Redox-Responsive Heparin–Chlorambucil Conjugate Polymeric Prodrug for Improved Anti-Tumor Activity
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
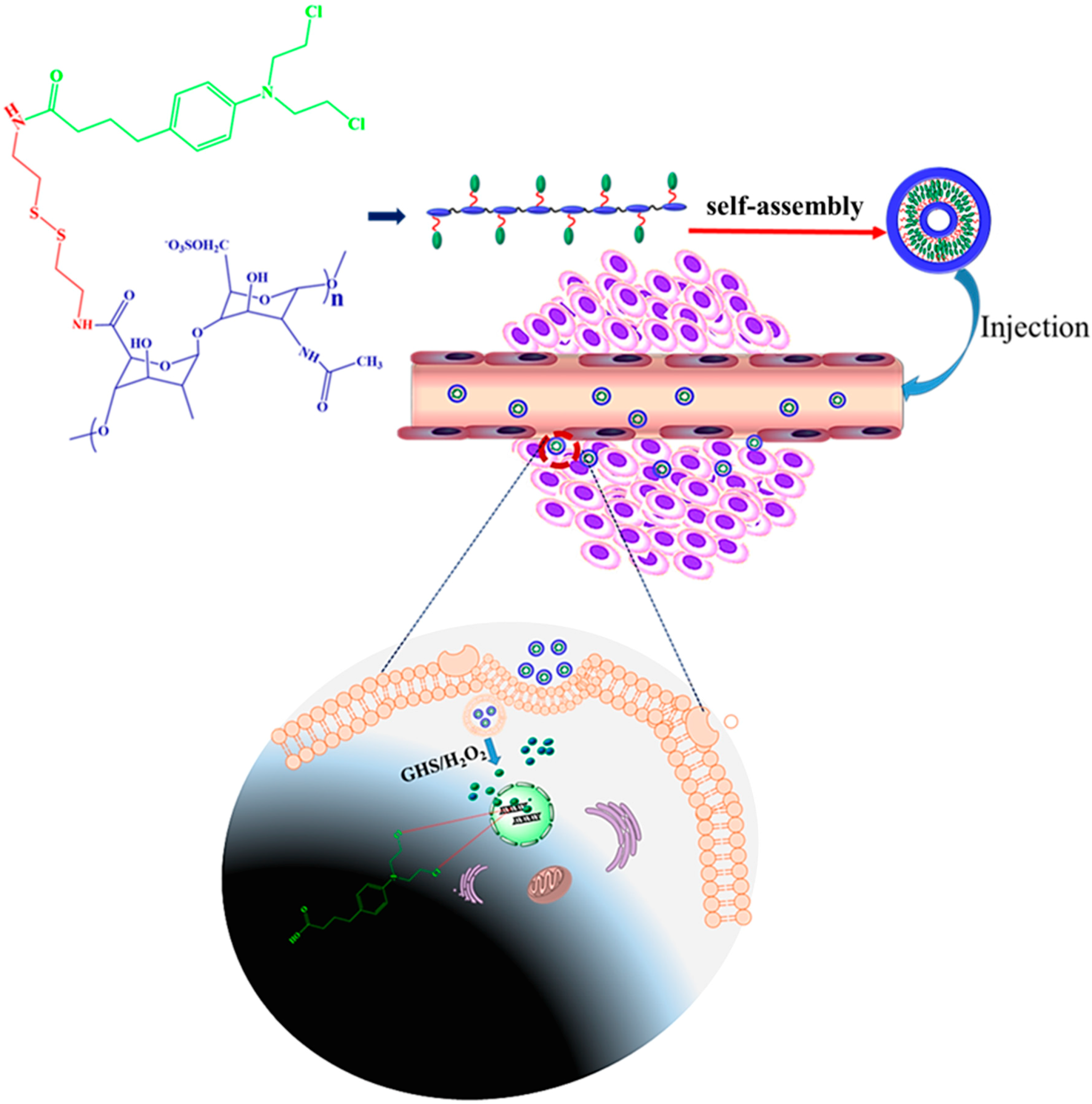
