Study on the Moisture Absorption and Thermal Properties of Hygroscopic Exothermic Fibers and Related Interactions with Water Molecules
Abstract
1. Introduction
2. Materials and Experimental
2.1. Physical Properties Test and Preparation of Fiber Batting Samples
2.2. IR Camera Test Analysis
2.3. Scanning Electron Microscopy (SEM) and FT-IR Analysis
2.4. Determination of Sorption Isotherms and Model Fitting
2.5. TGA-DSC Analysis
3. Results and Discussion
3.1. Thermal Management Test
3.2. Surface Characterization
3.3. Moisture Adsorption Equilibria and Isosteric Heats
3.4. Thermal Properties Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mei, T.; Guo, Q.H.; Wu, Y.Z. Study on the preparation and properties of high efficiency heat and light conversion polyester fiber. Ion Exch. Adsorpt. 2017, 33, 271–279. [Google Scholar]
- Xu, J.X.; Liu, L.; Li, J. Development and performance evaluation of electrically-heated textile based on silver-coated yarn. J. Text. Res. 2016, 37, 24–28. [Google Scholar]
- Chan, C.Y.L.; Burton, D.R. Local heating source for shallow water divers. J. Power Sources 1981, 6, 291–304. [Google Scholar] [CrossRef]
- Hu, H.B.; Qi, L. Properties of Hygroscopic Exothermic Type of Viscose Fibers. J. Funct. Polym. 2011, 24, 199–203. [Google Scholar]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Hu, H.B.; Qi, L. The development and application of hygroscopic and exothermic fiber. Synth. Fiber China 2010, 3, 13–16. [Google Scholar]
- Tokunaga, T.; Narushima, T.; Yonezawa, T. A method for accurate temperature measurement using infrared thermal camera. J. Electron Microsc. 2012, 61, 223–227. [Google Scholar] [CrossRef]
- Seiichi, K.; Tatsuo, I.; Yufu, A. Adsorption Science, 2nd ed.; Chemical Industry Press: Beijing, China, 2006; pp. 32–51. [Google Scholar]
- Zhao, Z.G. Principle of Adsorption Application; Chemical Industry Press: Beijing, China, 2005; pp. 75–132. [Google Scholar]
