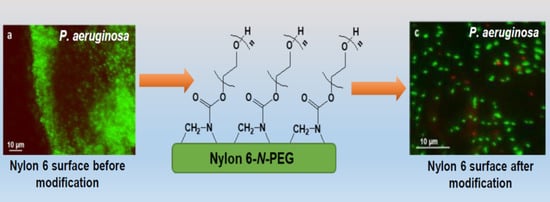
The Covalent Tethering of Poly(ethylene glycol) to Nylon 6 Surface via N,N′-Disuccinimidyl Carbonate Conjugation: A New Approach in the Fight against Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Samples
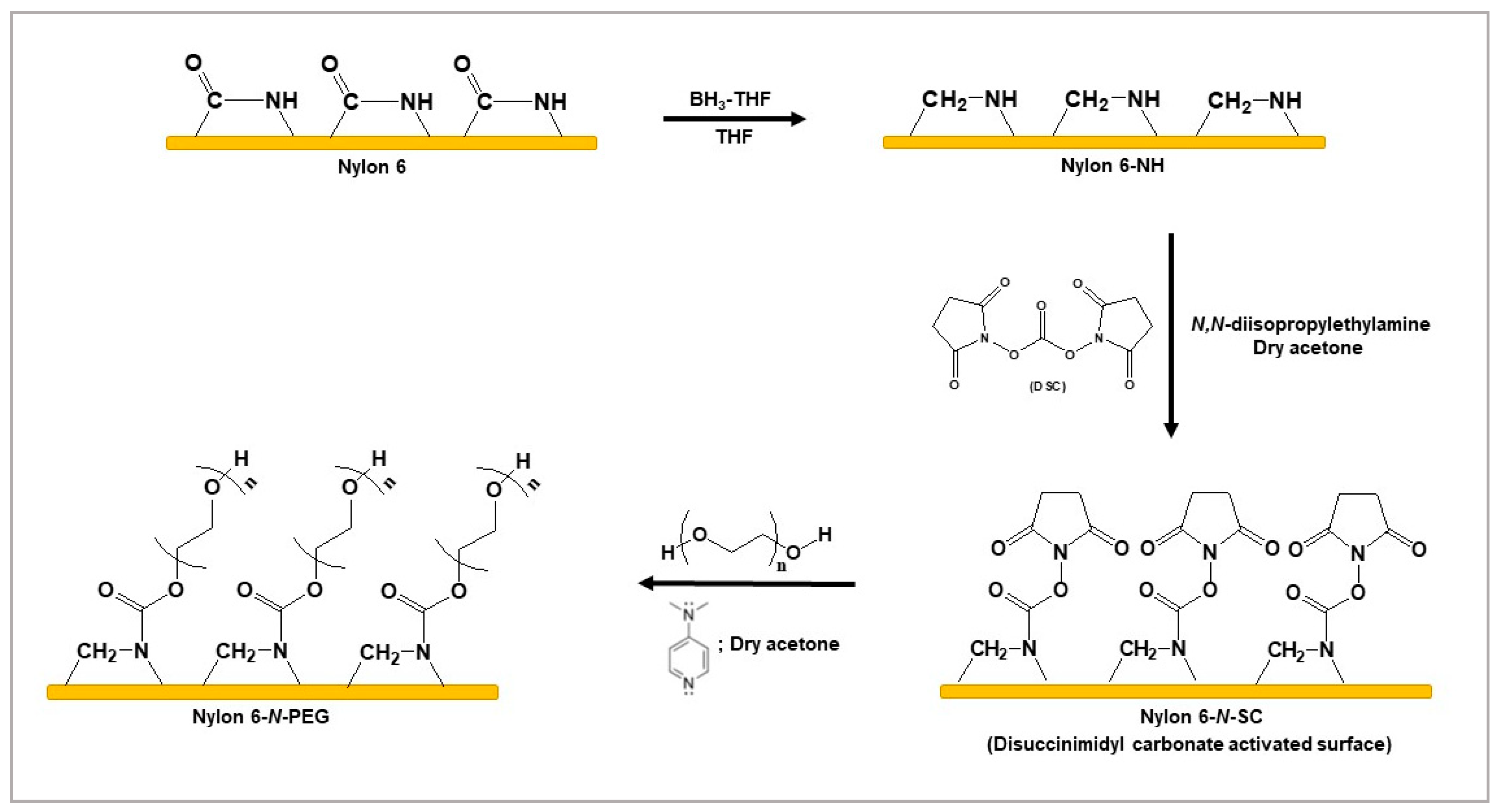
2.2.2. Reduction of Nylon 6 with BH3-THF
2.2.3. Tethering of PEG to the Nylon 6-NH Surface by Conjugating DSC
2.2.4. Surface Characterization
2.2.5. Bacterial Adhesion Tests
2.2.6. Cytotoxicity Assessment
3. Results
3.1. Tethering of PEG to the Nylon 6-NH Surface by Conjugating DSC
3.2. Characterization
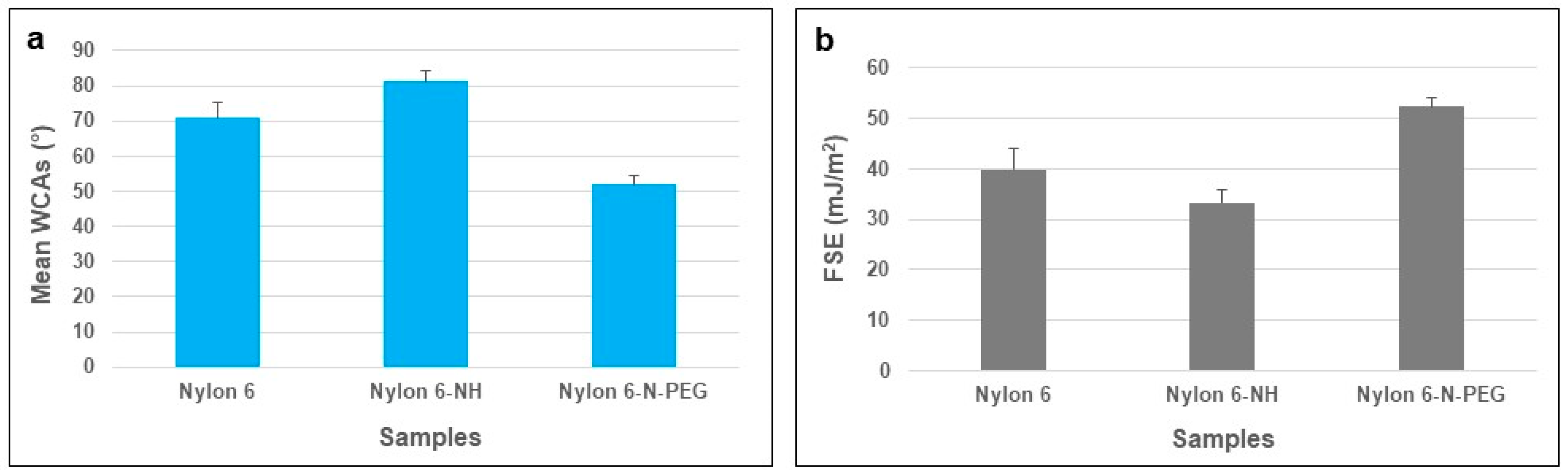
3.2.1. WCA and FSE Analyses of Pure and Modified Nylon 6 Samples
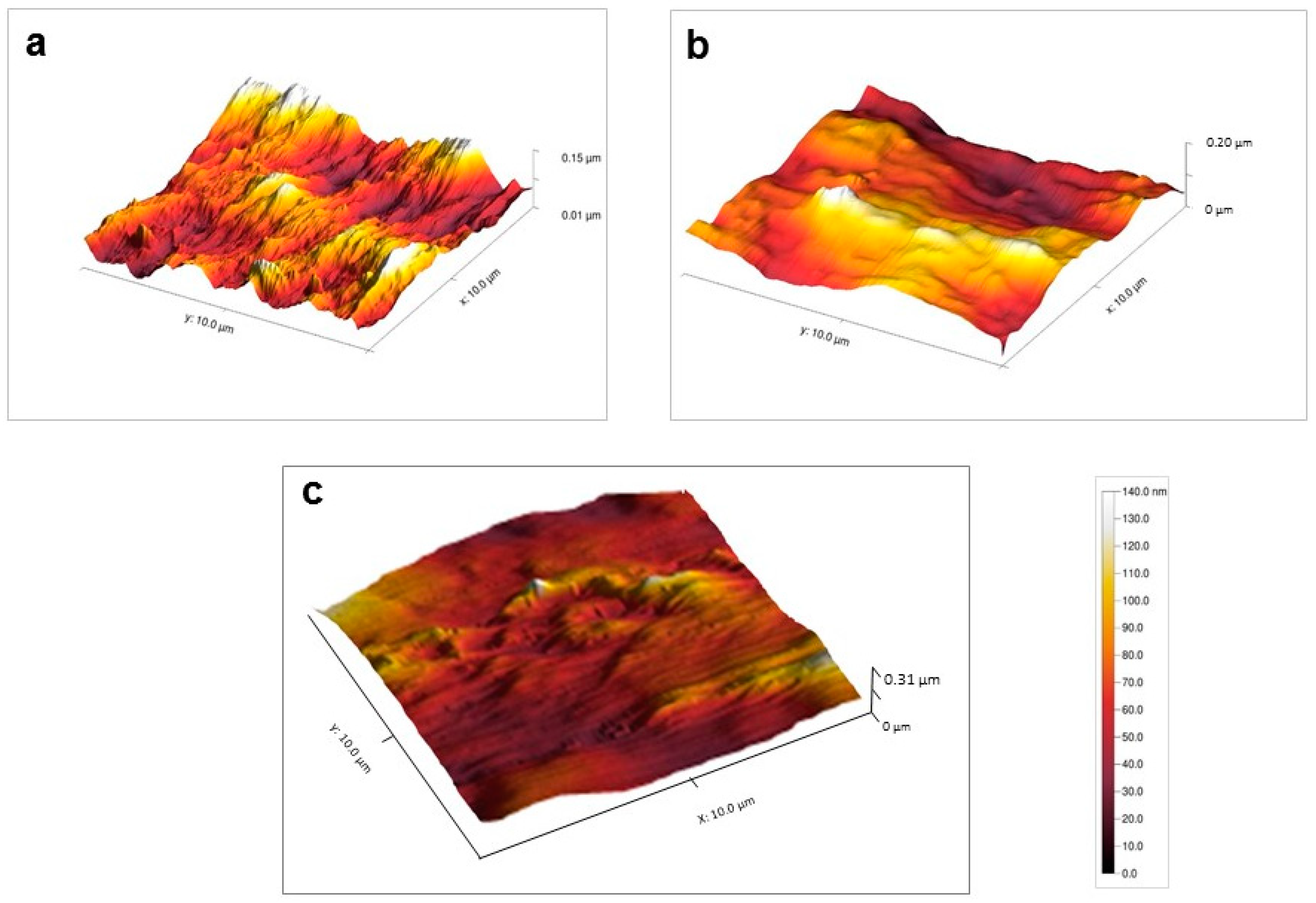
3.2.2. AFM Analyses
3.2.3. SEM Analyses
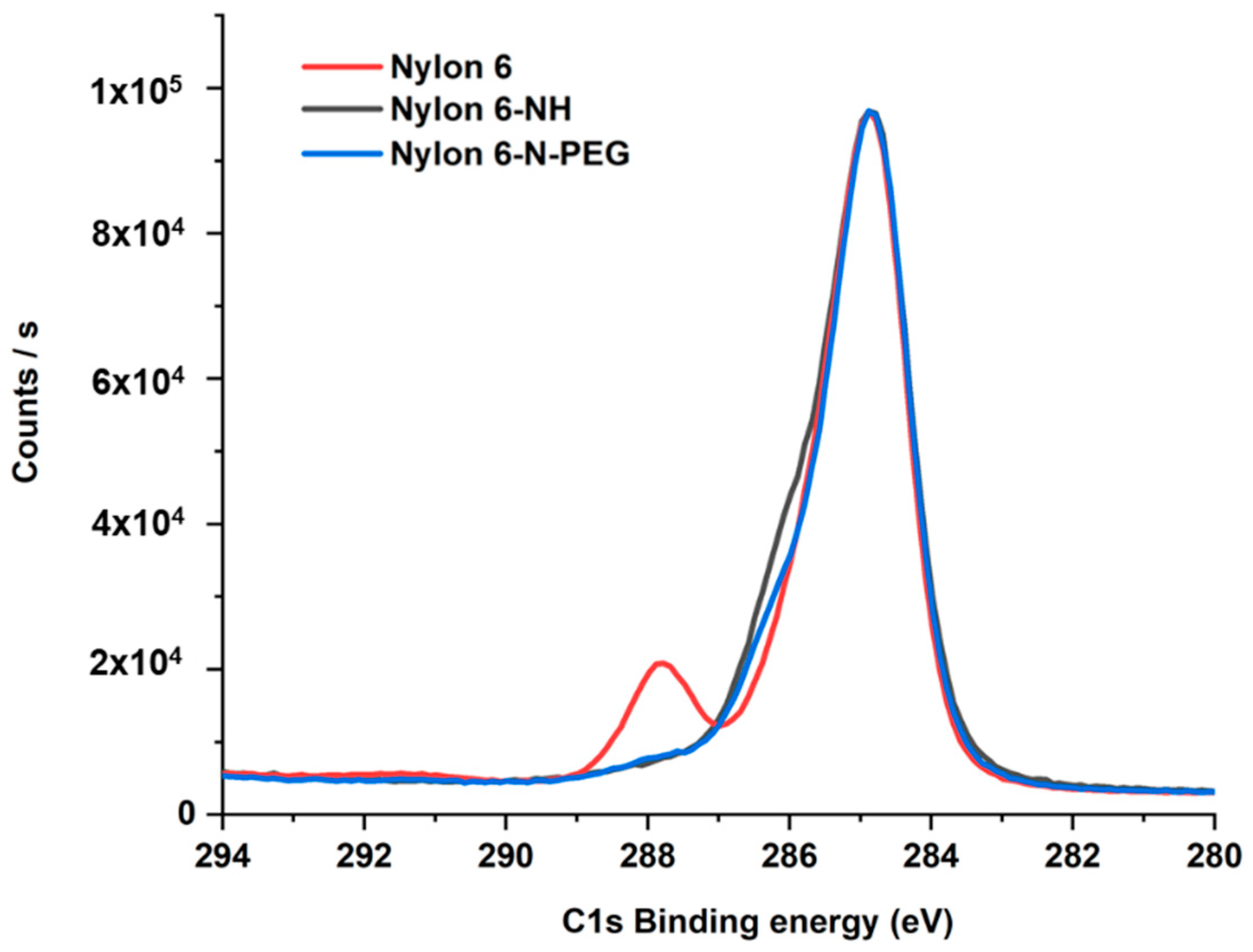
3.2.4. XPS Analyses
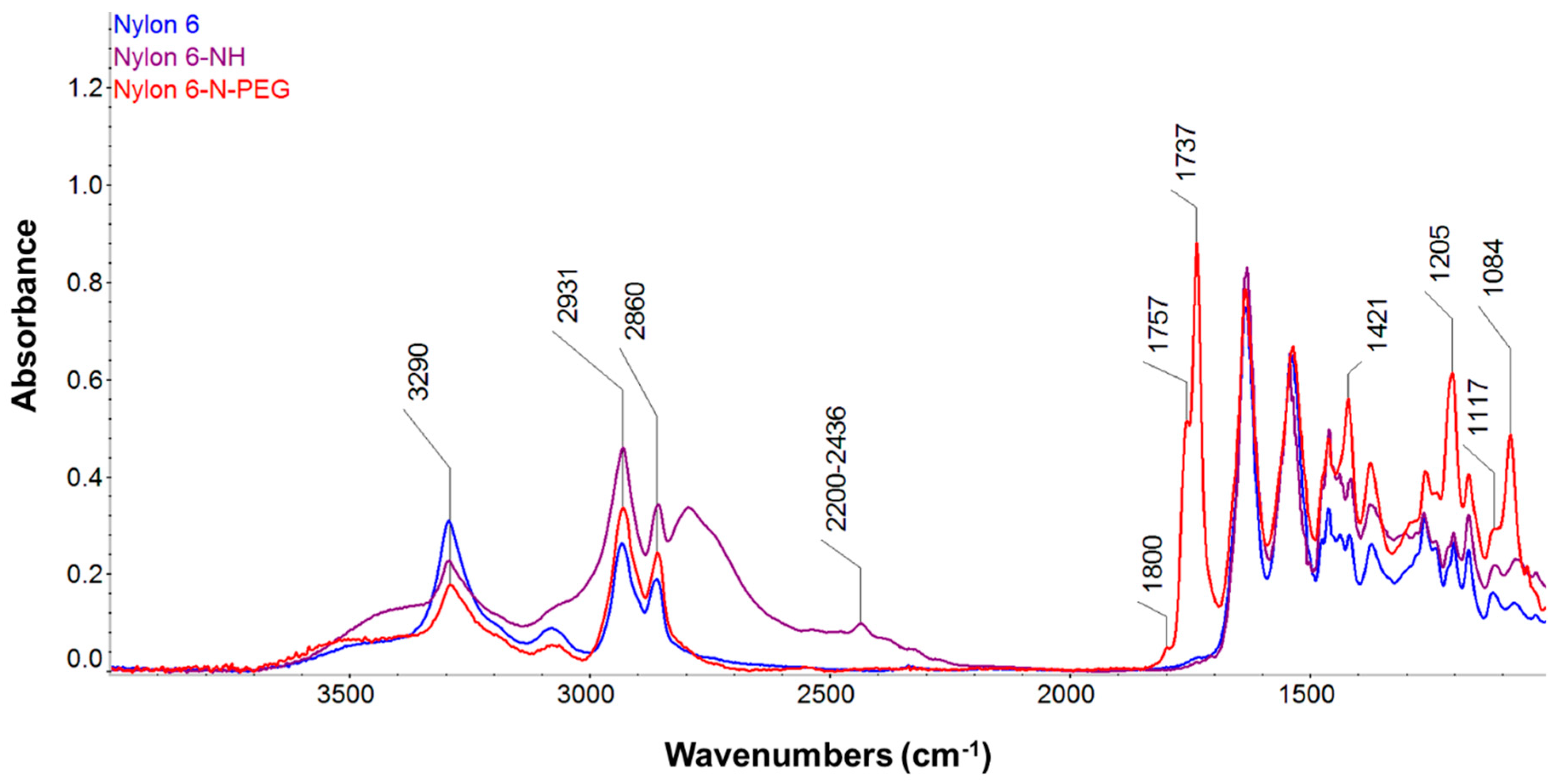
3.2.5. FT-IR Analyses
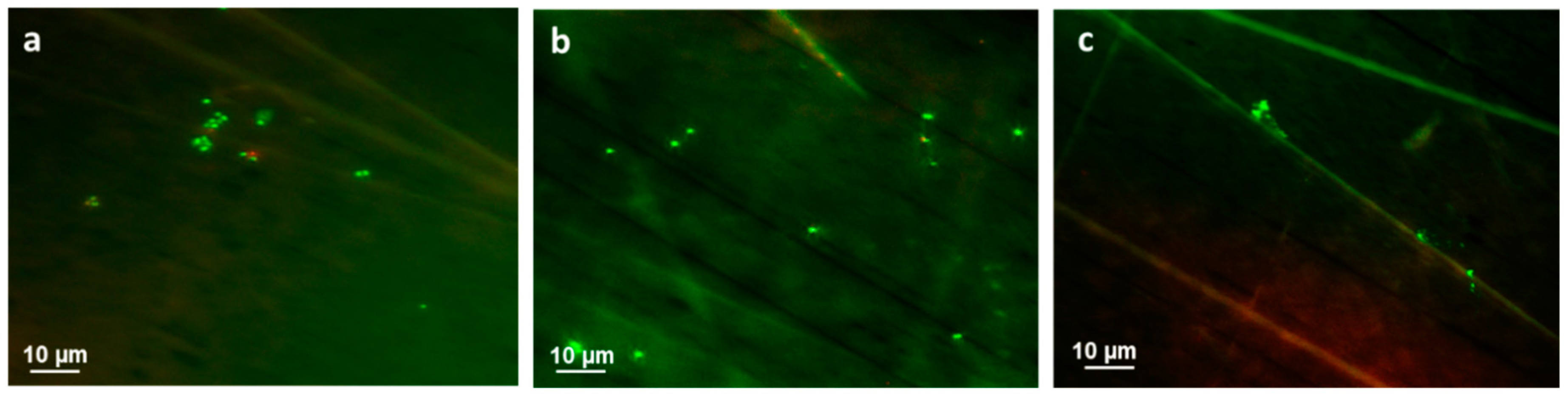
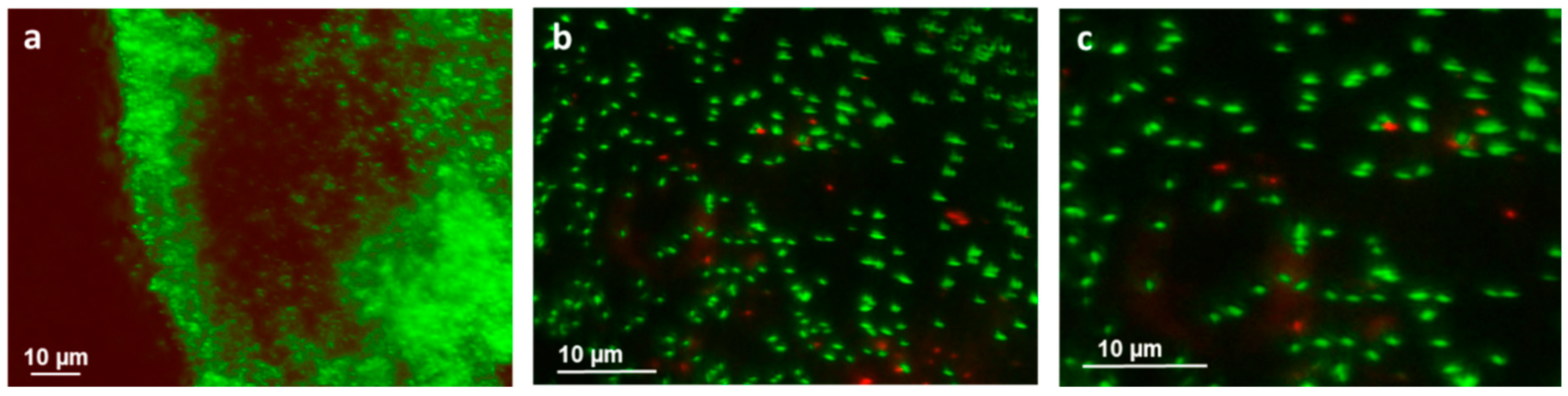
3.3. Bacterial Adhesion
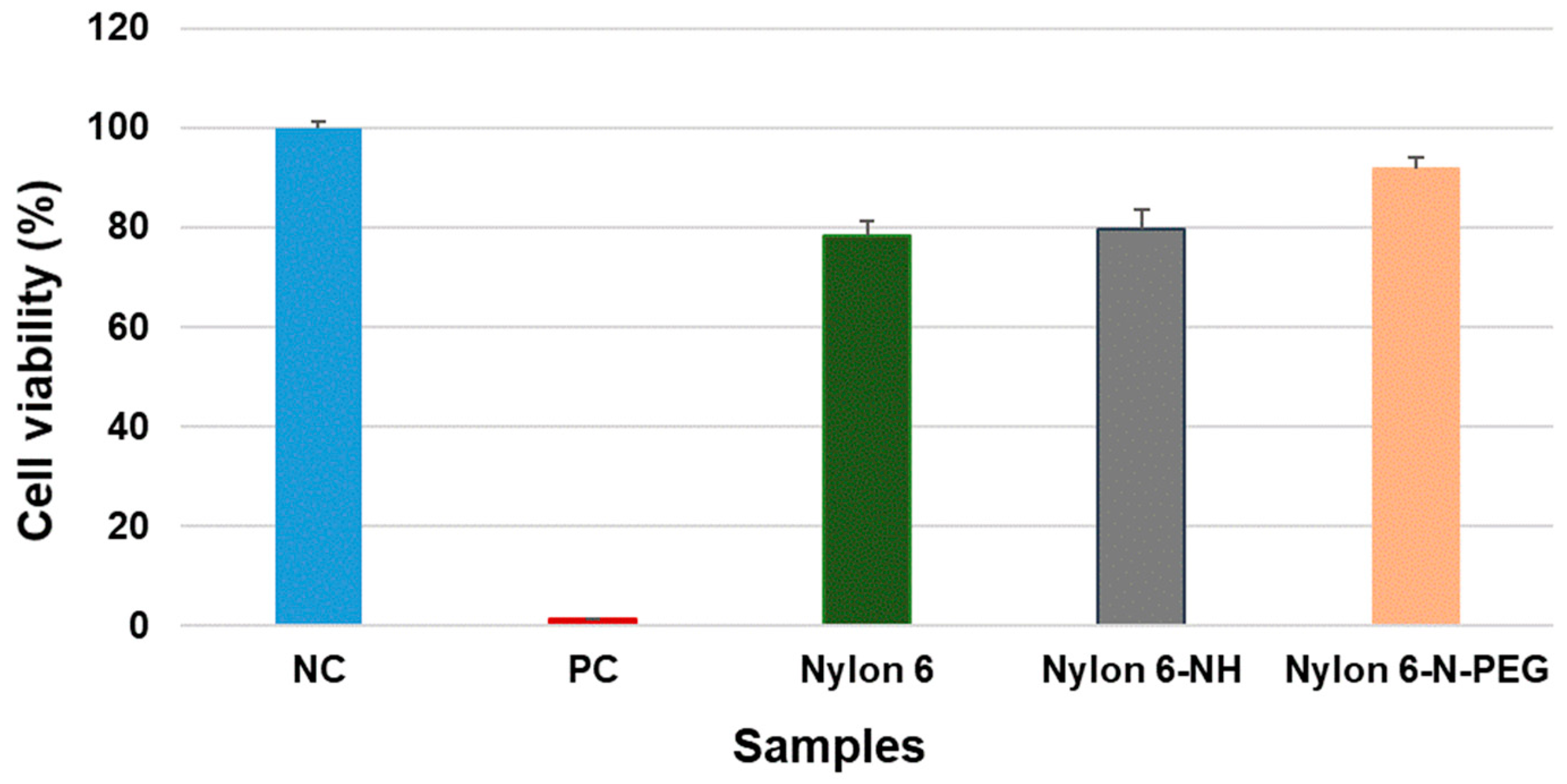
3.4. Cytotoxicity Assessment
3.4.1. Direct Contact Cytotoxicity
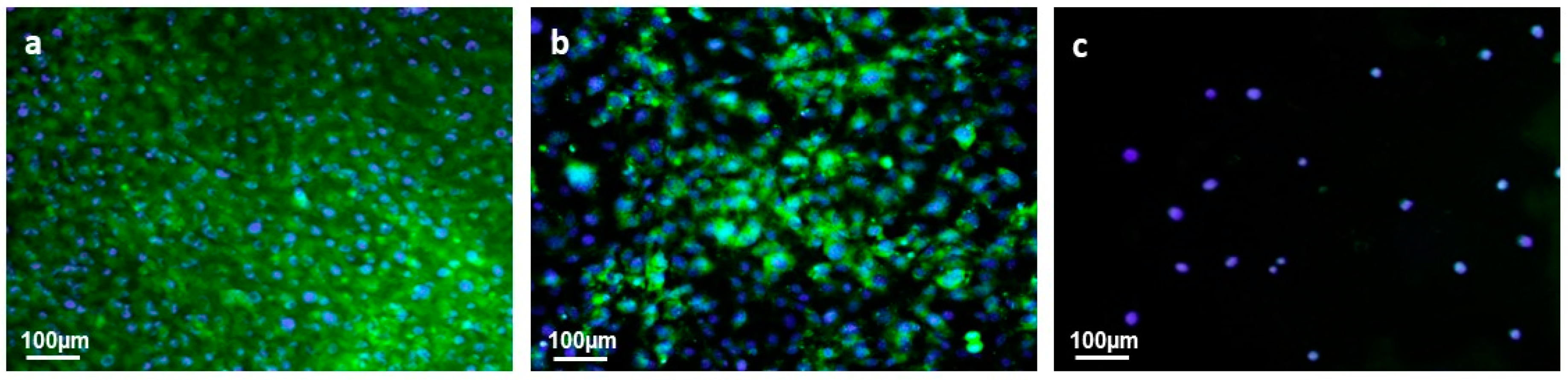
3.4.2. Cell Adhesion and Proliferation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Goor, O.J.; Brouns, J.E.; Dankers, P.Y. Introduction of anti-fouling coatings at the surface of supramolecular elastomeric materials via post-modification of reactive supramolecular additives. Polym. Chem. 2017, 