Changes in the Dimensions of Lignocellulose Nanofibrils with Different Lignin Contents by Enzymatic Hydrolysis
Abstract
1. Introduction
2. Materials and Methods:
2.1. Materials
2.2. Delignification
2.3. Preparation of LCNF
2.4. Enzymatic Hydrolysis by EG
2.5. Characteristics
3. Results and Discussion
3.1. Lignin Contents of LCNFs
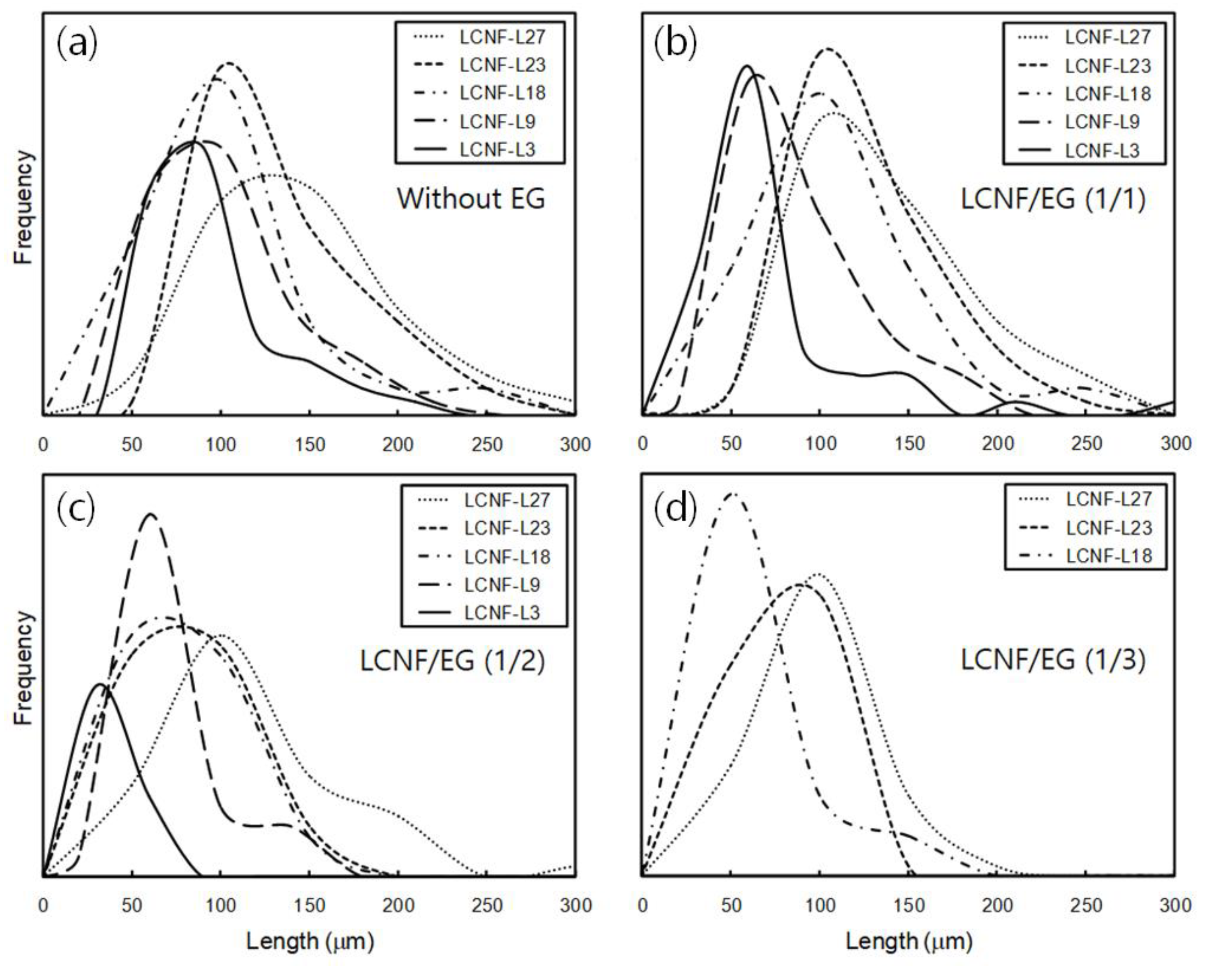
3.2. Morphology and Size of Insufficiently Defibrillated Products
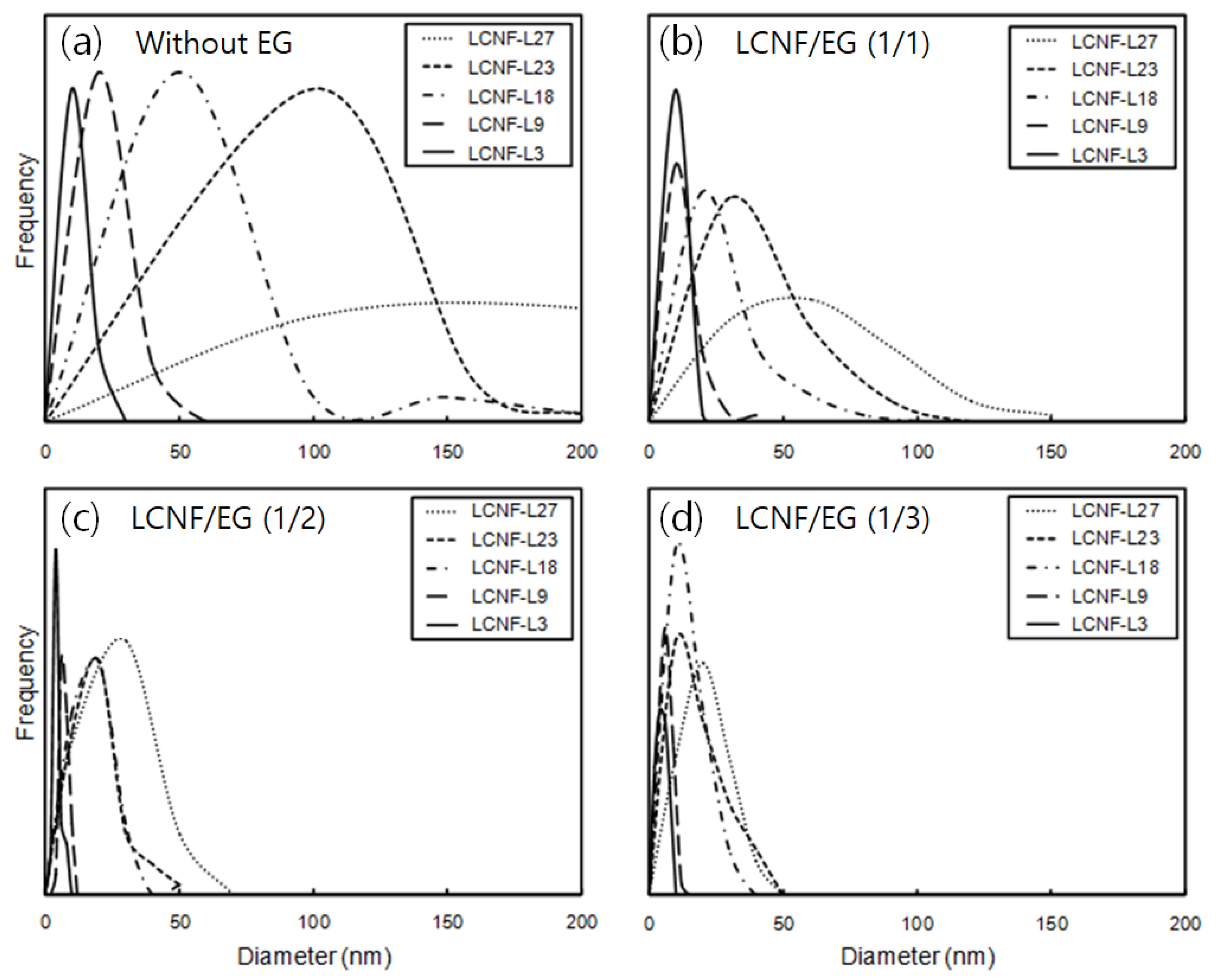
3.3. Morphology and Diameter Distribution of LCNFs
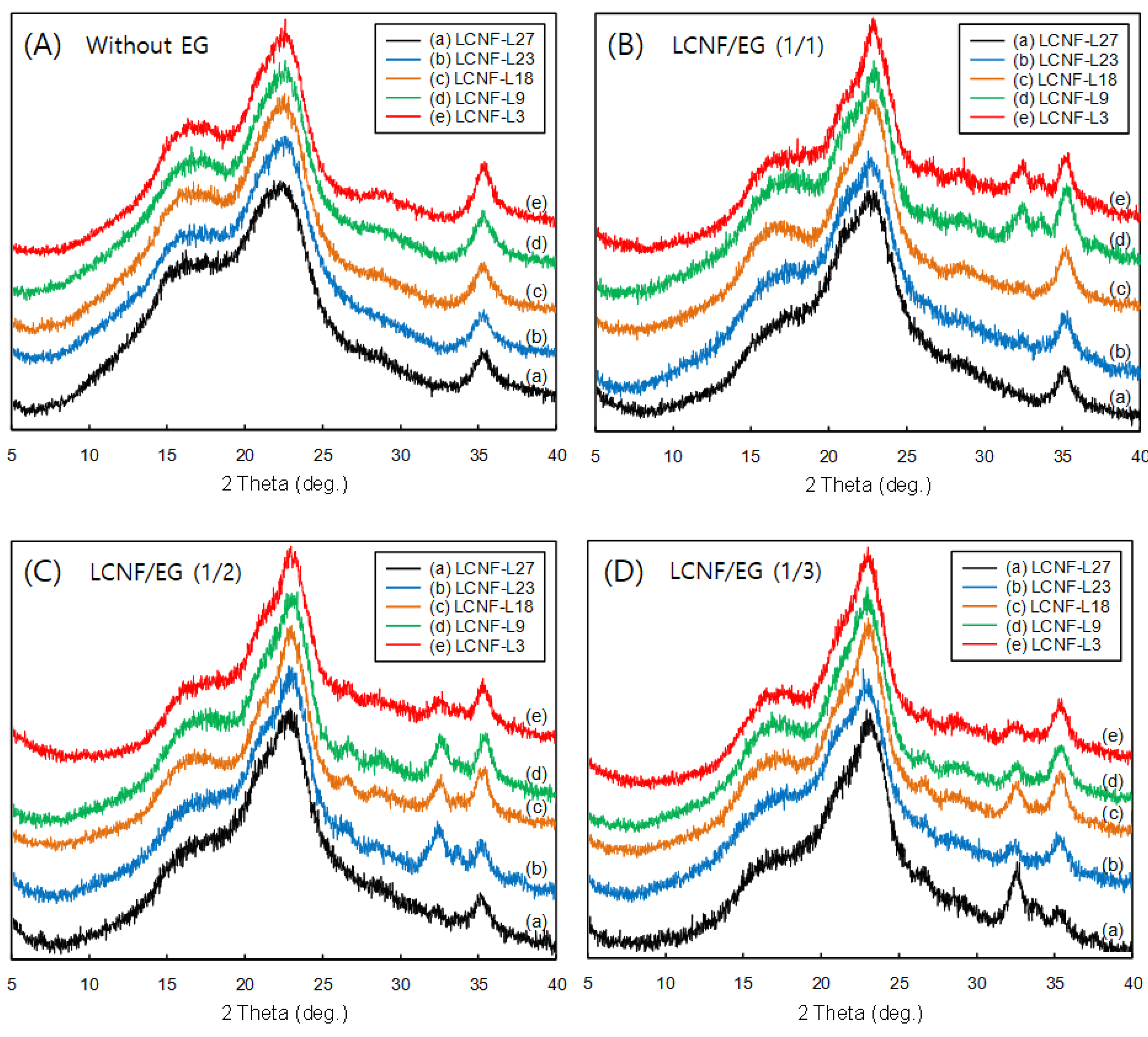
3.4. Crystalline Characteristics of LCNFs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-based bio- and nanocomposites: A review. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Osong, S.H.; Norgren, S.; Engstrand, P. An approach to produce nano-ligno-cellulose from mechanical pulp fine materials. Nord. Pulp Pap. Res. J. 2013, 28, 472–479. [Google Scholar] [CrossRef]
- Kumagai, A.; Lee, S.H.; Endo, T. Thin film of lignocellulosic nanofibrils with different chemical composition for QCM-D study. Biomacromolecules 2013, 14, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Diop, C.I.K.; Tajvidi, M.; Bilodeau, M.A.; Bousfield, D.W.; Hunt, J.F. Isolation of lignocellulose nanofibrils (LCNF) and application as adhesive replacement in wood composites: Example of fiberboard. Cellulose 2017, 24, 3037–3050. [Google Scholar] [CrossRef]