- Netzer, L.; Iscovici, R.; Sagiv, J. Adsorbed monolayers versus Langmuir-Blodgett monolayers—Why and how? II: Characterization of built-up films constructed by stepwise adsorption of individual monolayers. Thin Solid Films 1983, 100, 67–76. [Google Scholar] [CrossRef]
- Bartelt, M.C.; Privman, V. Kinetics of irreversible monolayer and multilayer adsorption. Int. J. Mod. Phys. B 1991, 5, 2883–2907. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Teller, E. On a theory of Van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Van den Berg, C.; Bruin, S. Water activity and its estimation in food systems. In Water Activity: Influences on Food Quality; Academic Press: New York, NY, USA, 1981; pp. 147–177. [Google Scholar]
- FerroFontan, C.; Chirife, J.; Sancho, E.; Iglesias, H.A. Analysis of a model for water sorption phenomena in foods. J. Food Sci. 1982, 47, 1590–1594. [Google Scholar] [CrossRef]
- Henderson, S.M. A basic concept of equilibrium moisture. Agric. Eng. 1952, 33, 29–32. [Google Scholar]
- Oswin, C.R. The kinetics of package life. III. Isotherm. J. Soc. Chem. Ind. 1946, 65, 419–421. [Google Scholar] [CrossRef]
- Smith, S.E. The sorption of water vapor by high polymers. J. Am. Chem. Soc. 1947, 69, 646. [Google Scholar] [CrossRef]
- Peleg, M. Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J. Food Process. Eng. 1993, 16, 21–37. [Google Scholar] [CrossRef]
- Jain, S.K.; Verma, R.C.; Sharma, G.P. Studies on moisture sorption isotherms for osmotically dehydrated papaya cubes and verification of selected models. J. Food Sci. Technol. 2010, 47, 343–346. [Google Scholar] [CrossRef]
- Shivhare, U.S. Models for sorption isotherms for foods: A review. Dry. Technol. 2006, 24, 917–930. [Google Scholar]
- Hatakeyama, T.; Nakamura, K.; Hatakeyama, H. Determination of bound water content in polymers by DTA, DSC and TG. Thermochim. Acta 1988, 123, 153–161. [Google Scholar] [CrossRef]