8, 5228–5238. [Google Scholar] [CrossRef] [Green Version]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. Rsc Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Abedini, F.; Ahmadi, A.; Yavari, A.; Hosseini, V.; Mousavi, S. Comparison of silver nylon wound dressing and silver sulfadiazine in partial burn wound therapy. Int. Wound J. 2013, 10, 573–578. [Google Scholar] [CrossRef]
- Chu, C.C.; Tsai, W.C.; Yao, J.Y.; Chiu, S.S. Newly made antibacterial braided nylon sutures. I. In vitro qualitative and in vivo preliminary biocompatibility study. J. Biomed. Mater. Res. 1987, 21, 1281–1300. [Google Scholar] [CrossRef]
- Thokala, N.; Kealey, C.; Kennedy, J.; Brady, D.B.; Farrell, J.B. Characterisation of polyamide 11/copper antimicrobial composites for medical device applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1179–1186. [Google Scholar] [CrossRef]
- Zhang, X.; Brodus, D.S.; Hollimon, V.; Hu, H. A brief review of recent developments in the designs that prevent bio-fouling on silicon and silicon-based materials. Chem. Cent. J. 2017, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Ozcelik, B.; Ho, K.K.K.; Glattauer, V.; Willcox, M.; Kumar, N.; Thissen, H. Poly(ethylene glycol)-based coatings combining low-biofouling and quorum-sensing inhibiting properties to reduce bacterial colonization. ASC Biomater. Sci. Eng. 2017, 3, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P. Protein Surface Interactions in the Presence of Polyethylene Oxide. