Stereocomplexation of Poly(lactic acid) and Chemical Crosslinking of Ethylene Glycol Dimethacrylate (EGDMA) Double-Crosslinked Temperature/pH Dual Responsive Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
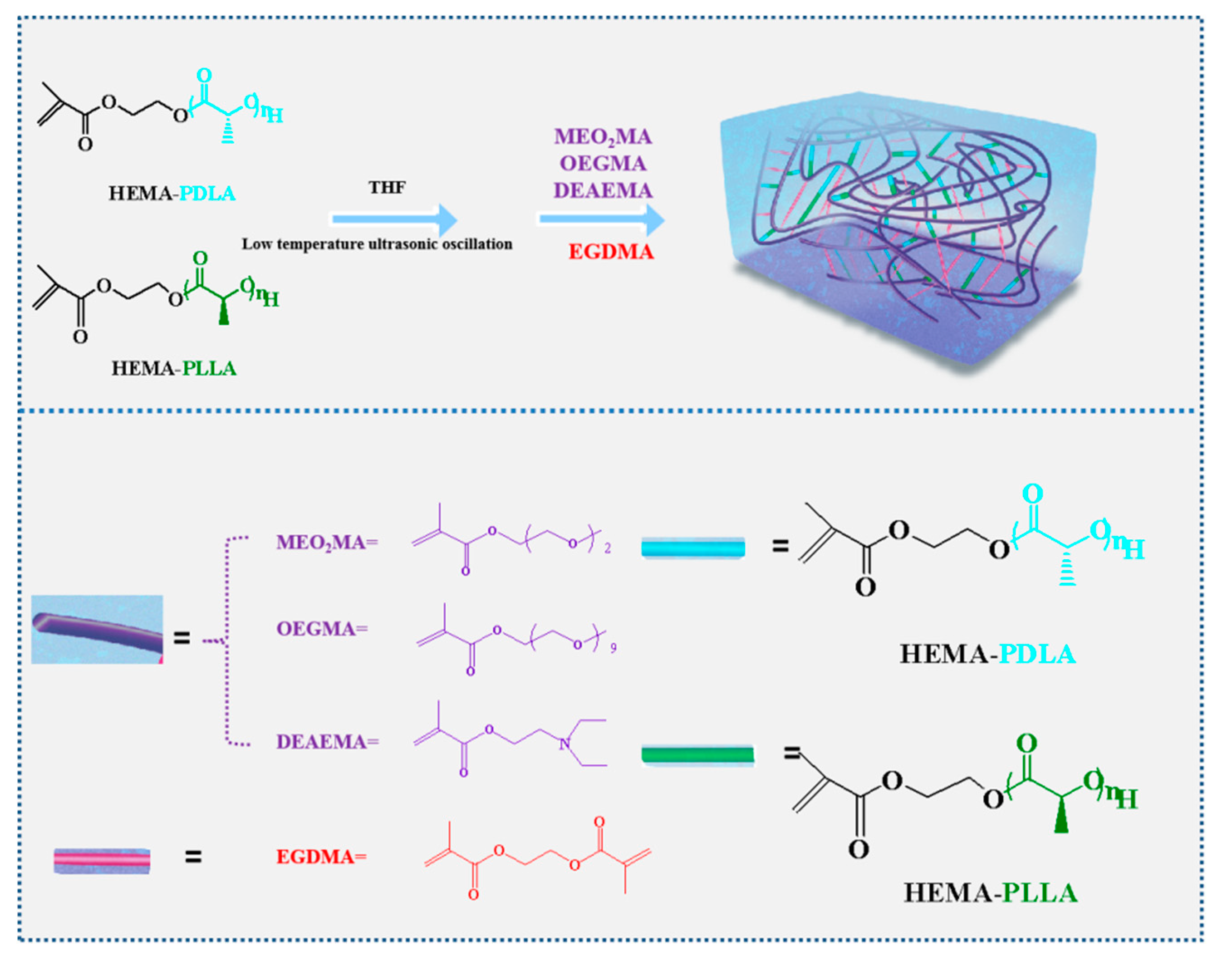
2.2. Synthesis
2.2.1. Synthesis of HEMA-PDLA Macromonomer
2.2.2. Synthesis of Double-Crosslinked Dual Responsive Hydrogels
2.2.3. Synthesis of Chemical Crosslinked Gel
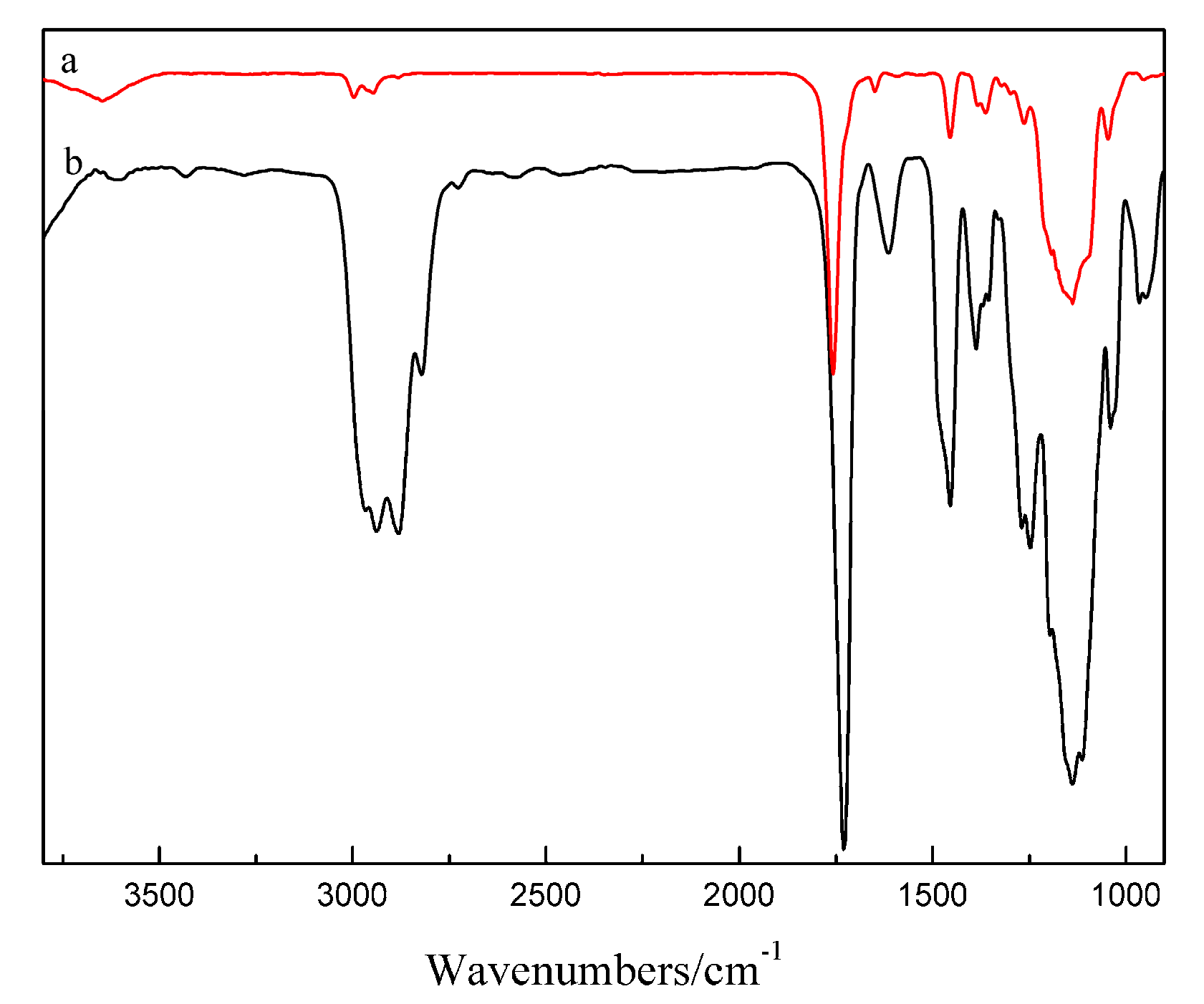
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
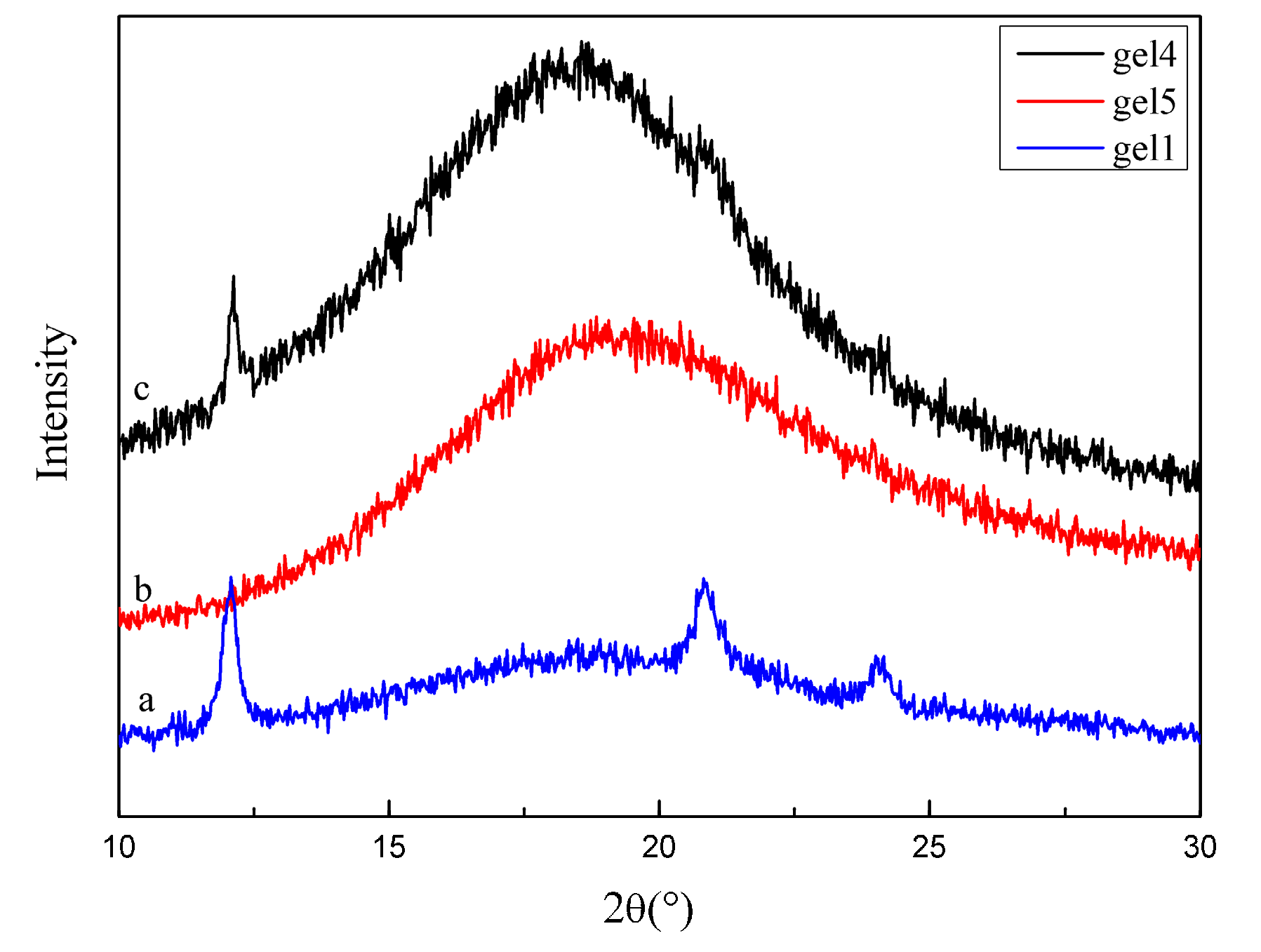
2.4. The Wide-Angle X-ray Diffraction (WAXD) Analysis of the Hydrogel
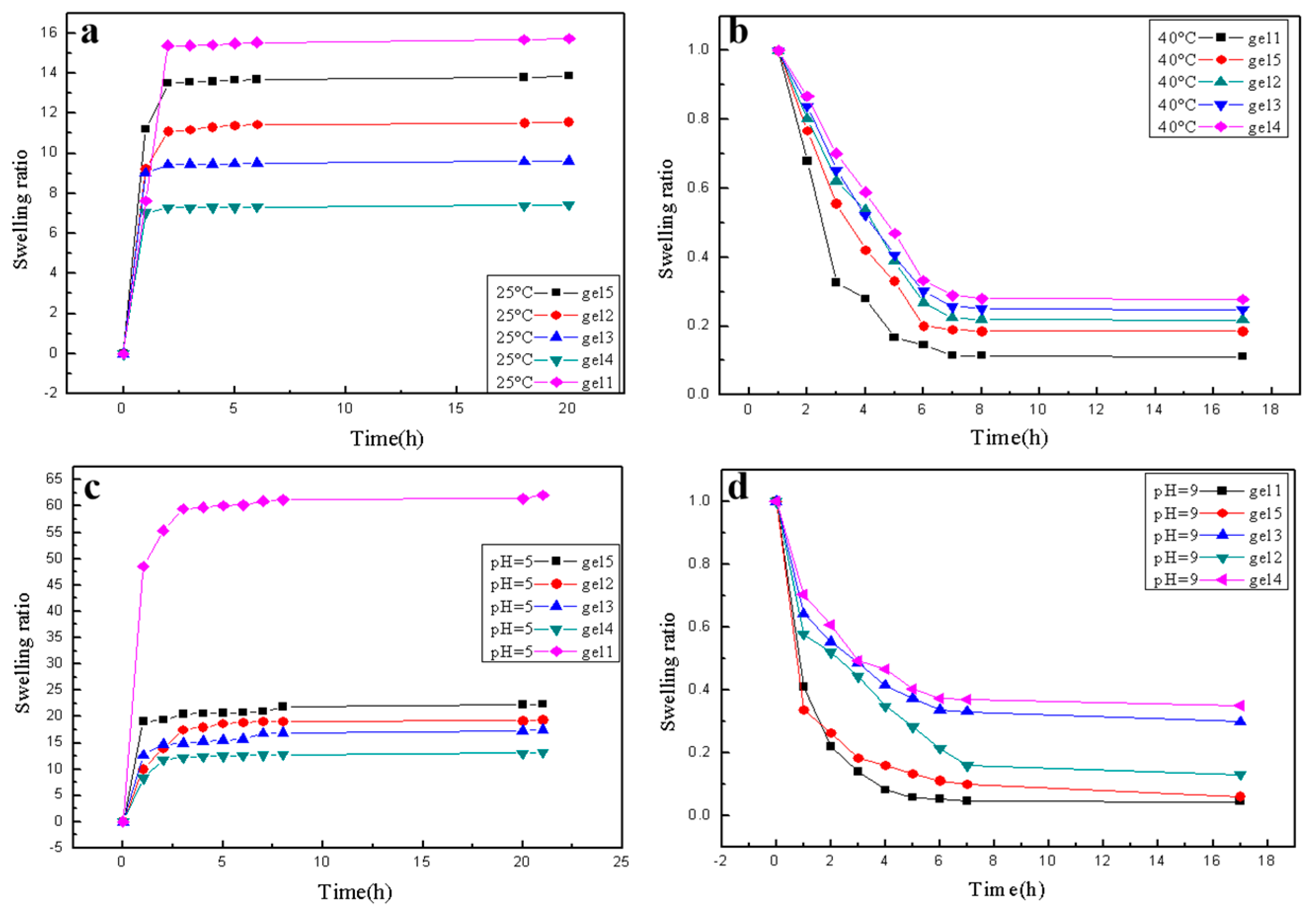
2.5. Swelling and Deswelling Properties of the Hydrogel
2.6. The Scanning Electron Microscope (SEM) Analysis of the Hydrogel
2.7. Thermal Analysis of the Hydrogel
2.8. Mechanical Properties Analysis of the Hydrogel
2.9. Drug Sustained Release Analysis of the Hydrogel
3. Results and Discussion
3.1. Synthesis and Characterization of Double-Crosslinked Dual Responsive Hydrogels
3.2. The Wide-Angle X-ray Diffraction (WAXD) Analysis of the Hydrogel
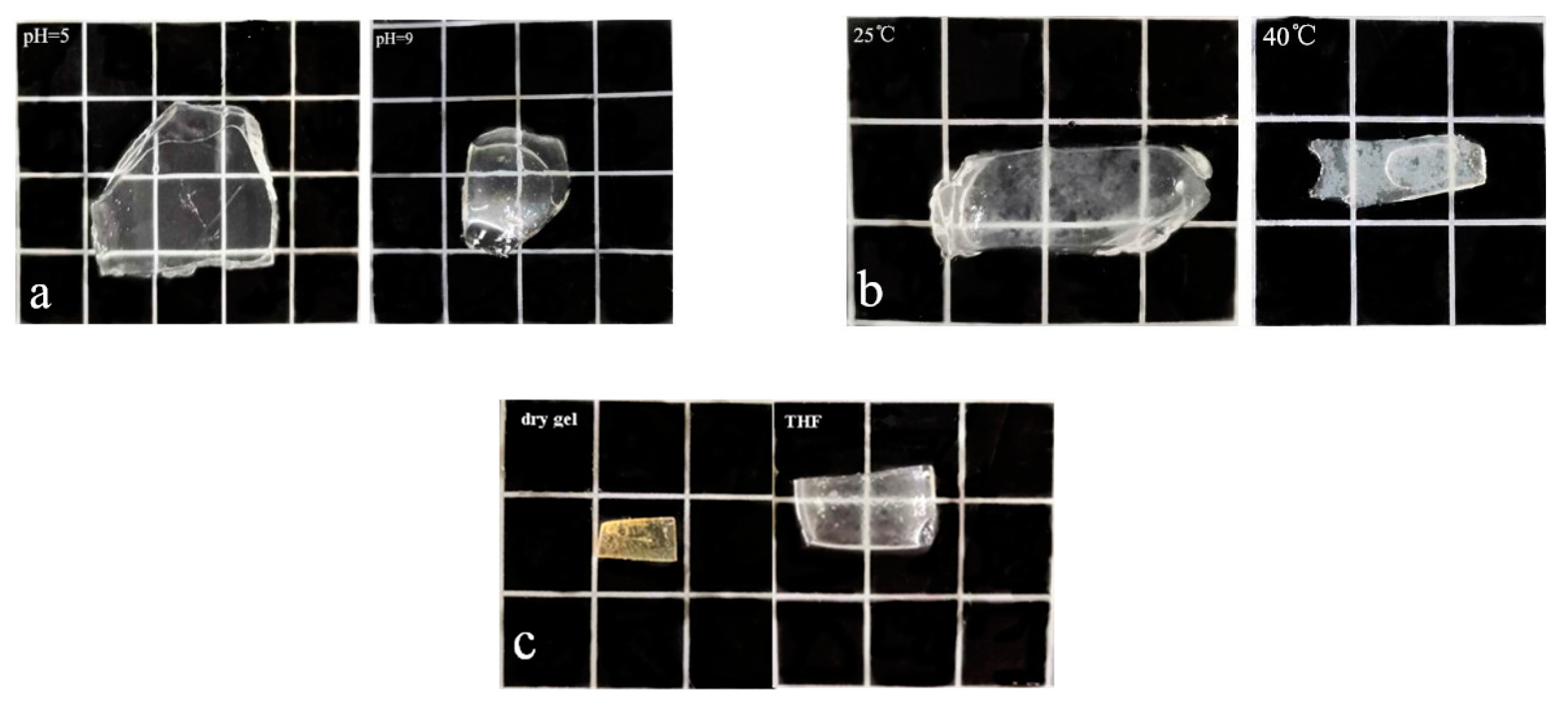
3.3. Temperature and pH Sensitivity of the Hydrogel
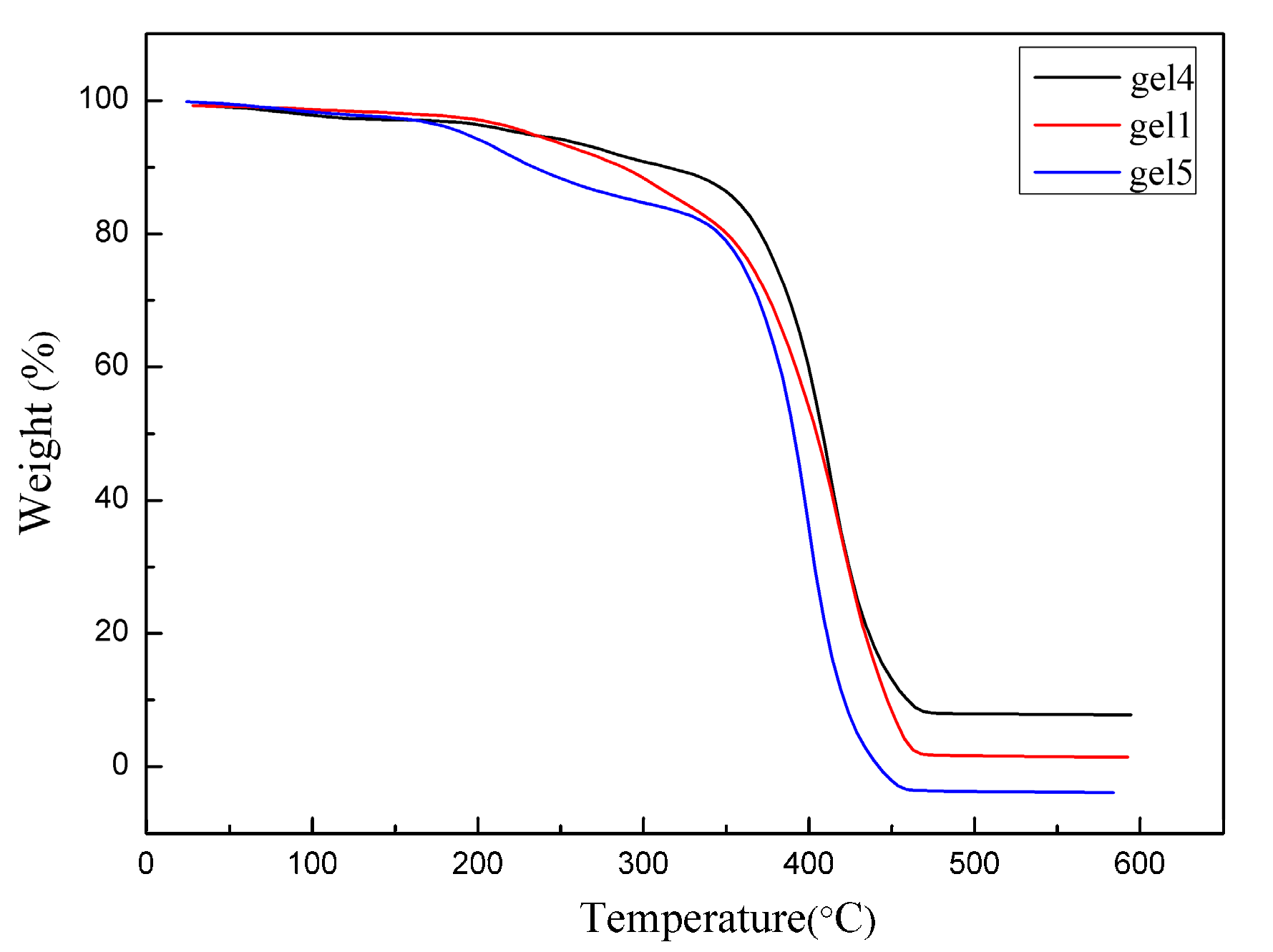
3.4. Thermal Analysis of the Hydrogel
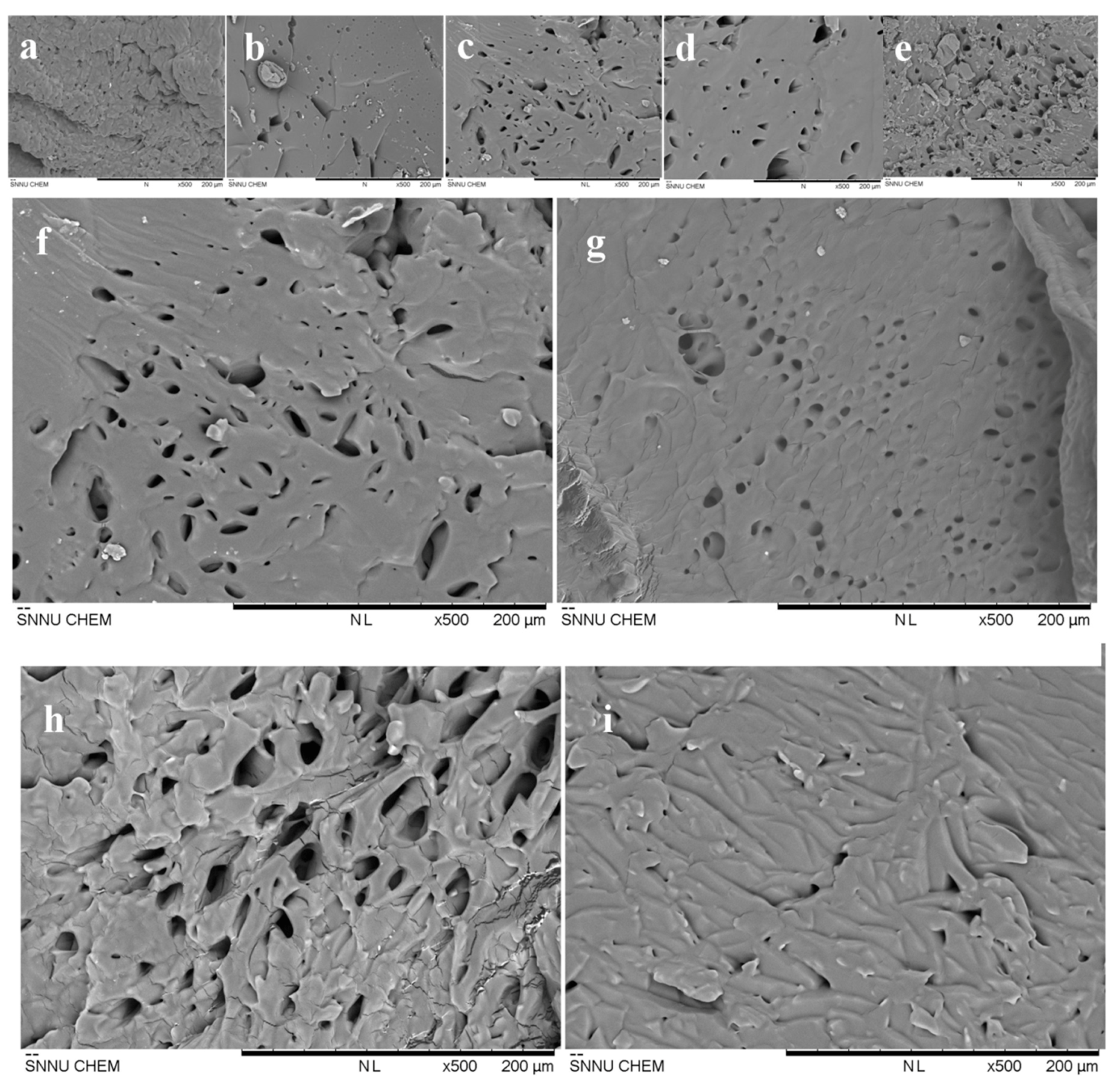
3.5. Scanning Electron Microscope (SEM) Analysis of the Hydrogel
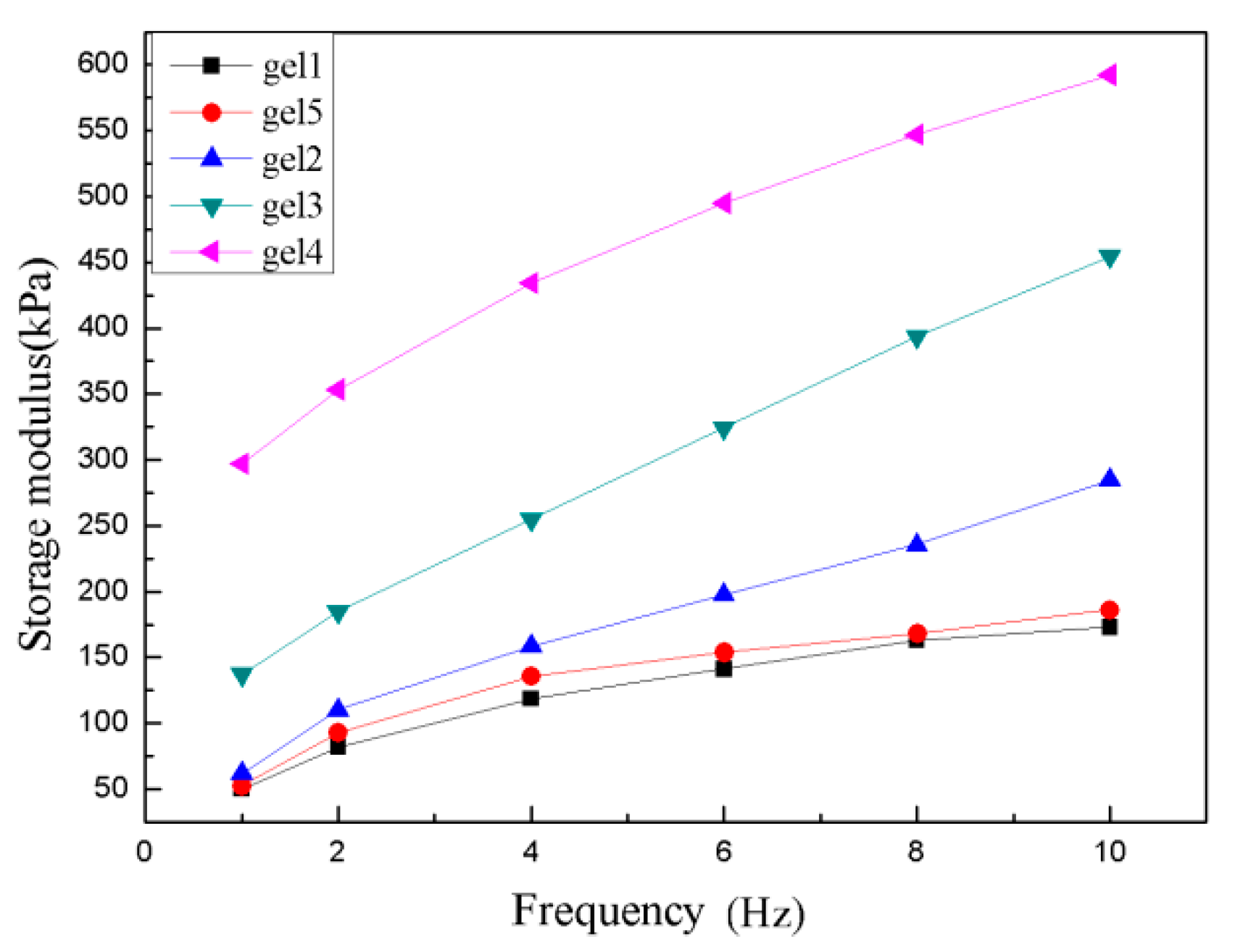