- Bedane, A.H.; Xiao, H. Structural and thermodynamic characterization of modified cellulose fiber-based materials and related interactions with water vapor. Appl. Surface Sci. 2015, 351, 725–737. [Google Scholar] [CrossRef]
- Shinyashiki, N.; Shimomura, M.; Ushiyama, T. Dynamics of water in partially crystallized polymer/water mixtures studied by dielectric spectroscopy. J. Phys. Chem. B 2007, 111, 10079–10087. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Hatakeyama, T. Interaction between water and hydrophilic polymers. Thermochim. Acta 1998, 308, 3–22. [Google Scholar] [CrossRef]
- O’Brien, M.F.E. The control of humidity by saturated salt solutions. J. Sci. Instrum. 1948, 25, 73–76. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Sun, L.J. Studies on the moisture adsorption isotherms of dried honey prepared by microwave-vacuum drying. Dry. Technol. Equip. 2007, 3, 31–35. [Google Scholar]
- Novak, I. From the Arrhenius to the Clausius—Clapeyron equation. Chem. Educ. 2002, 7, 347–348. [Google Scholar] [CrossRef]
- Rizvi, S.S.H.; Benado, A.L. Thermodynamic properties of dehydrated foods. Food Technol. 1984, 38, 83–92. [Google Scholar]
- Shan, X.L.; Liu, N. Study of testing method of textile’s moisture-absorption and heat-generating property. Knitt. Ind. 2015, 3, 67–69. [Google Scholar]
- Ping, Z.H.; Nguyen, Q.T.; Chen, S.M. States of water in different hydrophilic polymers-DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Goula, A.M.; Karapantsios, T.D.; Achilias, D.S. Water sorption isotherms and glass transition temperature of spray dried tomato pulp. J. Food Eng. 2008, 85, 73–83. [Google Scholar] [CrossRef]
- Timmermann, E.O. Multilayer sorption parameters: BET or GAB values. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 220, 235–260. [Google Scholar] [CrossRef]