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Zhang, T.-D.; Zhang, X.; Deng, X. Applications of protein-resistant polymer and hydrogel coatings on biosensors and biomaterials. Ann. Biotechnol. 2018, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sarita. Research on polyethylene glycol, crosslinked polyethylene glycol & polyethylene glycol chitosan conjugate coating for biomedical application. Int. J. Recent Technol. Eng. 2019, 8, 2921–2925. [Google Scholar] [CrossRef]
- Francolini, I.; Silvestro, I.; Di Lisio, V.; Martinelli, A.; Piozzi, A. Synthesis, characterization, and bacterial fouling-resistance properties of polyethylene glycol-grafted polyurethane elastomers. Int. J. Mol. Sci. 2019, 20, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Damodaran, V.B.; Murthy, S.N. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Chang, L.; Liu, X.; Lin, J.; Liu, H.; Han, B.; Wang, S. Antibacterial Property of a Polyethylene Glycol-Grafted Dental Material. ACS Appl. Mater. Interfaces 2017, 9, 17688–17692. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. Apmis 2017, 125, 392–417. [Google Scholar] [CrossRef] [Green Version]
- Branch, D.W.; Wheeler, B.C.; Brewer, G.J.; Leckband, D.E. Long-term stability of grafted polyethylene glycol surfaces for use with microstamped substrates in neuronal cell culture. Biomaterials 2001, 22, 1035–1047. [Google Scholar] [CrossRef]