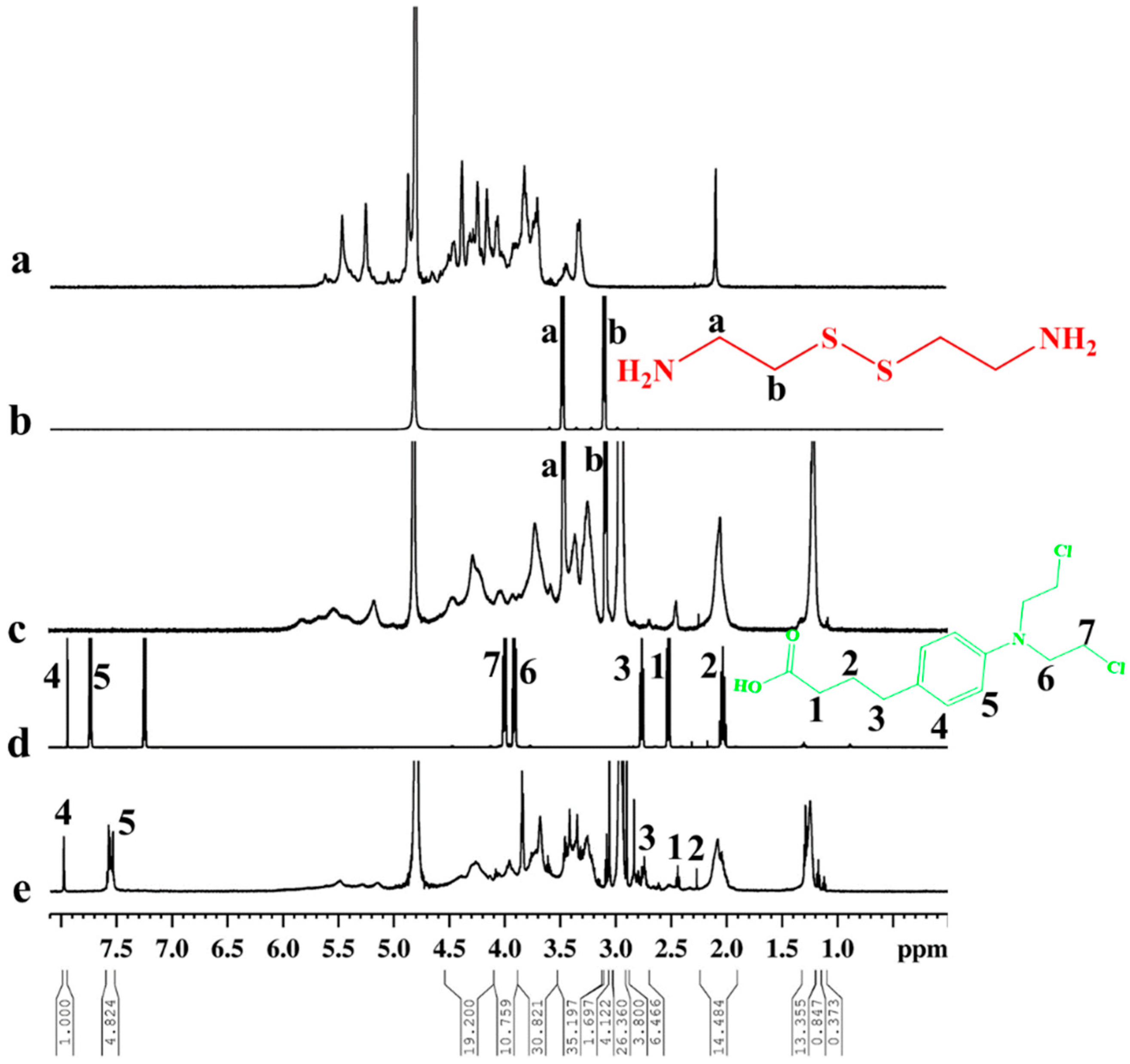
2.2. Conjugation of Hep–Chl
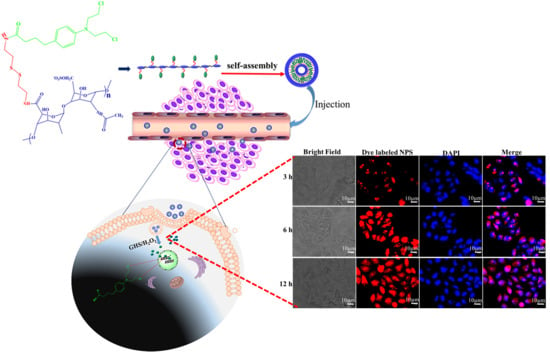
2.3. Preparation of the Hep–Chl Prodrug Self-Assembled Nanoparticle