- Sasaki, S. Elastic properties of swollen polyelectrolyte gels in aqueous salt solutions. J. Chem. Phys. 2006, 124, 94903. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Edwards, S.F. New model of polymer entanglement: Localized Gauss integral model. Plateau modulus GN, topological second virial coefficient A θ2 and physical foundation of the tube model. J. Chem. Phys. 1989, 90, 4567–4581. [Google Scholar] [CrossRef]
- Skouri, R.; Schosseler, F.; Munch, J.P. Swelling and elastic properties of polyelectrolyte gels. Macromolecules 1995, 28, 197–210. [Google Scholar] [CrossRef]
- Quirijns, E.J.; Van Boxtel, A.J.; Van Loon, W.K. Sorption isotherms, GAB parameters and isosteric heat of sorption. J. Sci. Food Agric. 2005, 85, 1805–1814. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Relationship between hydrogen bonding and bound water in polyhydroxystyrene derivatives. Polymer 1983, 24, 871–876. [Google Scholar] [CrossRef]
- Masuzawa, M.; Sterling, C. Gel-water relationships in hydrophilic polymers: Thermodynamics of sorption of water vapor. J. Appl. Polym. Sci. 2010, 12, 2023–2032. [Google Scholar] [CrossRef]



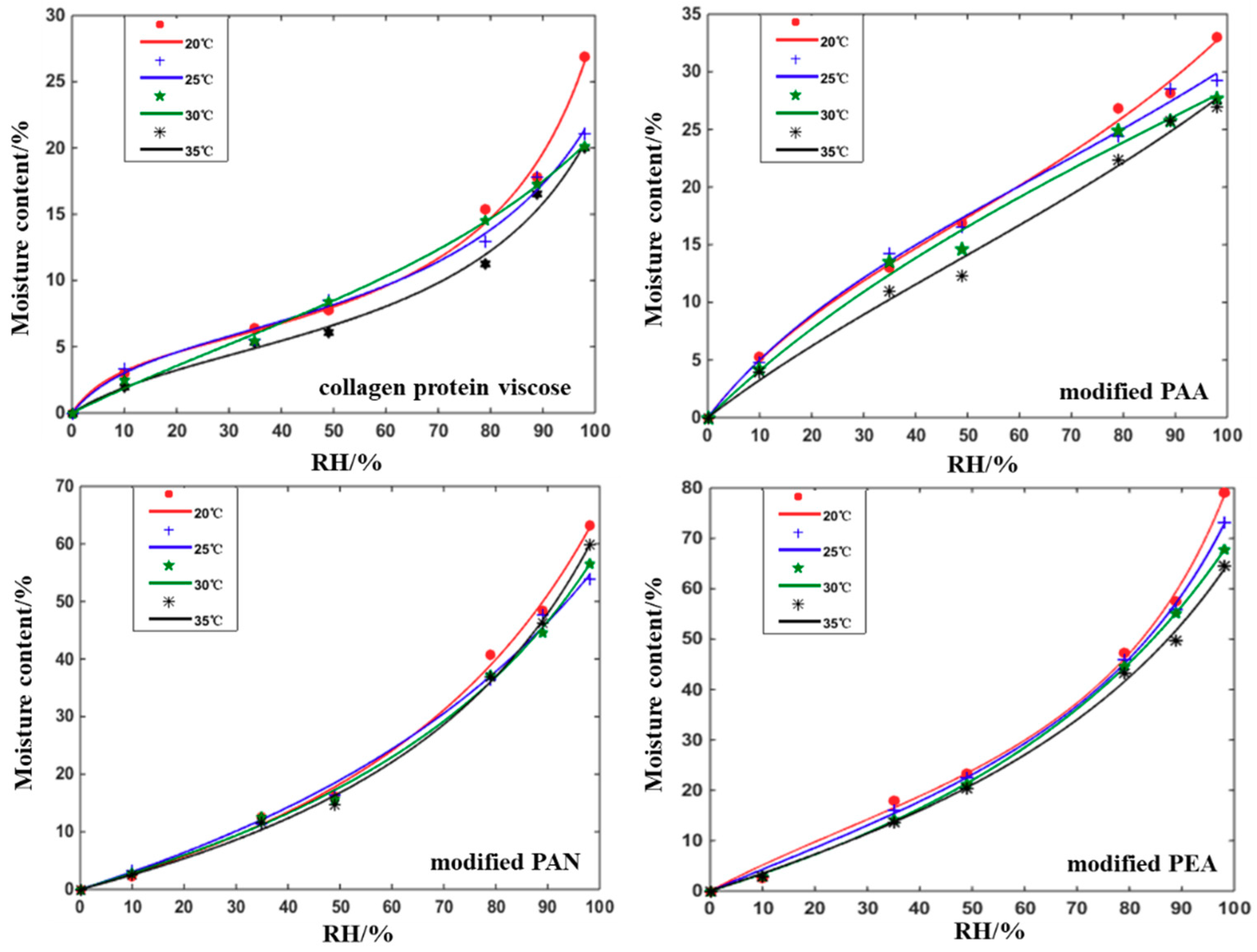
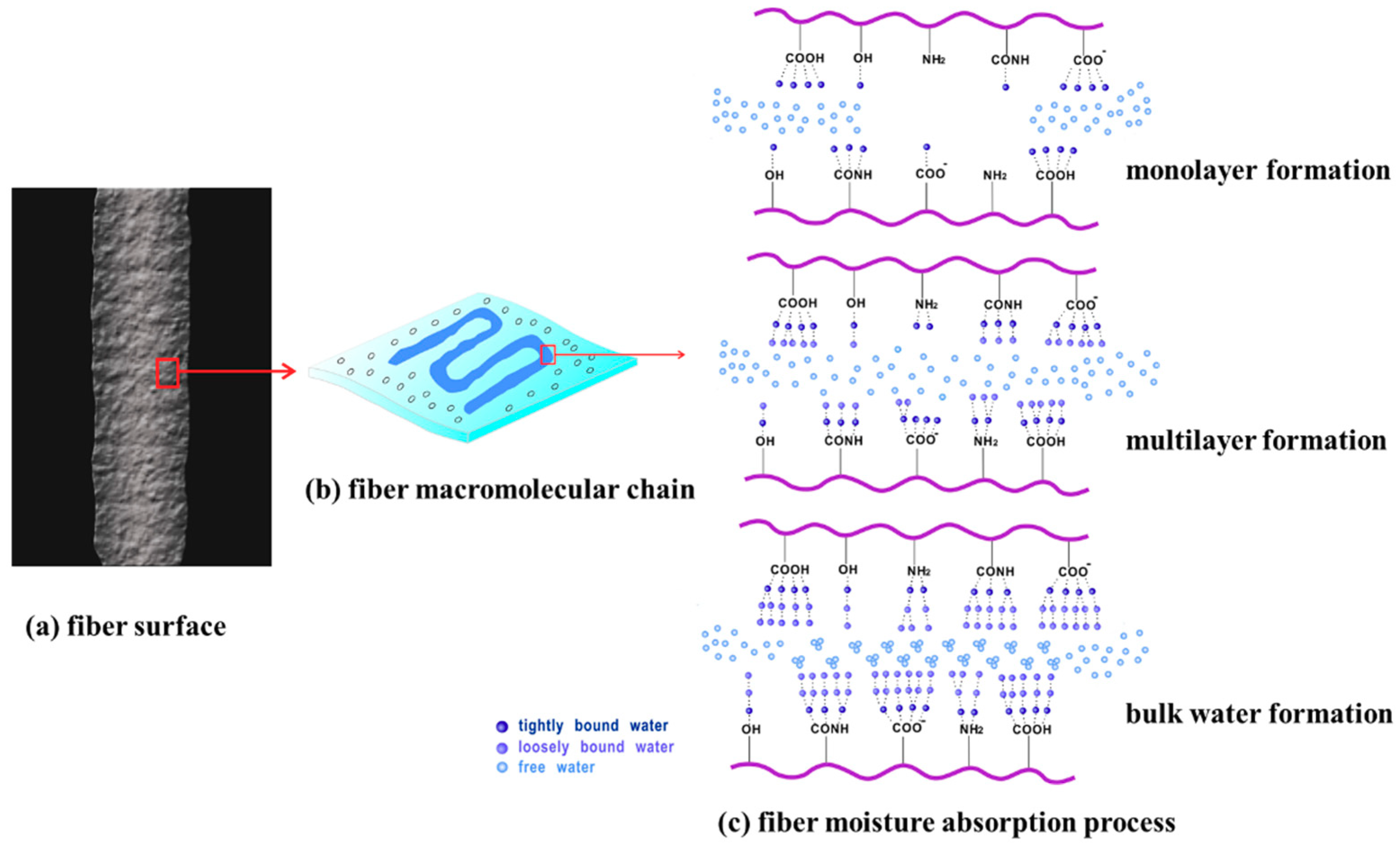

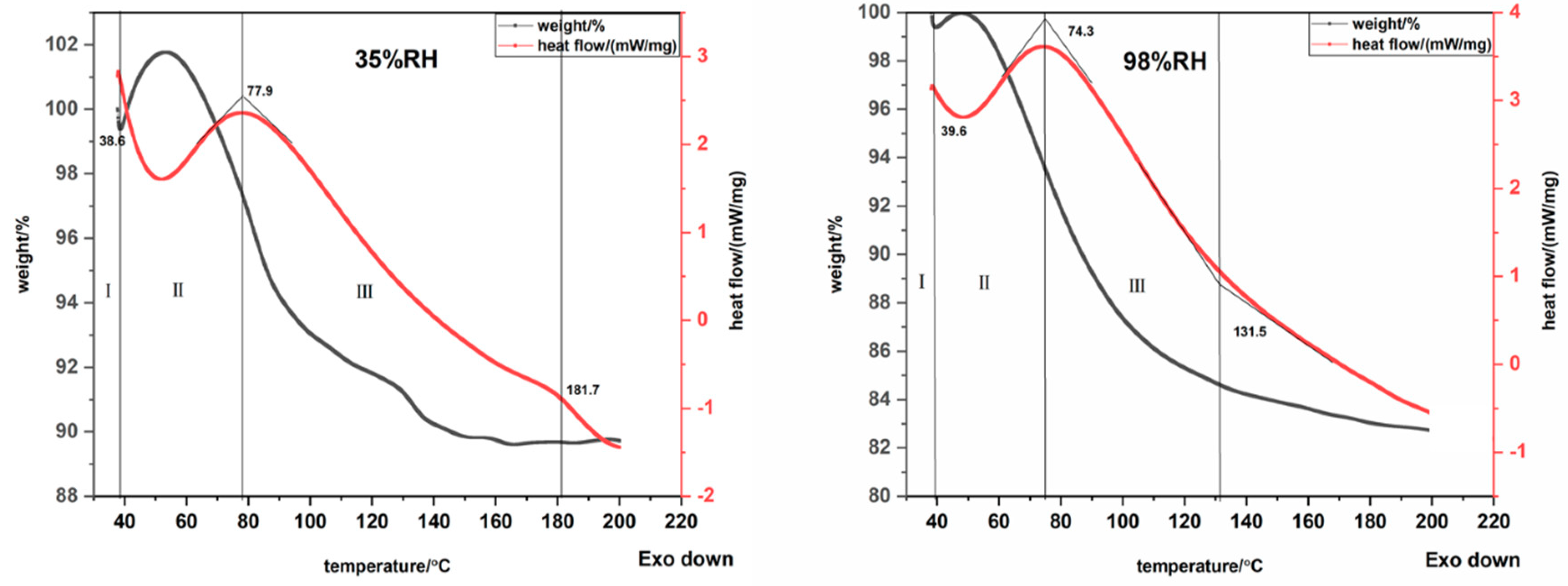

| Fiber Samples | Linear Density/(D) | Length/(mm) | Intensity/(cN/dtex) | Elongation/(%) | Moisture Regain/(%) | Grammage/(g/m2) | Thickness/(mm) |
|---|---|---|---|---|---|---|---|
| Collagen protein viscose | 1.5 | 38 | 2.34 | 19.77 | 12.05 | 2.23 | 8 |
| Modified PAA | 3.1 | 51 | 0.67 | 12.85 | 29.64 | 2.23 | 11 |
| Modified PEA | 1.3 | 33 | 0.72 | 22.4 | 35.36 | 2.63 | 9 |
| Modified PAN | 2.5 | 51 | 0.58 | 39.94 | 26.71 | 2.65 | 8 |
| Model | T/°C | Collagen Protein Viscose | Modified PAN | Modified PEA | Modified PAA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | SSE | RMSE | R2 | SSE | RMSE | R2 | SSE | RMSE | R2 | SSE | RMSE | ||
| GAB | 20 | 0.9951 | 0.000261 | 0.008071 | 0.9972 | 0.001001 | 0.01582 | 0.997 | 0.001562 | 0.01976 | 0.997 | 0.000273 | 0.008261 |