3.6. The Mechanical Properties of Hydrogels
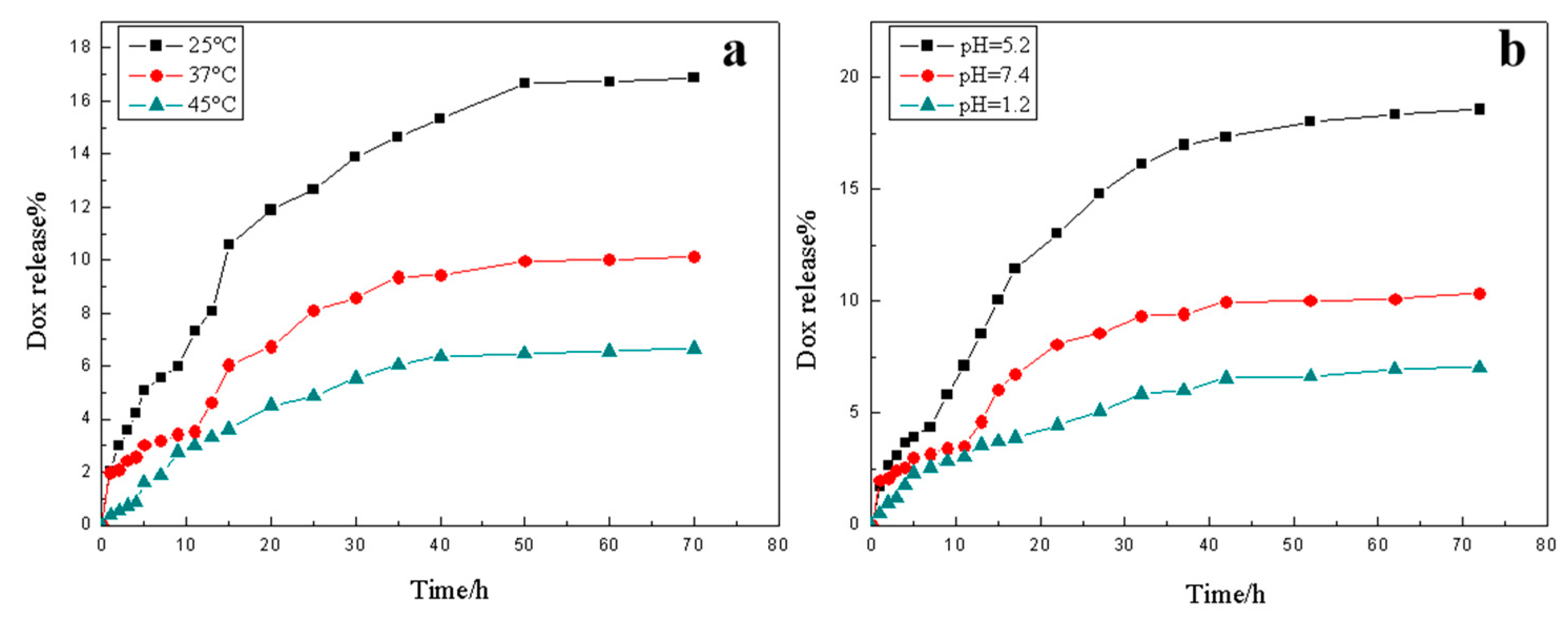
3.7. Drug Sustained Release of the Hydrogel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ning, H.R.; Wu, X.W.; Wu, Q.; Yu, W.L.; Wang, H.J.; Zheng, S.; Chen, Y.N.; Li, Y.Y.; Su, J.S. Microfiber-Reinforced Composite Hydrogels Loaded with Rat Adipose-Derived Stem Cells and BMP-2 for the Treatment of Medication-Related Osteonecrosis of the Jaw in a Rat Model. ACS Biomater. Sci. Eng. 2019, 5, 2430–2443. [Google Scholar] [CrossRef]
- Gao, G.R.; Wang, L.F.; Cong, Y.; Wang, Z.W.; Zhou, Y.; Wang, R.; Chen, J.; Fu, J. Synergistic pH and Temperature-Driven Actuation of Poly(NIPAM-co-DMAPMA)/Clay Nanocomposite Hydrogel Bilayers. ACS Omega 2018, 3, 17914–17921. [Google Scholar] [CrossRef]
- Paukkonena, H.; Kunnaria, M.; Lauréna, P.; Hakkarainen, T.; Auvinen, V.V.; Oksanen, T.; Koivuniemi, R.; Yliperttula, M.; Laaksonenac, T. Nanofibrillar cellulose hydrogels and reconstructed hydrogels as matrices for controlled drug release. Int. J. Pharm. 2017, 532, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.Q.; Tian, X.Z.; Deng, H.B.; Wang, Y.X.; Jiang, X. Dialdehyde-β-cyclodextrin-crosslinked carboxymethyl chitosan hydrogel for drug release. Carbohydr. Polym. 2020, 231, 115678. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Landfester, K. Stimuli-responsive microgels for the loading and release of functional compounds: Fundamental concepts and applications. Polymer 2012, 53, 5209–5231. [Google Scholar] [CrossRef]