- Park, C.W.; Han, S.Y.; Namgung, H.W.; Seo, P.N.; Lee, S.Y.; Lee, S.H. Preparation and characterization of cellulose nanofibrils with varying chemical compositions. BioResources 2017, 12, 5031–5044. [Google Scholar] [CrossRef]
- Park, C.W.; Youe, W.J.; Namgung, H.W.; Han, S.Y.; Seo, P.N.; Chae, H.M.; Lee, S.H. Effect of lignocellulose nanofibril and polymeric methylene diphenyl diisocyanate addition on plasticized lignin/polycaprolactone composites. BioResources 2018, 13, 6802–6817. [Google Scholar]
- Park, C.W.; Park, J.S.; Han, S.Y.; Lee, E.A.; Kwon, G.J.; Seo, Y.H.; Gwon, J.G.; Lee, S.Y.; Lee, S.H. Preparation and characteristics of wet-spun filament made of cellulose nanofibrils with different chemical compositions. Polymers 2020, 12, 949. [Google Scholar] [CrossRef]
- Park, C.W.; Han, S.Y.; Choi, S.K.; Lee, S.H. Preparation and properties of holocellulose nanofibrils with different hemicellulose content. BioResources 2017, 12, 6298–6308. [Google Scholar] [CrossRef]
