Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo
Abstract
1. Introduction
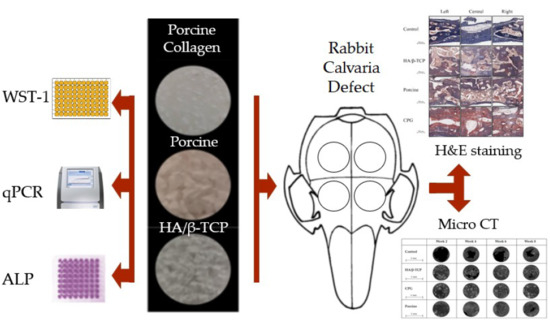
2. Materials and Methods
2.1. Graft Materials
2.2. Cell Culture and Seeding
2.3. Cell Cytotoxicity
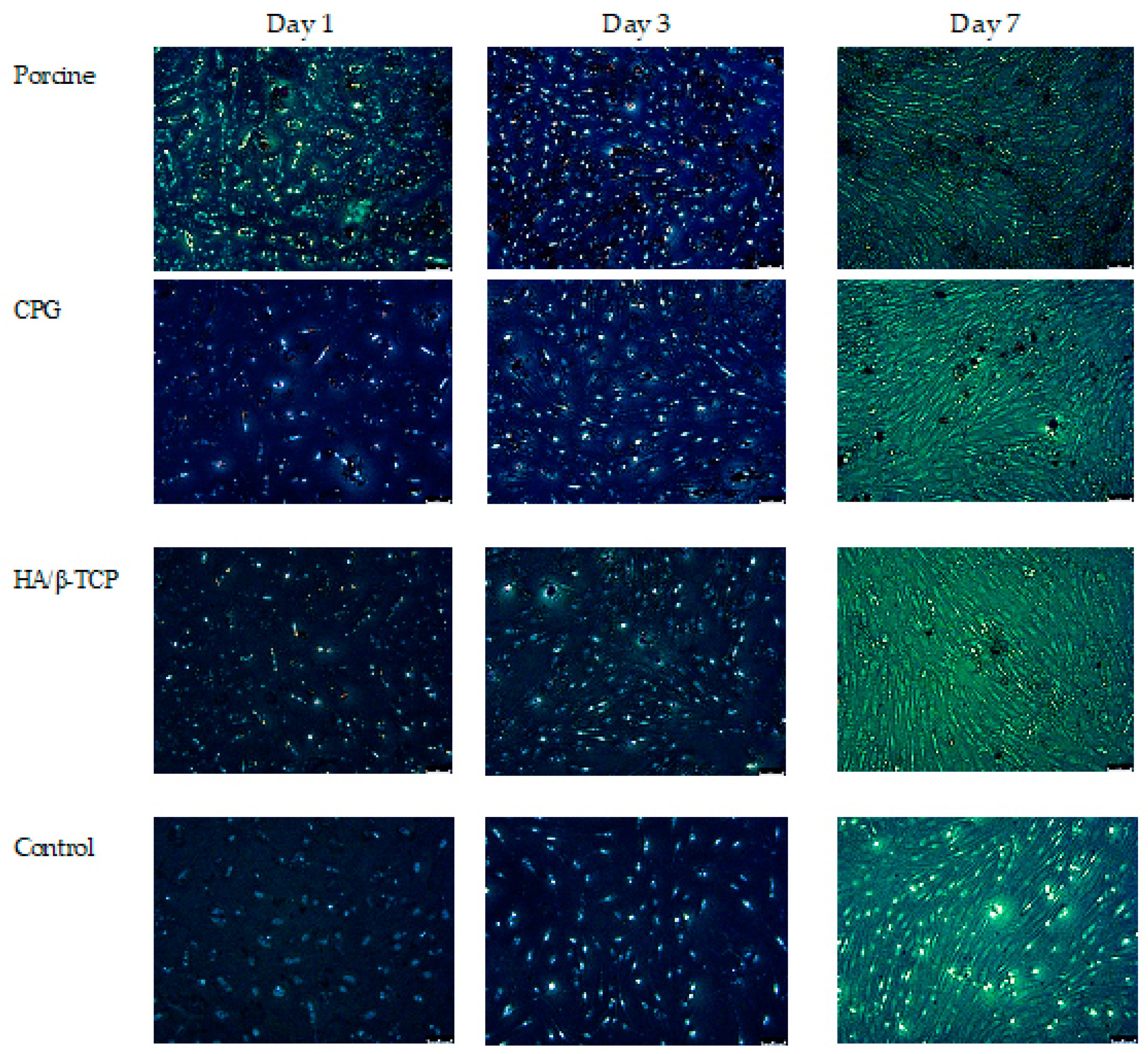
2.4. Assessment of Cell Morphology by Fluorescence Microscopy
2.5. Alkaline Phosphatase Activity
2.6. Real-Time Polymerase Chain Reaction (qPCR)
2.7. In Vivo Test
2.8. Micro-CT Scanning Cortical Defect Closure
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
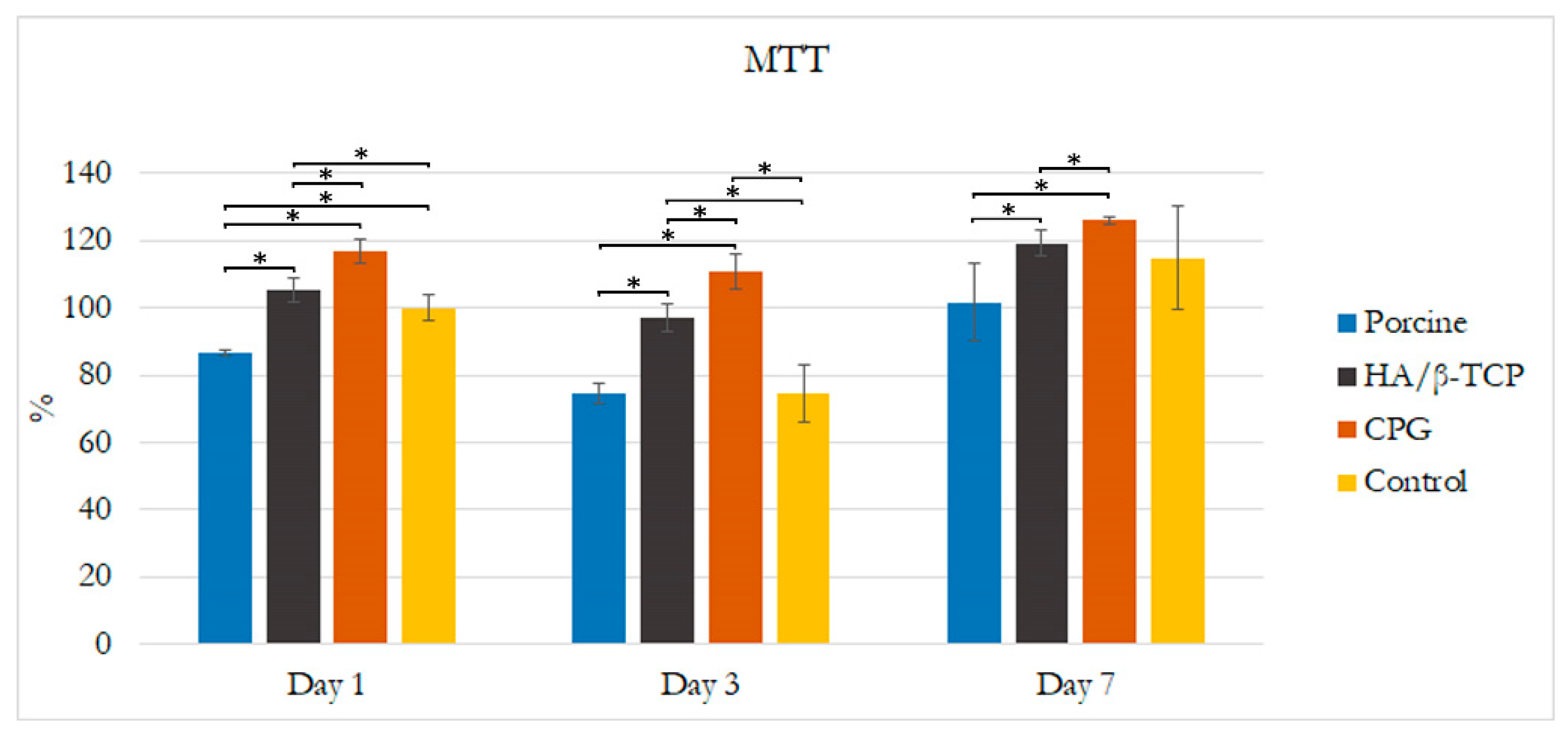
3.1. Cell Culture and Organization
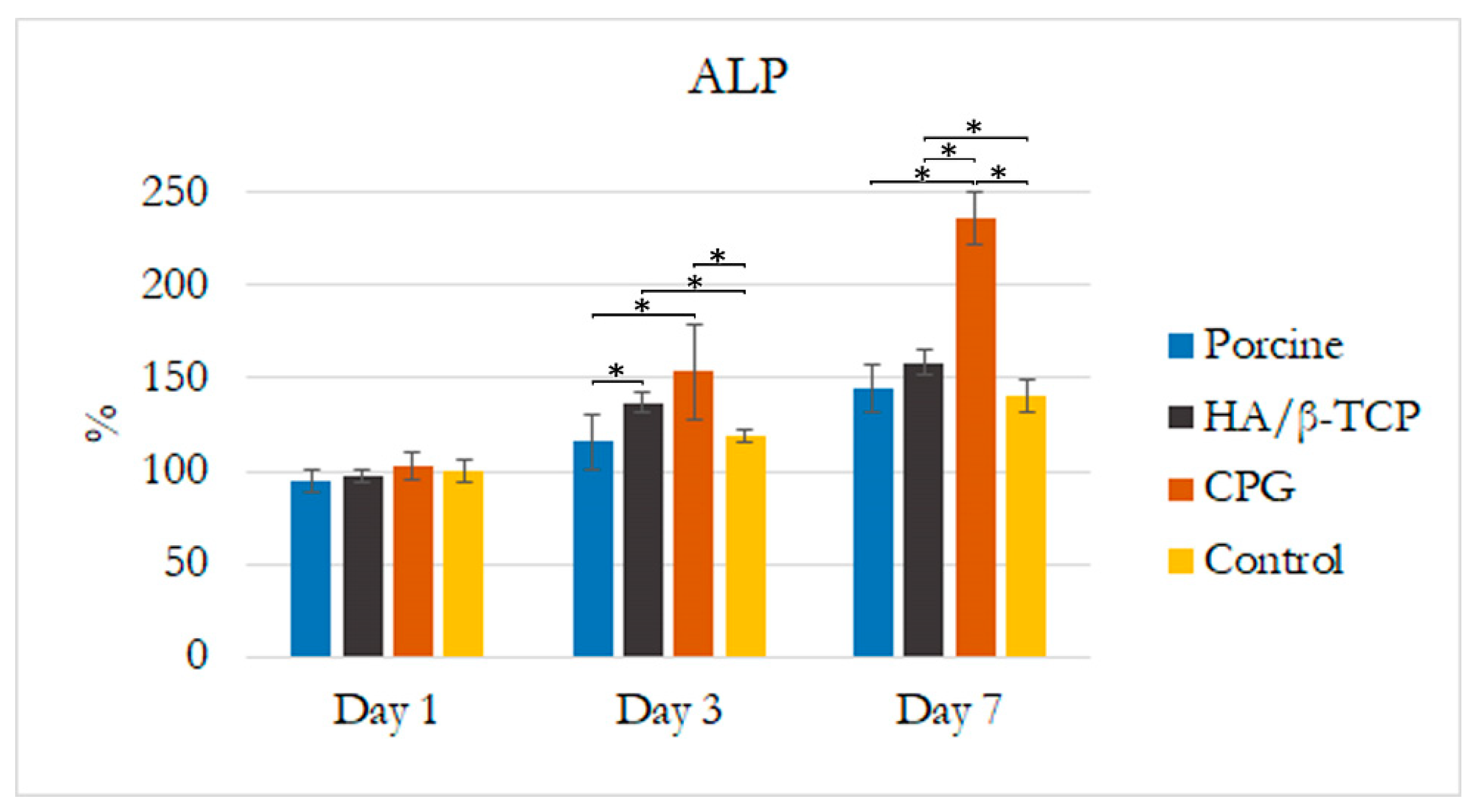
3.2. Alkaline Phosphatase Assay
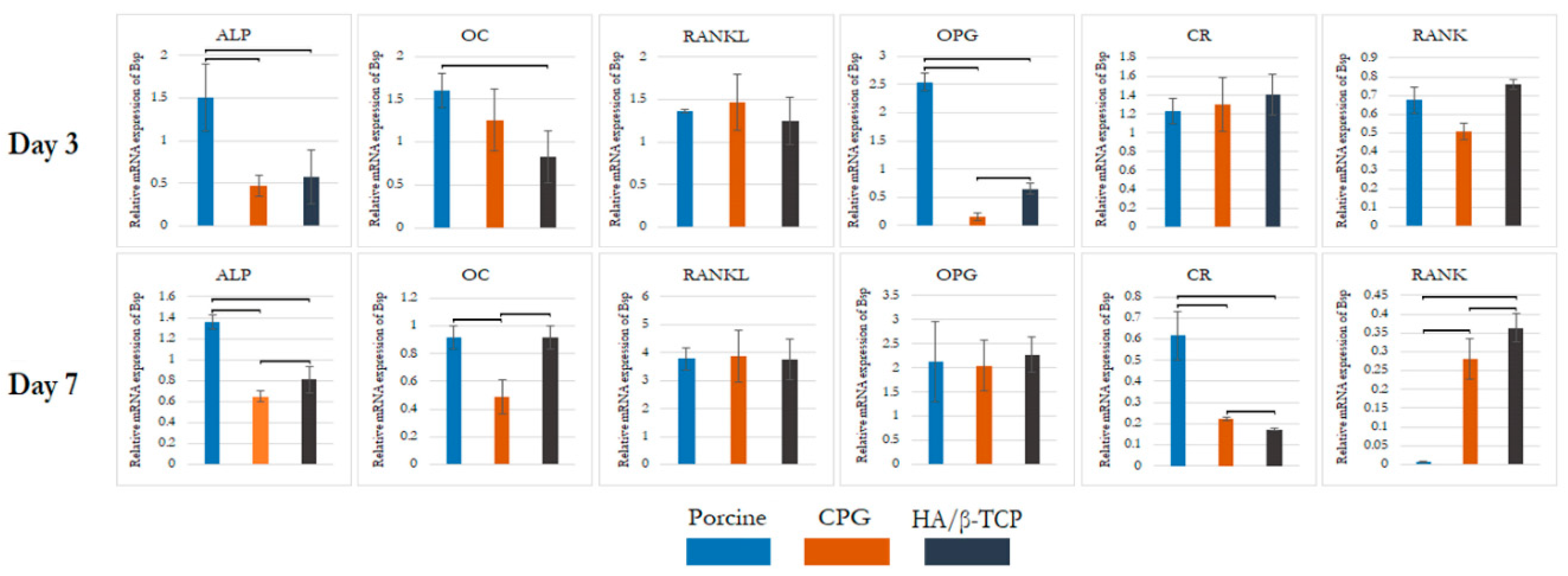
3.3. Real-Time Polymerase Chain Reaction (qPCR)
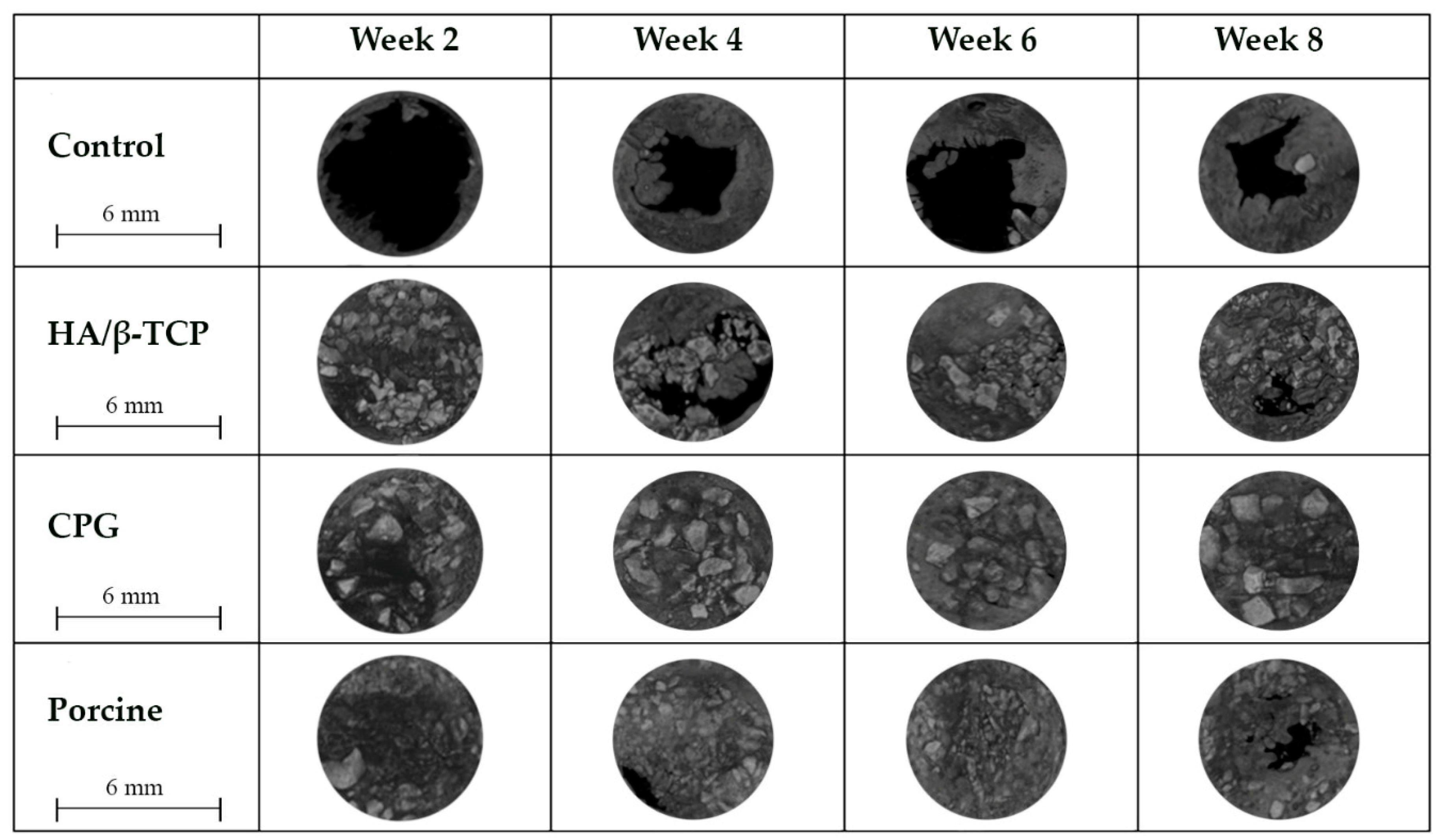
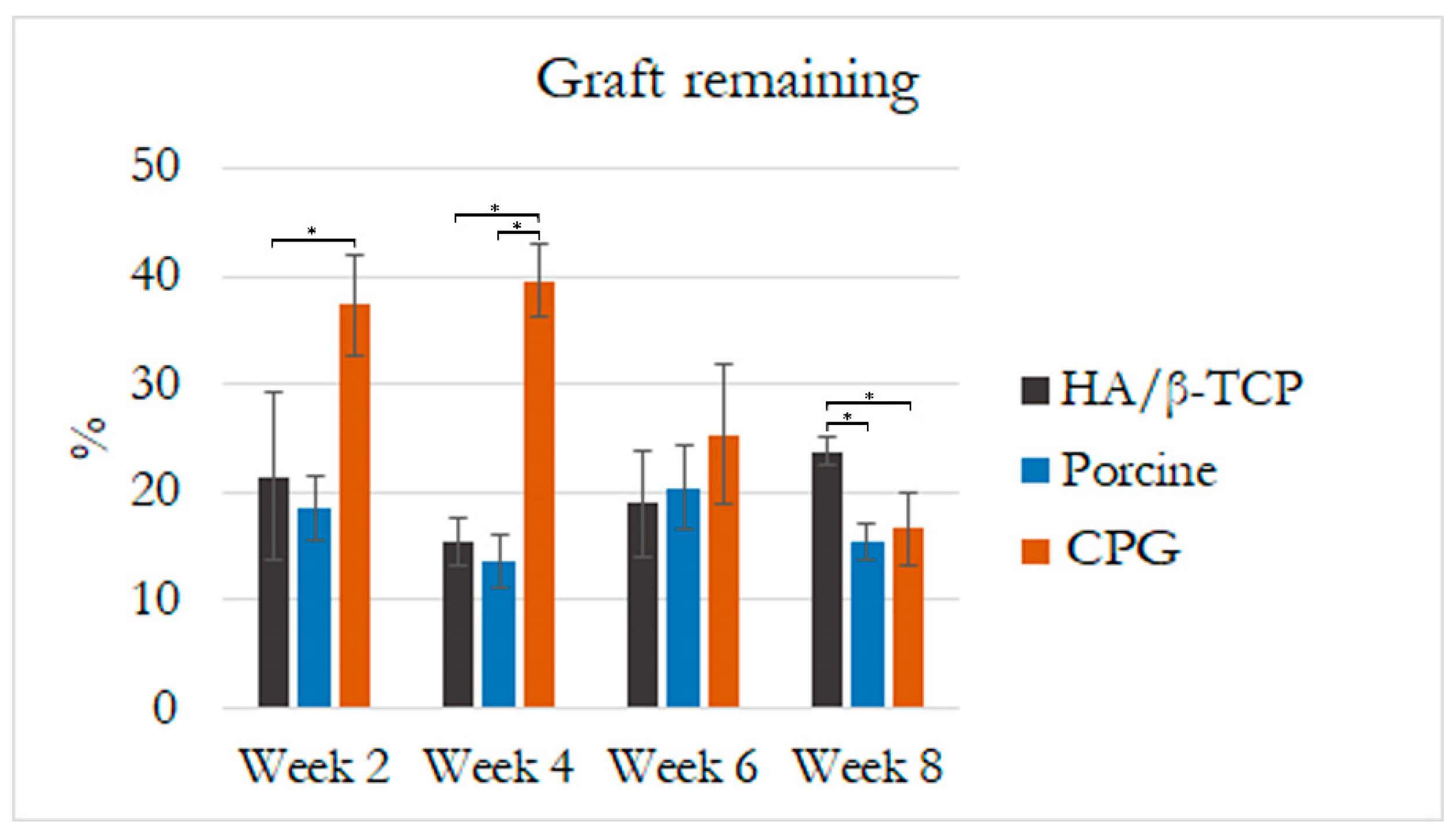
3.4. Defect Closure
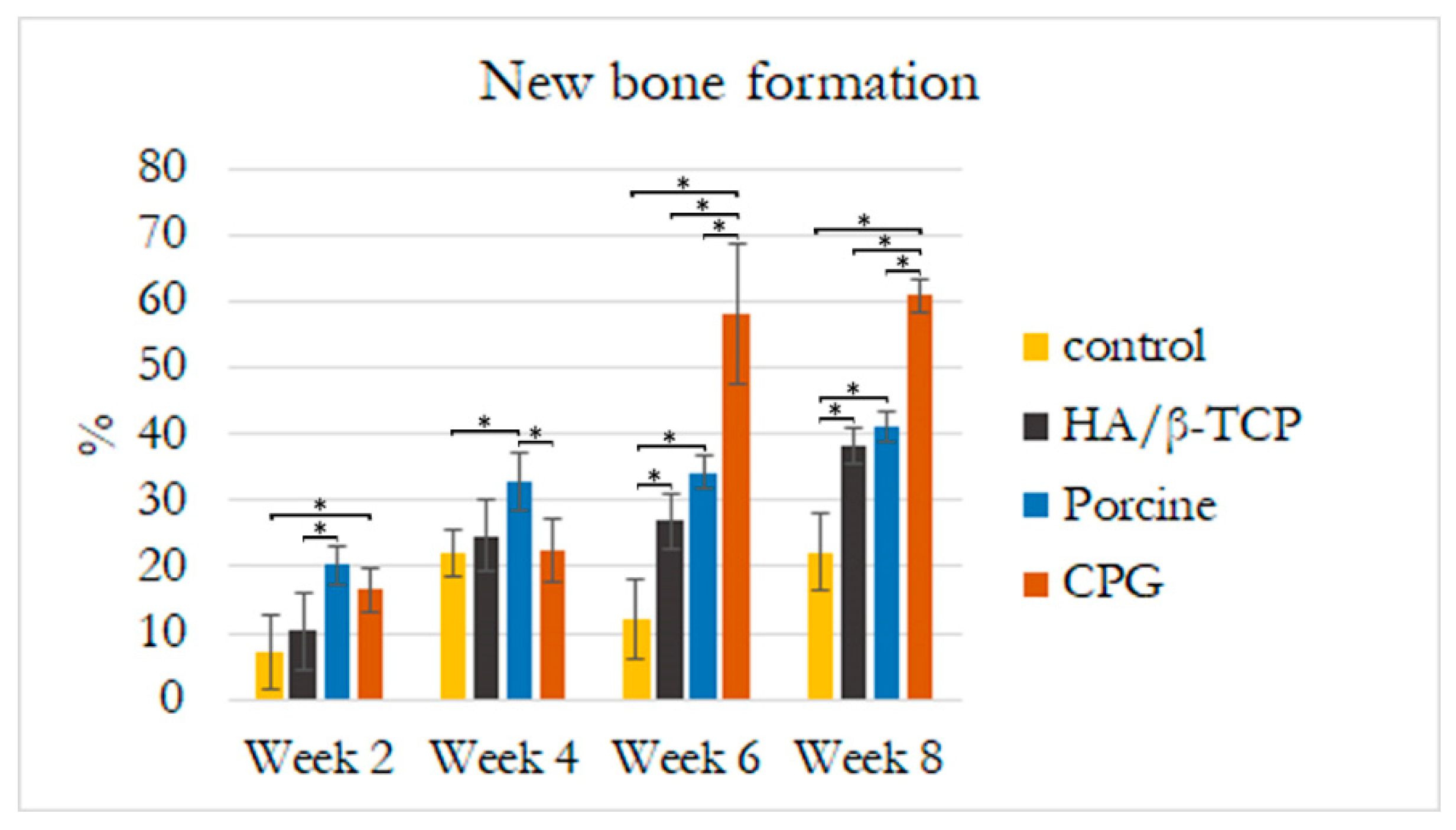
3.5. Histological and Histomorphometric Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Van Lieshout, E.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone substitutes in the Netherlands—A systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. JBJS 2002, 84, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Faour, O.; Dimitriou, R.; Cousins, C.A.; Giannoudis, P.V. The use of bone graft substitutes in large cancellous voids: Any specific needs? Injury 2011, 42, S87–S90. [Google Scholar] [CrossRef]