- Swar, S.; Máková, V.; Horáková, J.; Kejzlar, P.; Parma, P.; Stibor, I. A comparative study between chemically modified and copper nanoparticle immobilized Nylon 6 films to explore their efficiency in fighting against two types of pathogenic bacteria. Eur. Polym. J. 2020, 122, 109392. [Google Scholar] [CrossRef]
- Garrigou, A.; Laurent, C.; Berthet, A.; Colosio, C.; Jas, N.; Daubas-Letourneux, V.; Jackson Filho, J.M.; Jouzel, J.N.; Samuel, O.; Baldi, I.; et al. Critical review of the role of PPE in the prevention of risks related to agricultural pesticide use. Saf. Sci. 2020, 123, 104527. [Google Scholar] [CrossRef]
- Irzmańska, E.; Brochocka, A. Modified polymer materials for use in selected personal protective equipment products. Autex Res. J. 2017, 17, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Jiang, H.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-mediated grafting of poly (ethylene glycol) on polyamide and polyester surfaces and evaluation of antifouling ability of modified substrates. Langmuir 2007, 23, 7306–7313. [Google Scholar] [CrossRef] [PubMed]
- Wulf, K.; Teske, M.; Löbler, M.; Luderer, F.; Schmitz, K.P.; Sternberg, K. Surface functionalization of poly (ε-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. J. Biomed. Mater. Res.-Part. B Appl. Biomater. 2011, 98, 89–100. [Google Scholar] [CrossRef]
- Yang, M.; Tsang, E.M.W.; Wang, Y.A.; Peng, X.; Yu, H.Z. Bioreactive surfaces prepared via the self-assembly of dendron thiols and subsequent dendrimer bridging reactions. Langmuir 2005, 21, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123705013. [Google Scholar]