- Albornoz-Palma, G.; Ching, D.; Valerio, O.; Mendonça, R.T.; Pereira, M. Effect of lignin and hemicellulose on the properties of lignocellulose nanofibril suspensions. Cellulose 2020. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Kim, J.C. Nanocellulose applications for drug delivery: A review. J. For. Environ. Sci. 2019, 35, 141–149. [Google Scholar]
- Han, S.Y.; Park, C.W.; Kwon, G.J.; Kim, N.H.; Kim, J.C.; Lee, S.H. Ionic liquid pretreatment of lignocellulosic biomass. J. For. Environ. Sci. 2020, 36, 69–77. [Google Scholar]
- Jang, J.H.; Lee, S.H.; Kim, N.H. Effect of endoglucanase hydrolysis on the characteristics of microfibrillated cellulose. In Proceedings of the International Symposium on the Fundamentals and Applications of Nanocellulose, Seoul, Korea, 7 October 2014; pp. 52–65. [Google Scholar]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, I.C.; Nair, S.S.; Ragauskas, A.J.; Deng, Y.; Rojas, O.J.; Zhu, J.Y. Mechanical deconstruction of lignocellulose cell walls and their enzymatic saccharification. Cellulose 2013, 20, 807–818. [Google Scholar] [CrossRef]
- Qing, Y.; Sabo, R.; Zhu, J.Y.; Agarwal, U.; Cai, Z.; Wu, Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr. Polym. 2013, 97, 226–234. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Wang, L.; Song, X.; Qin, C.; Wang, S. Tuning of size and properties of cellulose nanofibers isolated from sugarcane bagasse by endoglucanase-assisted mechanical grinding. Ind. Crops Prod. 2020, 146, 112201. [Google Scholar] [CrossRef]