- Blank, A.T.; Riesgo, A.M.; Gitelis, S.; Rapp, T.B. Bone grafts, substitutes, and augments in benign orthopaedic conditions: Current concepts. Bull. NYU Hosp. Jt. Dis. 2017, 75, 119. [Google Scholar]
- Salamanca, E.; Hsu, C.C.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Pan, Y.H.; Chang, W.J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 984. [Google Scholar] [CrossRef]
- Murugan, R.; Rao, K.P.; Kumar, T.S. Heat-deproteinated xenogeneic bone from slaughterhouse waste: Physico-chemical properties. Bull. Mater. Sci. 2003, 26, 523–528. [Google Scholar] [CrossRef]
- Etok, S.E.; Valsami-Jones, E.; Wess, T.J.; Hiller, J.C.; Maxwell, C.A.; Rogers, K.D.; Manning, D.A.; White, M.L.; Lopez-Capel, E.; Collins, M.J.; et al. Structural and chemical changes of thermally treated bone apatite. J. Mater. Sci. 2007, 42, 9807–9816. [Google Scholar] [CrossRef]
- Cassetta, M.; Perrotti, V.; Calasso, S.; Piattelli, A.; Sinjari, B.; Iezzi, G. Bone formation in sinus augmentation procedures using autologous bone, porcine bone, and a 50: 50 mixture: A human clinical and histological evaluation at 2 months. Clin. Oral Implant. Res. 2015, 26, 1180–1184. [Google Scholar] [CrossRef]