| 25 | 0.993 | 0.000249 | 0.007899 | 0.9997 | 8.19 × 10−5 | 0.004526 | 0.9987 | 0.000594 | 0.01219 | 0.9963 | 0.00029 | 0.008512 | |
| 30 | 0.9978 | 7.96 × 10−5 | 0.00446 | 0.9977 | 0.000668 | 0.01293 | 0.9998 | 7.56 × 10−5 | 0.004349 | 0.9924 | 0.000544 | 0.01167 | |
| 35 | 0.993 | 0.000234 | 0.007643 | 0.9985 | 0.000469 | 0.01083 | 0.998 | 0.000754 | 0.01373 | 0.9923 | 0.000523 | 0.01143 | |
| Oswin | 20 | 0.9764 | 0.001254 | 0.01584 | 0.9151 | 0.03061 | 0.07825 | 0.942 | 0.03044 | 0.07802 | 0.9152 | 0.007765 | 0.03941 |
| 25 | 0.9423 | 0.002065 | 0.02032 | 0.9209 | 0.02568 | 0.07166 | 0.9276 | 0.03387 | 0.08231 | 0.8883 | 0.008761 | 0.04186 | |
| 30 | 0.9146 | 0.003034 | 0.02463 | 0.9143 | 0.0247 | 0.07028 | 0.9086 | 0.03856 | 0.08782 | 0.874 | 0.008996 | 0.04242 | |
| 35 | 0.9377 | 0.002081 | 0.0204 | 0.9198 | 0.02572 | 0.07172 | 0.9163 | 0.03102 | 0.07877 | 0.8821 | 0.008046 | 0.04011 | |
| Smith | 20 | 0.968 | 0.001702 | 0.01845 | 0.9358 | 0.02315 | 0.06804 | 0.9499 | 0.02626 | 0.07248 | 0.8085 | 0.01753 | 0.05922 |
| 25 | 0.9094 | 0.00324 | 0.02546 | 0.9413 | 0.01904 | 0.06171 | 0.9363 | 0.02981 | 0.07722 | 0.7472 | 0.01983 | 0.06298 | |
| 30 | 0.878 | 0.004337 | 0.02945 | 0.9267 | 0.02112 | 0.06499 | 0.9191 | 0.03409 | 0.08257 | 0.7406 | 0.01852 | 0.06086 | |
| 35 | 0.9355 | 0.002154 | 0.9355 | 0.943 | 0.01828 | 0.06047 | 0.9229 | 0.02859 | 0.07561 | 0.7964 | 0.01389 | 0.05271 | |
| peleg | 20 | 0.994 | 0.000318 | 0.0103 | 0.9927 | 0.002648 | 0.02971 | 0.9987 | 0.000669 | 0.01494 | 0.9955 | 0.000408 | 0.01166 |
| 25 | 0.9428 | 0.002046 | 0.02612 | 0.9926 | 0.002408 | 0.02833 | 0.9968 | 0.001367 | 0.02135 | 0.9955 | 0.000355 | 0.01088 | |
| 30 | 0.9993 | 2.45e-05 | 0.002857 | 0.9915 | 0.002452 | 0.02859 | 0.9963 | 0.001552 | 0.02275 | 0.9932 | 0.000485 | 0.01272 | |
| 35 | 0.9466 | 0.001783 | 0.02438 | 0.9987 | 0.000411 | 0.0117 | 0.9938 | 0.002307 | 0.02773 | 0.9939 | 0.000414 | 0.01175 | |
| Fiber Materials | T/°C | m0/% | C | K |