- Meschaninova, M.I.; Novopashina, D.S.; Semikolenova, O.A.; Silnikov, V.N.; Venyaminova, A.G. Novel convenient approach to the solid-phase synthesis of oligonucleotide conjugates. Molecules 2019, 24, 4266. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.K.; Doung, T.T.; McKee, S.P.; Thompson, W.J. N, N‘-dissuccinimidyl carbonate: A useful reagent for alkoxycarbonylation of amines. Tetrahedron Lett. 1992, 33, 2781–2784. [Google Scholar] [CrossRef]
- Diamanti, S.; Arifuzzaman, S.; Elsen, A.; Genzer, J.; Vaia, R.A. Reactive patterning via post-functionalization of polymer brushes utilizing disuccinimidyl carbonate activation to couple primary amines. Polymer 2008, 49, 3770–3779. [Google Scholar] [CrossRef]
- Gonçalves, I.; Abreu, A.S.; Matamá, T.; Ribeiro, A.; Gomes, A.C.; Silva, C.; Cavaco-Paulo, A. Enzymatic synthesis of poly(catechin)-antibiotic conjugates: An antimicrobial approach for indwelling catheters. Appl. Microbiol. Biotechnol. 2015, 99, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.; Chi, Y.S.; Lee, K.B.; Kim, Y.G.; Choi, I.S. Functionalization of poly(oligo(ethylene glycol) methacrylate) films on gold and Si/SiO2 for immobilization of proteins and cells: SPR and QCM studies. Biomacromolecules 2007, 8, 3922–3929. [Google Scholar] [CrossRef]