- Sandor, G.; Lindholm, T.; Clokie, C. Bone regeneration of the cranio-maxillofacial and dento-alveolar skeletons in the framework of tissue engineering. Top. Tissue Eng. 2003, 7, 1–46. [Google Scholar]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes—comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implant. 2011, 26, 866–872. [Google Scholar]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implant. Res. 2006, 17, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Tsai, C.Y.; Pan, Y.H.; Lin, Y.T.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Feng, S.W.; Chang, W.J. In vitro and in vivo study of a novel porcine collagen membrane for guided bone regeneration. Materials 2016, 9, 949. [Google Scholar] [CrossRef]
- Patel, P.P.; Buckley, C.; Taylor, B.L.; Sahyoun, C.C.; Patel, S.D.; Mont, A.J.; Mai, L.; Patel, S. and Freeman, J.W. Mechanical and Biological Evaluation of a Hydroxyapatite-Reinforced Scaffold for Bone Regeneration. J. Biomed. Mater. Res. Part A 2019, 107, 732–741. [Google Scholar] [CrossRef]
- Pagliani, L.; Andersson, P.; Lanza, M.; Nappo, A.; Verrocchi, D.; Volpe, S.; Sennerby, L. A collagenated porcine bone substitute for augmentation at Neoss implant sites: A prospective 1-year multicenter case series study with histology. Clin. Implant Dent. Relat. Res. 2012, 14, 746–758. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Negri, B.; Di Felice, R.; Marchionni, S.; Calvo-Guirado, J.L.; Covani, U.; et al. Clinical and Histological changes after ridge preservation with two xenografts: Preliminary results from a multicentre randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 204–214. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Menchini-Fabris, G.B.; Derchi, G.; Marconcini, S.; Covani, U. Extra oral digital scanning and imaging superimposition for volume analysis of bone remodeling after tooth extraction with and without 2 types of particulate porcine mineral insertion: A randomized controlled trial. Clin. Implant Dent. Relat. Res. 2017, 19, 750–759. [Google Scholar] [CrossRef]
- Salamanca, E.; Lee, W.F.; Lin, C.Y.; Huang, H.M.; Lin, C.T.; Feng, S.W.; Chang, W.J. A novel porcine graft for regeneration of bone defects. Materials 2015, 8, 2523–2536. [Google Scholar] [CrossRef]
- Le GUEHENNEC, L.; Goyenvalle, E.; Aguado, E.; Pilet, P.; D’Arc, M.B.; Bilban, M.; Spaethe, R.; Daculsi, G. MBCP® biphasic calcium phosphate granules and tissucol® fibrin sealant in rabbit femoral defects: The effect of fibrin on bone ingrowth. J. Mater. Sci. Mater. Med. 2005, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.-K.; Jeon, J.-Y.; Kim, Y.-J.; Kim, S.-G.; Hwang, K.