- Penttilä, P.A.; Várnai, A.; Pere, J.; Tammelin, T.; Salmén, L.; Siika-aho, M.; Viikari, L.; Serimaa, R. Xylan as limiting factor in enzymatic hydrolysis of nanocellulose. Bioresour. Technol. 2013, 129, 135–141. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N., Jr. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef]
- Pandey, J.K.; Saini, D.R.; Ahn, S.H. Degradation of cellulose-based polymer composites. In Cellulose Fibers: Bio- and Nano- Polymer Composites; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin, Germany, 2011; pp. 507–517. [Google Scholar]
- Leu, S.-Y.; Zhu, J.Y. Substrate-related factors affecting enzymatic saccharification of lignocellulose: Our recent understanding. Bioenergy Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Li, X.; Zhao, J. Binding and hydrolysis properties of engineered cellobiohydrolases and endoglucanases. Bioresour. Technol. 2018, 267, 235–241. [Google Scholar] [CrossRef]
- Beguin, P.; Aubert, J. The biological degradation of cellulose. FEMS Microbiol Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.C.; Monica, E.; Henriksson, G. Improved accessibility and reactivity of dissolving pulp for the viscose process: Pretreatment with monocomponent endoglucanase. Biomacromolecules 2006, 7, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Wise, L.E.; Murphy, M.; D’Addieco, A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 1946, 122, 35–43. [Google Scholar]
- Kumar, R.; Hu, F.; Hubbell, C.A.; Ragauskas, A.; Wyman, C.E. Comparison of laboratory delignification methods, their selectivity, and impacts on physiochemical characteristics of cellulosic biomass. Bioresour. Technol. 2013, 130, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Gomori, G. Preparation of buffers for use in enzyme studies. Methods Enzymol. 1955, 1, 138–146. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin AEJr Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A comparative study of the suitability of different cereal straws for lignocellulose nanofibers isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef]
- He, W.; Jiang, S.; Zhang, Q.; Pan, M. Isolation and characterization of cellulose nanofibers from Bambusa rigida. BioResources 2013, 8, 5678–5689. [Google Scholar] [CrossRef]
- Goyal, A.; Ghosh, B.; Eveleigh, D. Characteristics of fungal cellulases. Bioresour. Technol. 1991, 36, 37–50. [Google Scholar] [CrossRef]
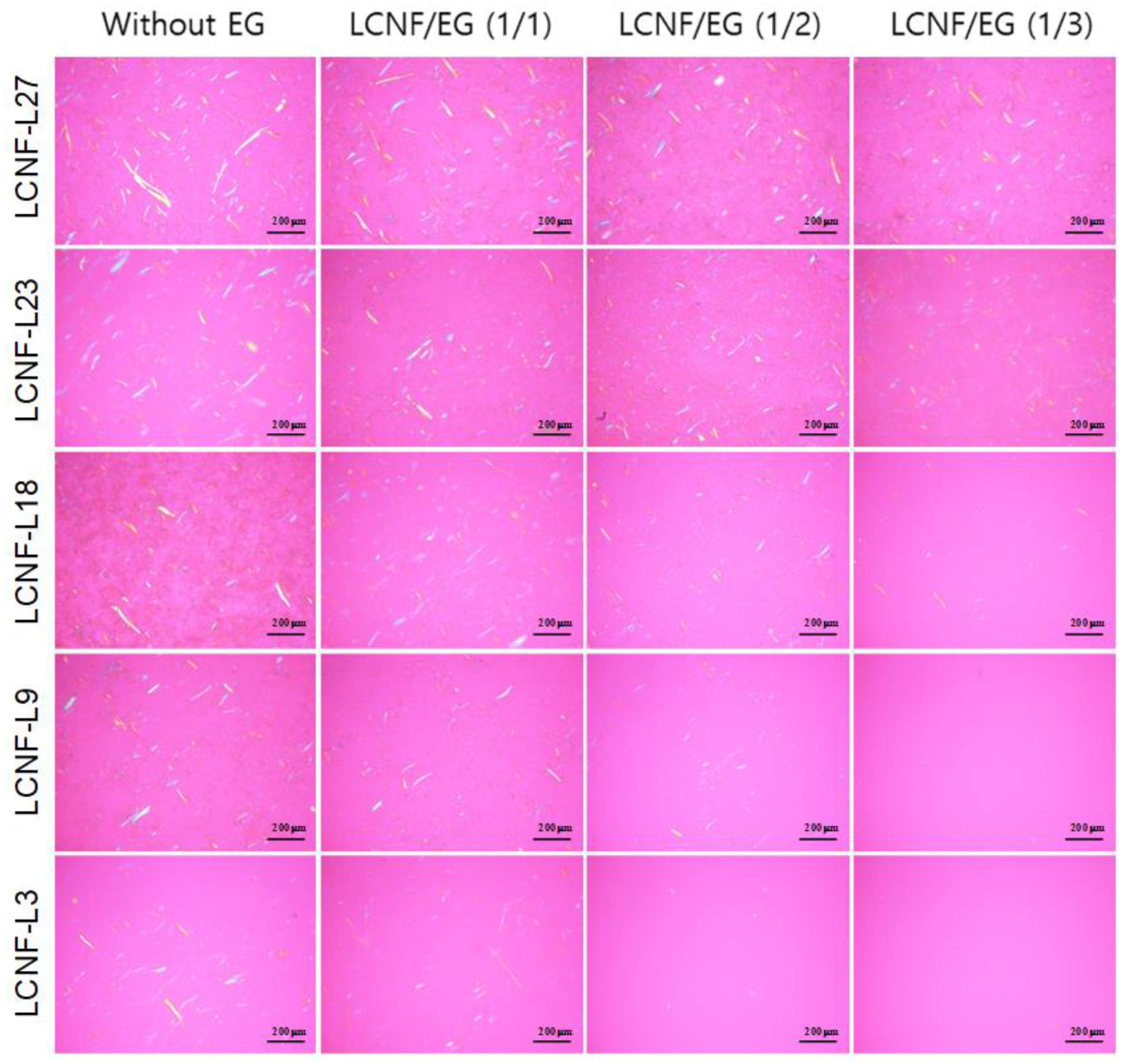