- Cai, L.L.; Liu, P.; Li, X.; Huang, X.; Ye, Y.Q.; Chen, F.Y.; Yuan, H.; Hu, F.Q.; Du, Y.Z. RGD peptide-mediated chitosan-based polymeric micelles targeting delivery for integrin-overexpressing tumor cells. Int. J. Nanomed. 2011, 6, 3499–3508. [Google Scholar]
- Chen, H.; Zhang, Y.; Li, D.; Hu, X.; Wang, L.; McClung, W.G.; Brash, J.L. Surfaces having dual fibrinolytic and protein resistant properties by immobilization of lysine on polyurethane through a PEG spacer. J. Biomed. Mater. Research. Part. A 2009, 90, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Swar, S.; Zajícová, V.; Müllerová, J.; Šubrtová, P.; Horáková, J.; Dolenský, B.; Řezanka, M.; Stibor, I. Effective poly(ethylene glycol) methyl ether grafting technique onto Nylon 6 surface to achieve resistance against pathogenic bacteria Staphylococcus aureus and Pseudomonas aeruginosa. J. Mater. Sci. 2018, 53, 14104–14120. [Google Scholar] [CrossRef]
- Jia, X.; Herrera-Alonso, M.; McCarthy, T.J. Nylon surface modification. Part 1. Targeting the amide groups for selective introduction of reactive functionalities. Polymer 2006, 14, 4916–4924. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, L.; An, X.; Liu, C.; Hu, Y. Surface Engineering of Thin Film Composite Polyamide Membranes with Silver Nanoparticles through Layer-by-Layer Interfacial Polymerization for Antibacterial Properties. ACS Appl. Mater. Interfaces 2017, 9, 40987–40997. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopaminemodified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and celladhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Vaillard, V.A.; Menegon, M.; Neuman, N.I.; Vaillard, S.E. mPEG–NHS carbonates: Effect of alkyl spacerson the reactivity: Kinetic and mechanistic insights. Appl. Polym. Sci. 2019, 136, 47028. [Google Scholar] [CrossRef]
- Romero-Vargas Castrillón, S.; Lu, X.; Shaffer, D.L.; Elimelech, M. Amine enrichment and poly(ethylene glycol) (PEG) surface modification of thin-film composite forward osmosis membranes for organic fouling control. J. Membr. Sci. 2014, 450, 331–339. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Timm, D.A. Relationship of substratum wettability measurements and initial Staphylococcus aureau adhesion to films and fabrics. J. Colloid Interface Sci. 1988, 123, 275–286. [Google Scholar] [CrossRef]
- Pei, J.; Hall, H.; Spencer, N.D. The role of plasma proteins in cell adhesion to PEG surface-density-gradient-modified titanium oxide. Biomaterials 2011, 32, 8968–8978. [Google Scholar] [CrossRef] [PubMed]
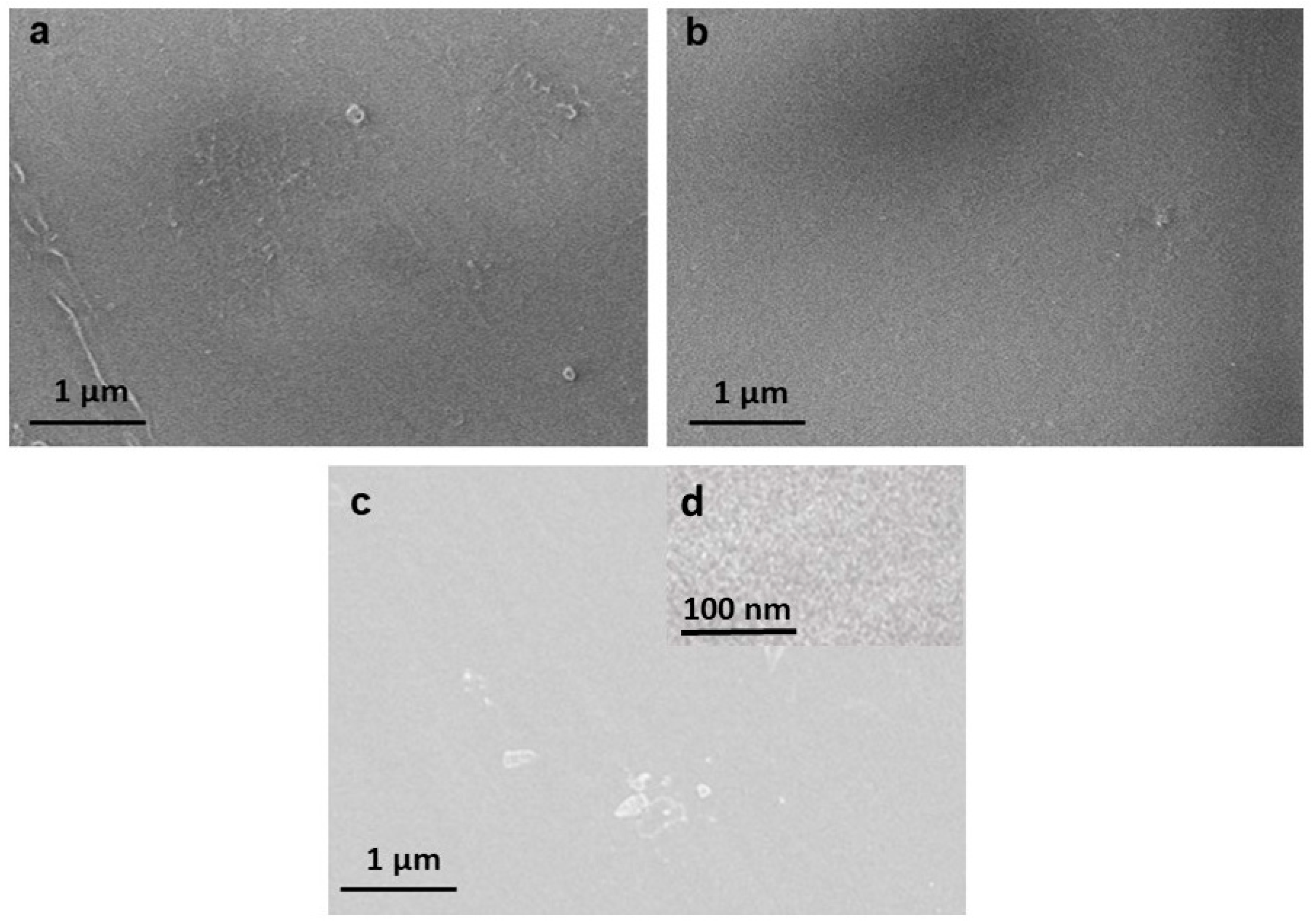
| Samples | Surface Area | |
|---|---|---|
| (1 × 1) µm2 | (10 × 10) µm2 | |
| Nylon 6 | 5.3 ± 0.4 nm | 35.7 ± 4.6 nm |
| Nylon 6-NH | 1.4 ± 0.3 nm | 7.5 ± 0.2 nm |
| Nylon 6-N-PEG | 3.8 ± 0.2 nm | 18.4 ± 1.1 nm |
| Sample | Elemental Composition of Sample Surface (%) | ||
|---|---|---|---|
| C | N | O | |
| Nylon 6 | 76.11 | 11.15 | 12.74 |
| Nylon 6-NH | 81.98 | 10.94 | 7.08 |
| Nylon 6-N-PEG | 74.88 | 10.53 | 14.59 |