-G. Cell attachment and proliferation of osteoblast-like MG63 cells on silk fibroin membrane for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 17. [Google Scholar] [CrossRef] [PubMed]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. Kobe J. Med. Sci. 2007, 53, 25–35. [Google Scholar]
- Bimboim, H.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef]
- Vogelstein, B.; Gillespie, D. Preparative and analytical purification of DNA from agarose. Proc. Natl. Acad. Sci. USA 1979, 76, 615–619. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sollazzo, V.; Palmieri, A.; Scapoli, L.; Martinelli, M.; Girardi, A.; Alviano, F.; Pellati, A.; Perrotti, V.; Carinci, F. Bio-Oss® acts on Stem cells derived from Peripheral Blood. Oman Med. J. 2010, 25, 26. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, G.; Kim, C.; Lee, J.S.; Lee, I.G.; An, S.H.; Yun, W.S.; Kim, S.Y.; Shim, J.H. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, 1800025. [Google Scholar] [CrossRef]
- Vincent, D.H.; Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Mondal, S.C.; Jana, S. Influenced of Biofield Energy Healing Treatment on Vitamin D3 for the Assessment of Bone Health Parameters in MG-63 cells. viXra 2018. viXra:1807.0213. [Google Scholar]
- Ongaro, A.; Pellati, A.; Bagheri, L.; Rizzo, P.; Caliceti, C.; Massari, L.; De Mattei, M. Characterization of notch signaling during osteogenic differentiation in human osteosarcoma cell line MG63. J. Cell. Physiol. 2016, 231, 2652–2663. [Google Scholar] [CrossRef]