- Khodeir, M.; Ernould, B.; Brassinne, J.; Ghiassinejad, S.; Jia, H.; Antoun, S.; Friebe, C.; Schubert, U.S.; Kochovski, Z.; Lu, Y.; et al. Synthesis and characterisation of redox hydrogels based on stable nitroxide radicals. Soft Matter 2019, 15, 6418–6426. [Google Scholar] [CrossRef]
- Willner, I. Stimuli-Controlled Hydrogels and Their Applications. Acc. Chem. Res. 2017, 50, 657–658. [Google Scholar] [CrossRef]
- Lago, G.L.; Felisberti, M.I. pH and thermo-responsive hybrid hydrogels based on PNIPAAM and keratin. Eur. Polym. J. 2020, 125, 109538. [Google Scholar] [CrossRef]
- Avais, M.; Chattopadhyay, S. Waterborne pH responsive hydrogels: Synthesis, characterization and selective pH responsive behavior around physiological pH. Polymer 2019, 180, 121701. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Fan, L.X.; Yan, S.; Li, F.B.; Li, H.J.; Tang, J.G. Tough and electro-responsive hydrogel actuators with bidirectional bending behavior. Nanoscale 2019, 11, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.M.; Liu, M.Y.; Li, H. A transient simulation to predict the kinetic behavior of magnetic-sensitive hydrogel responsive to magnetic stimulus. Int. J. Mech. Sci. 2020, 182, 105765. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.S.; Jung, Y.S.; Na, K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly(L-lactide) copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.Q.; Xu, F.; Xu, Z.H.; Wei, D.Q.; Feng, Y.J.; Wang, Y.M.; Jia, D.C.; Zhou, Y. UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery. Carbohydr. Polym. 2017, 174, 904. [Google Scholar] [CrossRef]