| Sample | Cell Count (Number of Cells/cm2) | |||
|---|---|---|---|---|
| 1 Day | 7 Days | |||
| Mean | SD | Mean | SD | |
| Nylon 6 | 15,911 | ±3970 | uncountable | - |
| Nylon 6-NH | 12,254 | ±2350 | 318,202 | ±40,285 |
| Nylon 6-N-PEG | 493 | ±110 | 61,912 | ±1405 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swar, S.; Máková, V.; Stibor, I. The Covalent Tethering of Poly(ethylene glycol) to Nylon 6 Surface via N,N′-Disuccinimidyl Carbonate Conjugation: A New Approach in the Fight against Pathogenic Bacteria. Polymers 2020, 12, 2181. https://doi.org/10.3390/polym12102181
Swar S, Máková V, Stibor I. The Covalent Tethering of Poly(ethylene glycol) to Nylon 6 Surface via N,N′-Disuccinimidyl Carbonate Conjugation: A New Approach in the Fight against Pathogenic Bacteria. Polymers. 2020; 12(10):2181. https://doi.org/10.3390/polym12102181
Chicago/Turabian StyleSwar, Sumita, Veronika Máková, and Ivan Stibor. 2020. "The Covalent Tethering of Poly(ethylene glycol) to Nylon 6 Surface via N,N′-Disuccinimidyl Carbonate Conjugation: A New Approach in the Fight against Pathogenic Bacteria" Polymers 12, no. 10: 2181. https://doi.org/10.3390/polym12102181
APA StyleSwar, S., Máková, V., & Stibor, I. (2020). The Covalent Tethering of Poly(ethylene glycol) to Nylon 6 Surface via N,N′-Disuccinimidyl Carbonate Conjugation: A New Approach in the Fight against Pathogenic Bacteria. Polymers, 12(10), 2181. https://doi.org/10.3390/polym12102181