- Cappagli, V.; Potes, C.S.; Ferreira, L.B.; Tavares, C.; Eloy, C.; Elisei, R.; Sobrinho-Simões, M.; Wookey, P.J.; Soares, P. Calcitonin receptor expression in medullary thyroid carcinoma. PeerJ 2017, 5, 3778. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. The Rankl/Rank/Opg Pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.W.; Ahn, J.; Hankenson, K.D. Notch signaling promotes osteoclast maturation and resorptive activity. J. Cell. Biochem. 2015, 116, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, S.C.S.X.B.; Pereira, C.L.; Mazzonetto, R.; de Moraes, M.; Moreira, R.W.F. Histological and histomorphometric analyses of calcium phosphate cement in rabbit calvaria. J. Cranio-Maxillofac. Surg. 2008, 36, 354–359. [Google Scholar] [CrossRef]
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C 2013, 33, 3506–3513. [Google Scholar] [CrossRef]
- Nannmark, U.; Sennerby, L. The bone tissue responses to prehydrated and collagenated cortico-cancellous porcine bone grafts: A study in rabbit maxillary defects. Clin. Implant Dent. Relat. Res. 2008, 10, 264–270. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Ravera, L.; Mortellaro, C.; Piattelli, A. Bone regeneration in iliac crestal defects: An experimental study on sheep. BioMed Res. Int. 2016, 2016, 4086870. [Google Scholar] [CrossRef]