- Khodeir, M.; Antoun, S.; Ruymbeke, E.; Gohy, J.F. Temperature and Redox-Responsive Hydrogels Based on Nitroxide Radicals and Oligoethyleneglycol Methacrylate. Macromol. Chem. Phys. 2020, 221, 1900550. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.L.; Wang, D.J. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Wu, J.H.; Li, P.F.; Dong, C.L.; Jiang, H.J.; Xue, B.; Gao, X.; Qin, M.; Wang, W.; Chen, B.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Narayanan, J.; Liu, X.Y.; Chong, T.K.; Chen, S.B.; Chung, T.S. Topology Evolution and Gelation Mechanism of Agarose Gel. J. Phys. Chem. 2005, 109, 5638–5643. [Google Scholar] [CrossRef]
- Fan, H.L.; Wang, J.H.; Jin, Z.X. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-CrossLinked Hydrogels Based on Polymer-Tannic Acid Multiple Hydrogen Bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Haraguchi, K.; Shimizu, S.; Tanaka, S. Instant Strong Adhesive Behavior of Nanocomposite Gels toward Hydrophilic Porous Materials. Langmuir 2018, 34, 8480–8488. [Google Scholar] [CrossRef]
- Li, S.B.; Yi, J.J.; Yu, X.M.; Shi, H.J.; Zhu, J.; Wang, L. Preparation and Characterization of Acid Resistant Double CrossLinked Hydrogel for Potential Biomedical Applications. ACS Biomater. Sci. Eng. 2018, 4, 872–883. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, Y.X.; Wang, Q.Y.; Yang, J.Q.; Hu, S.Q.; Chen, L.Y. Mechanically Strong and Highly Tough Prolamin Protein Hydrogels Designed from Double-Cross-Linked Assembled Networks. ACS Appl. Polym. Mater. 2019, 1, 1272–1279. [Google Scholar] [CrossRef]
- Hua, H.X.; Ye, B.H.; Lv, Y.H.; Zhang, Q. Preparing antibacterial and in-situ formable double crosslinking chitosan/hyaluronan composite hydrogels. Mater. Lett. 2019, 254, 17–20. [Google Scholar] [CrossRef]
- Ye, L.; Lv, Q.; Sun, X.Y.; Liang, Y.Z.; Fang, P.W.; Yuan, X.Y.; Li, M.; Zhang, X.Z.; Shang, X.F.; Liang, H.Y. Fully physically cross-linked double network hydrogels with strong mechanical properties, good recovery and self-healing properties. Soft Matter 2020, 16, 1840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Cheng, Y.R.; Hou, X.J.; Yang, B.; Guo, F. Ionic effects on the mechanical and swelling properties of a poly(acrylic acid/acrylamide) double crosslinking hydrogen. New J. Chem. 2018, 42, 9151–9158. [Google Scholar] [CrossRef]
- Quesada-Pe’rez, M.; Maroto-Centeno, J.A.; Forcada, J.; Hidalgo-Alvarez, R.; Hidalgo-Alvarez, R. Gel swelling theories: The classical formalism and recent approaches. Soft Matter 2011, 7, 10536–10547. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Wang, X.N.; Wang, Y.; Yan, M.R.; Liang, X.Y.; Zhao, N.; Ma, Y.T.; Gao, Y.C. Thermosensitive Hydrogel Based on Poly(2-Ethyl-2-Oxazoline)–Poly(D,L-Lactide)–Poly(2-Ethyl-2-Oxazoline) for Sustained Salmon Calcitonin Delivery. AAPS PharmSciTech 2020, 21, 71. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex Formation between Enantiomeric Poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Wu, J.; Shi, X.Y.; Wang, Z.D.; Song, F.; Gao, W.L.; Liu, S.X. Stereocomplex Poly(Lactic Acid) Amphiphilic Conetwork Gel with Temperature and pH Dual Sensitivity. Polymers 2019, 11, 1940. [Google Scholar] [CrossRef]
- Shi, X.Y.; Wu, J.; Wang, Z.D.; Song, F.; Gao, W.L.; Liu, S.X. Synthesis and properties of a temperature-sensitive hydrogel based on physical crosslinking via stereocomplexation of PLLA-PDLA. RSC Adv. 2020, 10, 19759–19769. [Google Scholar] [CrossRef]
- Tai, H.Y.; Howard, D.; Takae, S.; Wang, W.X.; Vermonden, T.; Hennink, W.E.; Stayton, P.S.; Hoffman, A.S.; Endruweit, A.; Alexander, C.; et al. Photo-Cross-Linked Hydrogels from Thermoresponsive PEGMEMA-PPGMA-EGDMA Copolymers Containing Multiple Methacrylate Groups: Mechanical Property, Swelling, Protein Release, and Cytotoxicity. Biomacromolecules 2009, 10, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.Y.; Wang, W.X.; Vermonden, T.; Heath, F.; Hennink, W.E.; Alexander, C.; Shakesheff, K.M.; Howdle, S.M. Thermoresponsive and Photocrosslinkable PEGMEMA-PPGMA-EGDMA Copolymers from a One-Step ATRP Synthesis. Biomacromolecules 2009, 10, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, K.; Nie, J.J.; Du, B.Y.; Tang, G.P. Poly(N-vinylpyrrolidinone) Microgels: Preparation, Biocompatibility, and Potential Application as Drug Carriers. Biomacromolecules 2014, 15, 2285–2293. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo (ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Park, C.; Heo, J.; Lee, J.; Kim, T.; Kim, S.Y. Well-Defifined Dual Light- and Thermo-Responsive Rod-Coil Block Copolymers Containing an Azobenzene, MEO2MA and OEGMA. Polymers 2020, 12, 284. [Google Scholar] [CrossRef]
- Anderson, C.R.; Gnopo, Y.D.; Gambinossi, F.; Mylon, S.E.; Ferri, J.K. Modulation of cell responses to Ag-(MeO2MA-co-OEGMA): Effects of nanoparticle surface hydrophobicity and serum proteins on cellular uptake and toxicity. J. Biomed. Mater. Res. 2018, 106, 1061–1071. [Google Scholar] [CrossRef]
- Tan, B.H.; Hussain, H.; Lin, T.T.; Chua, Y.C.; Leong, Y.W.; Tjiu, W.W.; Wong, P.K.; HeStable, C.B. Dispersions of Hybrid Nanoparticles Induced by Stereocomplexation between Enantiomeric Poly (lactide) Star Polymers. Langmuir 2011, 27, 10538–10547. [Google Scholar] [CrossRef]
- Shang, P.; Wu, J.; Shi, X.Y.; Wang, Z.D.; Song, F.; Liu, S.X. Synthesis of Thermo-Responsive Block-Graft Copolymer Based on PCL and PEG Analogs and Preparation of Hydrogel via Click Chemistry. Polymers 2019, 11, 765. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
| Sample | n(HEMA-PLLA20) (mol) | n(HEMA-PDLA20) (mol) | [DEAEMA]/[(OEGMA+MEO2MA)] (n:n) | EGDMA (%) |