|---|---|---|---|---|
| collagen protein viscose | 20 | 5.20 | 14.05 | 0.8238 |
| 25 | 5.88 | 11.14 | 0.7488 | |
| 30 | 10.41 | 2.98 | 0.5781 | |
| 35 | 5.05 | 6.36 | 0.7785 | |
| modified PAN | 20 | 39.8 | 1.03 | 0.6215 |
| 25 | 27.6 | 1.41 | 0.6773 | |
| 30 | 32.55 | 1.33 | 0.6225 | |
| 35 | 29.92 | 1.20 | 0.6662 | |
| modified PEA | 20 | 22.14 | 3.33 | 0.7587 |
| 25 | 27.09 | 2.29 | 0.7028 | |
| 30 | 51.19 | 1.12 | 0.5686 | |
| 35 | 35.25 | 1.56 | 0.6208 | |
| modified PAA | 20 | 19.38 | 6.08 | 0.5032 |
| 25 | 23.5 | 6.49 | 0.3822 | |
| 30 | 35.73 | 5.39 | 0.2281 | |
| 35 | 23.43 | 3.53 | 0.4113 |
| Relative Humidity/% | Heats of Desorption of Fiber Samples (J/g) | |||
|---|---|---|---|---|
| Collagen Protein Viscose | Modified PAA | Modified PEA | Modified PAN | |
| 35 | 16.7 | 140.7 | 95.0 | 85.3 |
| 98 | 191.5 | 243.6 | 501.1 | 477.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Gao, S.; Zhang, R.; Cheng, L.; Yu, J. Study on the Moisture Absorption and Thermal Properties of Hygroscopic Exothermic Fibers and Related Interactions with Water Molecules. Polymers 2020, 12, 98. https://doi.org/10.3390/polym12010098
Cui Y, Gao S, Zhang R, Cheng L, Yu J. Study on the Moisture Absorption and Thermal Properties of Hygroscopic Exothermic Fibers and Related Interactions with Water Molecules. Polymers. 2020; 12(1):98. https://doi.org/10.3390/polym12010098
Chicago/Turabian StyleCui, Yi, Shuyi Gao, Ruiyun Zhang, Longdi Cheng, and Jianyong Yu. 2020. "Study on the Moisture Absorption and Thermal Properties of Hygroscopic Exothermic Fibers and Related Interactions with Water Molecules" Polymers 12, no. 1: 98. https://doi.org/10.3390/polym12010098
APA StyleCui, Y., Gao, S., Zhang, R., Cheng, L., & Yu, J. (2020). Study on the Moisture Absorption and Thermal Properties of Hygroscopic Exothermic Fibers and Related Interactions with Water Molecules. Polymers, 12(1), 98. https://doi.org/10.3390/polym12010098





