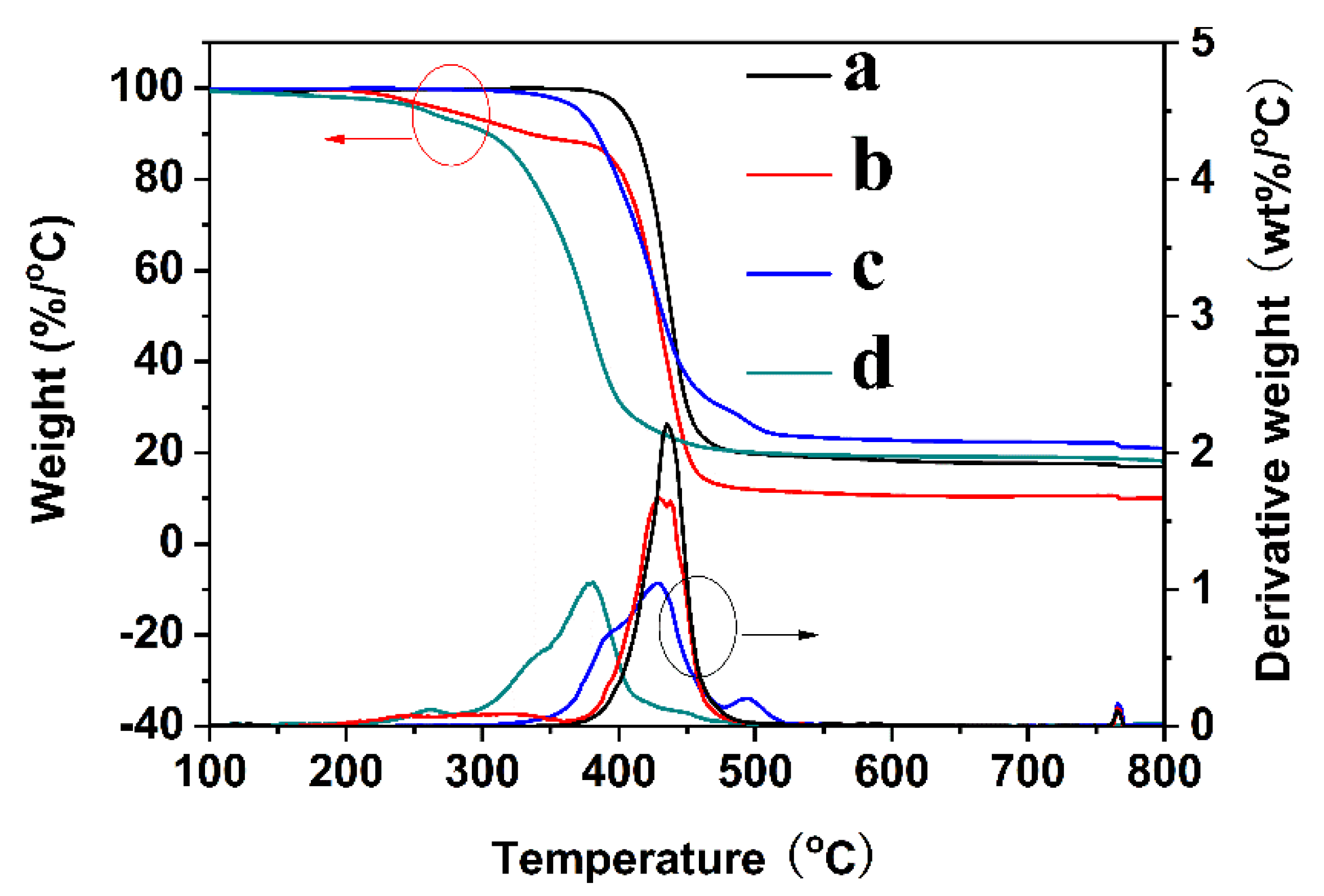

| Sample | Tdi (°C) | Tmax (°C) | Intensity | Yc at 800 °C (%) |
|---|---|---|---|---|
| PET fabric | 403 | 435 | 2.22 | 17.1 |
| PET-g-PMAPS | 278 | 429 | 1.65 | 10.2 |
| ZnO-NWs@PET | 371 | 430 | 1.05 | 21.2 |
| PDMS@ZnO-NWs@PET | 260 | 381 | 1.05 | 19.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Zhang, M.; He, Y.; Zhang, M.; Shen, R.; Zhang, Y.; Wang, M.; Wu, G. Fabrication and Potential Applications of Highly Durable Superhydrophobic Polyethylene Terephthalate Fabrics Produced by In-Situ Zinc Oxide (ZnO) Nanowires Deposition and Polydimethylsiloxane (PDMS) Packaging. Polymers 2020, 12, 2333. https://doi.org/10.3390/polym12102333
Hu J, Zhang M, He Y, Zhang M, Shen R, Zhang Y, Wang M, Wu G. Fabrication and Potential Applications of Highly Durable Superhydrophobic Polyethylene Terephthalate Fabrics Produced by In-Situ Zinc Oxide (ZnO) Nanowires Deposition and Polydimethylsiloxane (PDMS) Packaging. Polymers. 2020; 12(10):2333. https://doi.org/10.3390/polym12102333
Chicago/Turabian StyleHu, Jiangtao, Mingxing Zhang, Yulong He, Maojiang Zhang, Rongfang Shen, Yumei Zhang, Minglei Wang, and Guozhong Wu. 2020. "Fabrication and Potential Applications of Highly Durable Superhydrophobic Polyethylene Terephthalate Fabrics Produced by In-Situ Zinc Oxide (ZnO) Nanowires Deposition and Polydimethylsiloxane (PDMS) Packaging" Polymers 12, no. 10: 2333. https://doi.org/10.3390/polym12102333
APA StyleHu, J., Zhang, M., He, Y., Zhang, M., Shen, R., Zhang, Y., Wang, M., & Wu, G. (2020). Fabrication and Potential Applications of Highly Durable Superhydrophobic Polyethylene Terephthalate Fabrics Produced by In-Situ Zinc Oxide (ZnO) Nanowires Deposition and Polydimethylsiloxane (PDMS) Packaging. Polymers, 12(10), 2333. https://doi.org/10.3390/polym12102333