- Covani, U.; Cornelini, R.; Barone, A. Buccal bone augmentation around immediate implants with and without flap elevation: A modified approach. Int. J. Oral Maxillofac. Implant. 2008, 23, 25. [Google Scholar]
- Barone, A.; Cornelini, R.; Ciaglia, R.; Covani, U. Implant placement in fresh extraction sockets and simultaneous osteotome sinus floor elevation: A case series. Int. J. Periodontics Restor. Dent. 2008, 28, 3. [Google Scholar]

| Gene Symbol | Forward primer sequence (5′ > 3′) | Reverse primer sequence (5′ > 3′) |
|---|---|---|
| ALP | AGCCTTCCTGAAAGAGGATTGG | GCCAGTACTTGGGGTCTTTCT |
| OC | TCCTTTGGGGTTTGGCCTAC | CCAGCCTCCAGCACTGTTTA |
| RANKL | ACTGGCCTCTCACCTTTTCTG | AGCCATCCACCATCGCTTTC |
| CR | TTGCTGCCCGCAATTTATGA | TGCTGGCAAGATACTCAGGT |
| OPG | CTGGAACCCCAGAGCGAAAT | GCCTCCTCACACAGGGTAAC |
| RANK | GAAGGTGGACTGGCTACCAC | TTTCCTTCCCCTCCCCAGAA |
| GAPDH | CCTCCTGTTCGACAGTCAGC | CCTAGCCTCCCGGGTTTCTC |
| Defect Upper Side | Defect Lower Side | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 Weeks | 4 Weeks | 6 Weeks | 8 Weeks | 2 Weeks | 4 Weeks | 6 Weeks | 8 Weeks | |
| Control | 6.25 ± 7.99 * | 57.95 ± 17.04 * | 56.98 ± 35.34 * | 72.19 ± 24.08 * | 14.67 ± 11.48 * | 55.22 ± 15.8 * | 49.28 ± 31.01 * | 64.63 ± 19.04 * |