|---|---|---|---|---|
| gel1 | 0.08 | 0.08 | 0.3 | 0 |
| gel2 | 0.08 | 0.08 | 0.3 | 3 |
| gel3 | 0.08 | 0.08 | 0.3 | 5 |
| gel4 | 0.08 | 0.08 | 0.3 | 7 |
| gel5 | 0.16 | 0 | 0.3 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wu, J.; Shi, X.; Song, F.; Gao, W.; Liu, S. Stereocomplexation of Poly(lactic acid) and Chemical Crosslinking of Ethylene Glycol Dimethacrylate (EGDMA) Double-Crosslinked Temperature/pH Dual Responsive Hydrogels. Polymers 2020, 12, 2204. https://doi.org/10.3390/polym12102204
Wang Z, Wu J, Shi X, Song F, Gao W, Liu S. Stereocomplexation of Poly(lactic acid) and Chemical Crosslinking of Ethylene Glycol Dimethacrylate (EGDMA) Double-Crosslinked Temperature/pH Dual Responsive Hydrogels. Polymers. 2020; 12(10):2204. https://doi.org/10.3390/polym12102204
Chicago/Turabian StyleWang, Zhidan, Jie Wu, Xiaoyu Shi, Fei Song, Wenli Gao, and Shouxin Liu. 2020. "Stereocomplexation of Poly(lactic acid) and Chemical Crosslinking of Ethylene Glycol Dimethacrylate (EGDMA) Double-Crosslinked Temperature/pH Dual Responsive Hydrogels" Polymers 12, no. 10: 2204. https://doi.org/10.3390/polym12102204
APA StyleWang, Z., Wu, J., Shi, X., Song, F., Gao, W., & Liu, S. (2020). Stereocomplexation of Poly(lactic acid) and Chemical Crosslinking of Ethylene Glycol Dimethacrylate (EGDMA) Double-Crosslinked Temperature/pH Dual Responsive Hydrogels. Polymers, 12(10), 2204. https://doi.org/10.3390/polym12102204