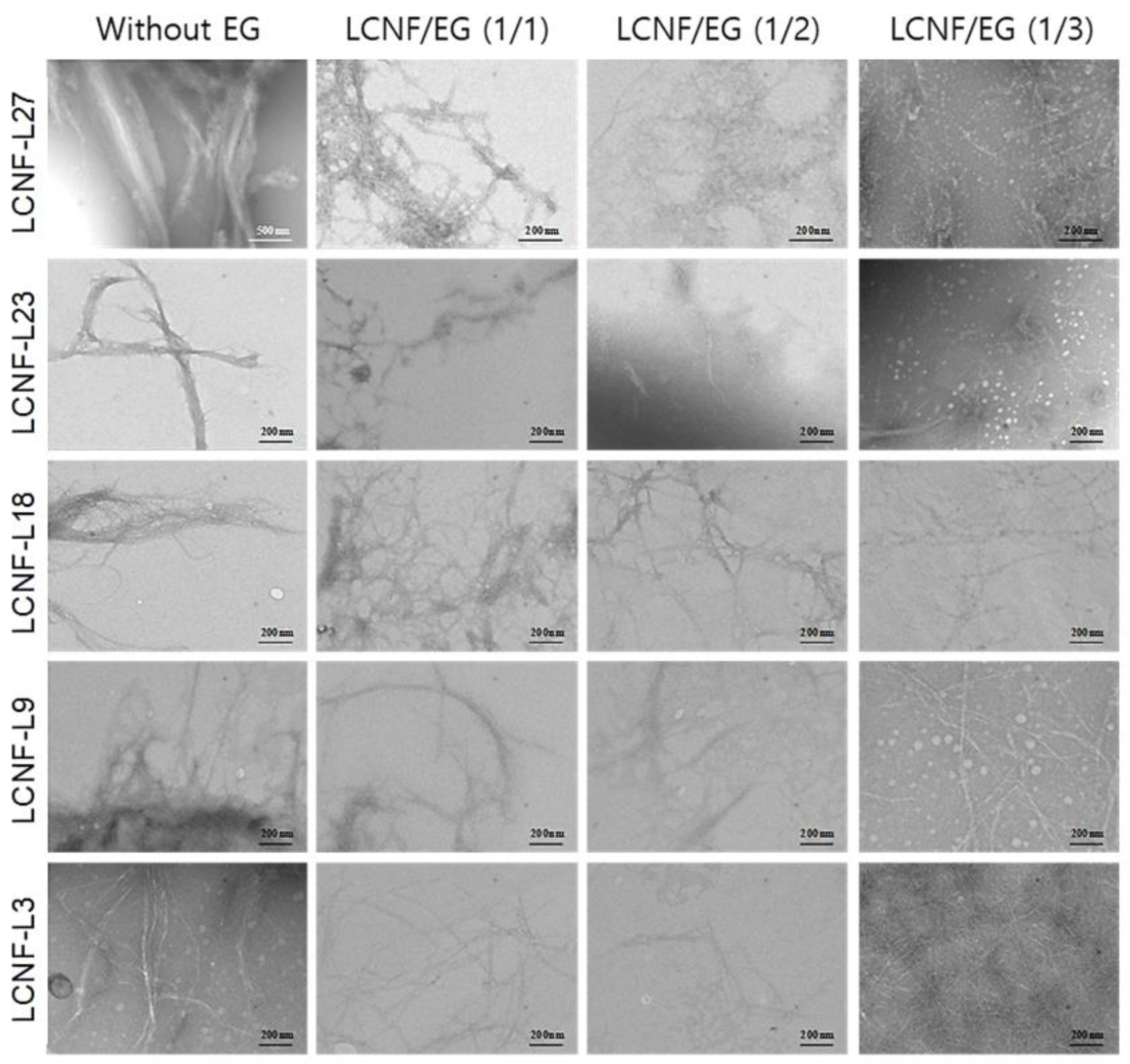
| Sample Code | LCNF-L27 | LCNF-L23 | LCNF-L18 | LCNF-L9 | LCNF-L3 |
|---|---|---|---|---|---|
| Lignin contents (%) | 27 | 23 | 18 | 9 | 3 |
| Sample Code | Without EG | Ratio of Substrate/Enzyme (LCNF/EG) | ||
|---|---|---|---|---|
| 1/1 | 1/2 | 1/3 | ||
| LCNF-L27 | 118.6 ± 41.8 | 104.4 ± 32.3 | 89.5 ± 32.9 | 71.4 ± 20.1 |
| LCNF-L23 | 101.4 ± 33.9 | 95.2 ± 29.9 | 57.3 ± 16.2 | 56.1 ± 15.9 |
| LCNF-L18 | 77.8 ± 26.5 | 80.1 ± 33.5 | 53.9 ± 22.1 | 40.9 ± 10.6 |
| LCNF-L9 | 74.8 ± 24.1 | 62.6 ± 26.0 | 44.2 ± 17.0 | N/A |
| LCNF-L3 | 59.6 ± 15.6 | 48.0 ± 20.4 | 21.4 ± 8.4 | N/A |
| Sample Code | Without EG | Ratio of Substrate/Enzyme (LCNF/EG) | ||
|---|---|---|---|---|
| 1/1 | 1/2 | 1/3 | ||
| LCNF-L27 | 200.2 ± 119.2 | 49.5 ± 21.5 | 18.0 ± 8.2 | 17.3 ± 7.6 |
| LCNF-L23 | 45.3 ± 31.6 | 24.9 ± 11.2 | 16.3 ± 10.0 | 12.6 ± 6.4 |
| LCNF-L18 | 20.8 ± 15.8 | 16.6 ± 9.5 | 12.1 ± 4.9 | 9.9 ± 4.0 |
| LCNF-L9 | 12.3 ± 5.8 | 7.9 ± 1.8 | 6.5 ± 1.1 | 4.4 ± 1.6 |
| LCNF-L3 | 6.7 ± 2.4 | 4.4 ± 1.3 | 3.9 ± 0.7 | 3.8 ± 1.5 |
| Sample Code | Without EG | Ratio of Substrate/Enzyme (LCNF/EG) | ||
|---|---|---|---|---|
| 1/1 | 1/2 | 1/3 | ||
| LCNF-L27 | 32.2 | 38.9 | 50.0 | 56.0 |
| LCNF-L23 | 39.3 | 44.7 | 52.7 | 50.9 |