| HA/β-TCP | 65.56 ± 20.66 | 84.22 ± 6.95 | 93.48 ± 13.31 | 97.19 ± 1.65 | 61.74 ± 7.99 | 78.73 ± 12.45 | 93.99 ± 12.18 | 97.73 ± 1.57 |
| CPG | 73.65 ± 12.77 | 99.17 ± 1.06 | 100 ± 0 | 99.42 ± 1.3 ¶ | 52.88 ± 17.95 | 98.84 ± 1.28 | 100 ± 0 | 99.49 ± 1.14 ¶ |
| Porcine | 63.32 ± 18.44 | 94.58 ± 10.31 | 95.59 ± 7.37 | 94.66 ± 5.3 | 65.02 ± 20.42 | 95.56 ± 8.57 | 95.98 ± 6.77 | 95.64 ± 3.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca, E.; Hsu, C.C.; Yao, W.L.; Choy, C.S.; Pan, Y.H.; Teng, N.-C.; Chang, W.-J. Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers 2020, 12, 93. https://doi.org/10.3390/polym12010093
Salamanca E, Hsu CC, Yao WL, Choy CS, Pan YH, Teng N-C, Chang W-J. Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers. 2020; 12(1):93. https://doi.org/10.3390/polym12010093
Chicago/Turabian StyleSalamanca, Eisner, Chia Chen Hsu, Wan Ling Yao, Cheuk Sing Choy, Yu Hwa Pan, Nai-Chia Teng, and Wei-Jen Chang. 2020. "Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo" Polymers 12, no. 1: 93. https://doi.org/10.3390/polym12010093
APA StyleSalamanca, E., Hsu, C. C., Yao, W. L., Choy, C. S., Pan, Y. H., Teng, N.-C., & Chang, W.-J. (2020). Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers, 12(1), 93. https://doi.org/10.3390/polym12010093