| LCNF-L18 | 40.9 | 47.4 | 58.3 | 58.0 |
| LCNF-L9 | 41.7 | 48.6 | 52.7 | 54.5 |
| LCNF-L3 | 42.2 | 54.0 | 57.7 | 59.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.-H.; Hayashi, N.; Han, S.-Y.; Park, C.-W.; Febrianto, F.; Lee, S.-H.; Kim, N.-H. Changes in the Dimensions of Lignocellulose Nanofibrils with Different Lignin Contents by Enzymatic Hydrolysis. Polymers 2020, 12, 2201. https://doi.org/10.3390/polym12102201
Jang J-H, Hayashi N, Han S-Y, Park C-W, Febrianto F, Lee S-H, Kim N-H. Changes in the Dimensions of Lignocellulose Nanofibrils with Different Lignin Contents by Enzymatic Hydrolysis. Polymers. 2020; 12(10):2201. https://doi.org/10.3390/polym12102201
Chicago/Turabian StyleJang, Jae-Hyuk, Noriko Hayashi, Song-Yi Han, Chan-Woo Park, Fauzi Febrianto, Seung-Hwan Lee, and Nam-Hun Kim. 2020. "Changes in the Dimensions of Lignocellulose Nanofibrils with Different Lignin Contents by Enzymatic Hydrolysis" Polymers 12, no. 10: 2201. https://doi.org/10.3390/polym12102201
APA StyleJang, J.-H., Hayashi, N., Han, S.-Y., Park, C.-W., Febrianto, F., Lee, S.-H., & Kim, N.-H. (2020). Changes in the Dimensions of Lignocellulose Nanofibrils with Different Lignin Contents by Enzymatic Hydrolysis. Polymers, 12(10), 2201. https://doi.org/10.3390/polym12102201