2.4. Characterization of the Hep–Chl Prodrugs
2.5. Drug Release in the Simulated Redox Environment
2.6. Cell Viability Assay
2.7. Examination of In Vitro Cellular Uptake of Hep–Chl Nanoparticles
3. Results and Discussion
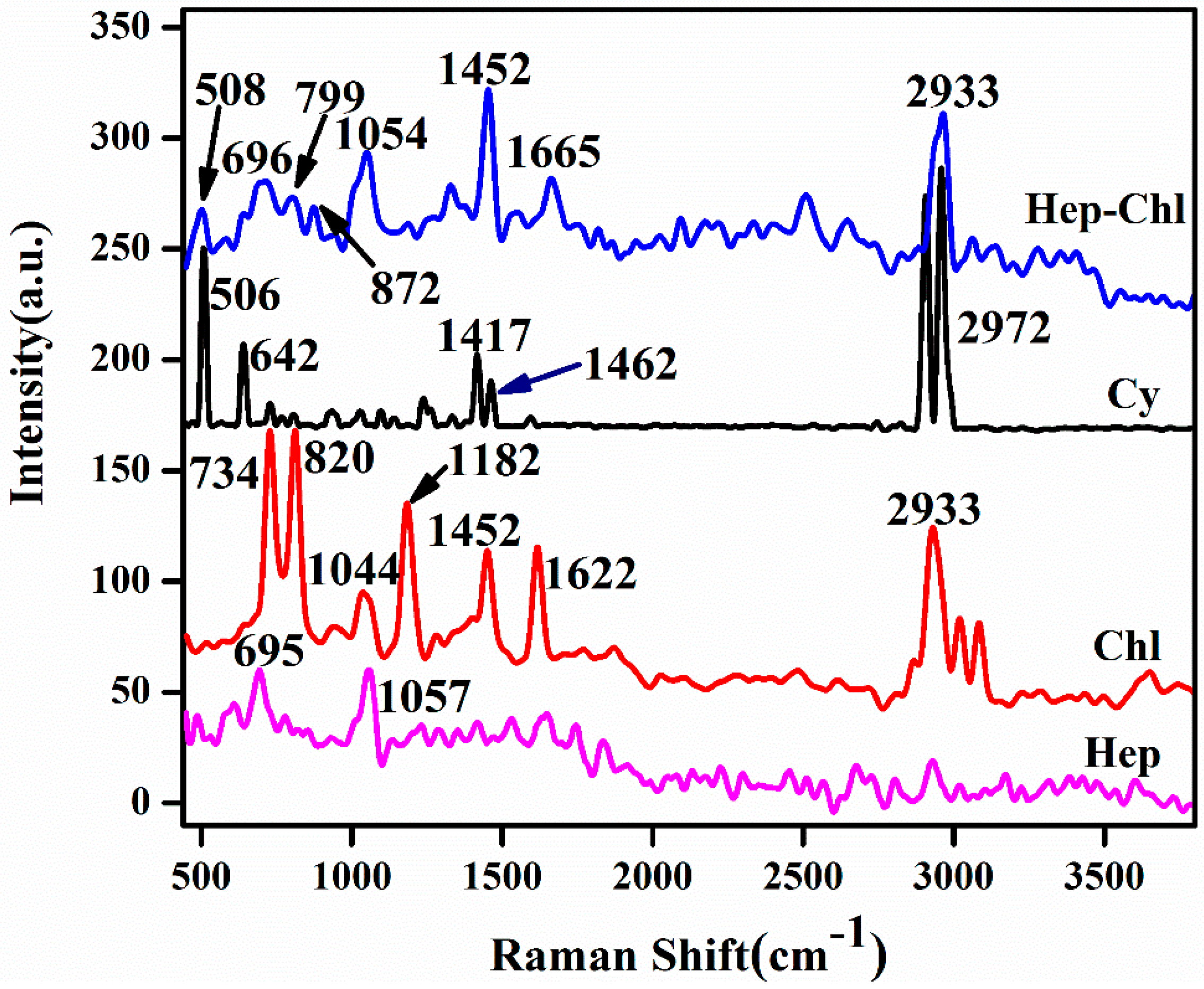
3.1. Synthesis of the Hep–Chl Prodrug
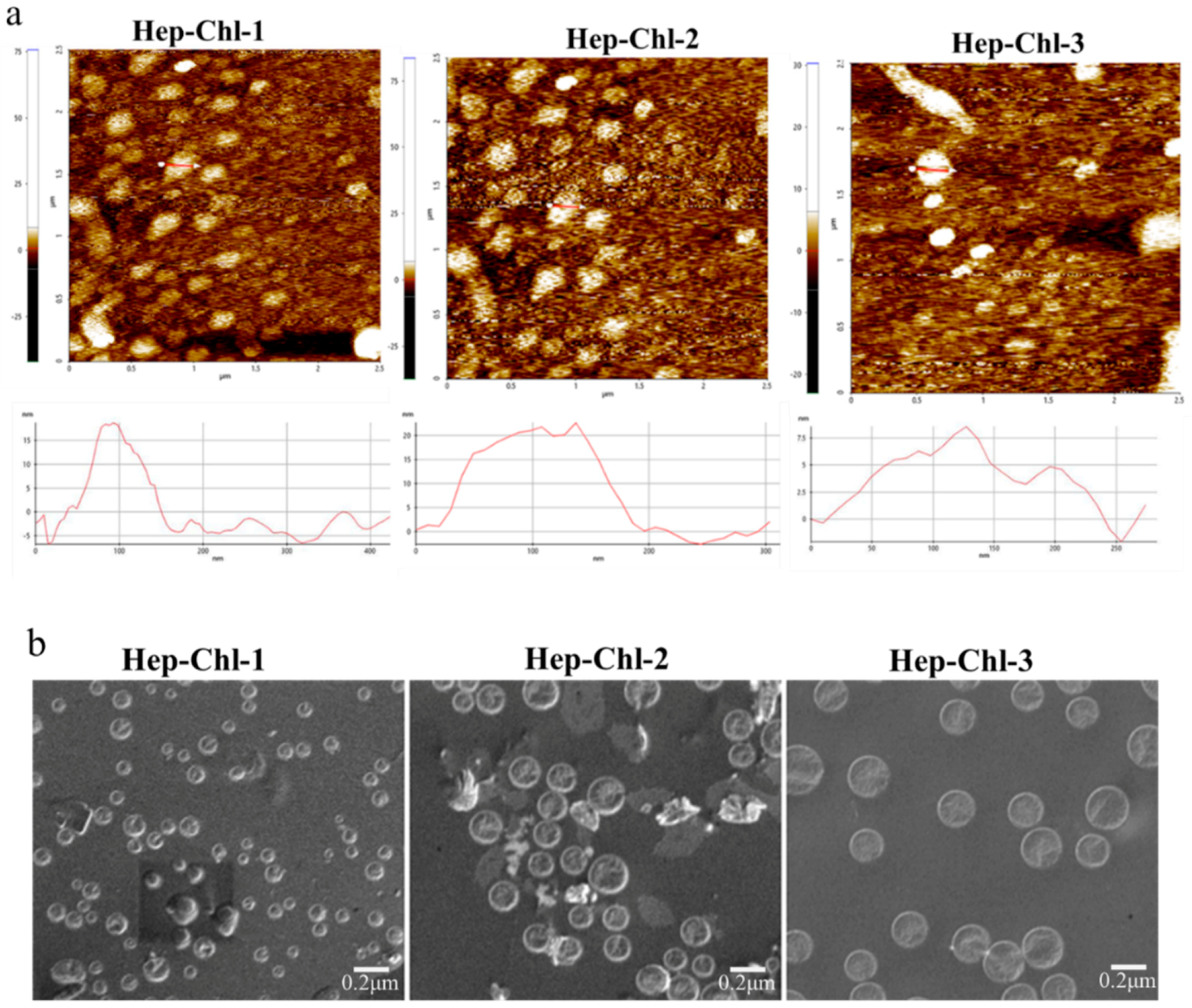
3.2. Formation of the Self-Assembled Prodrug
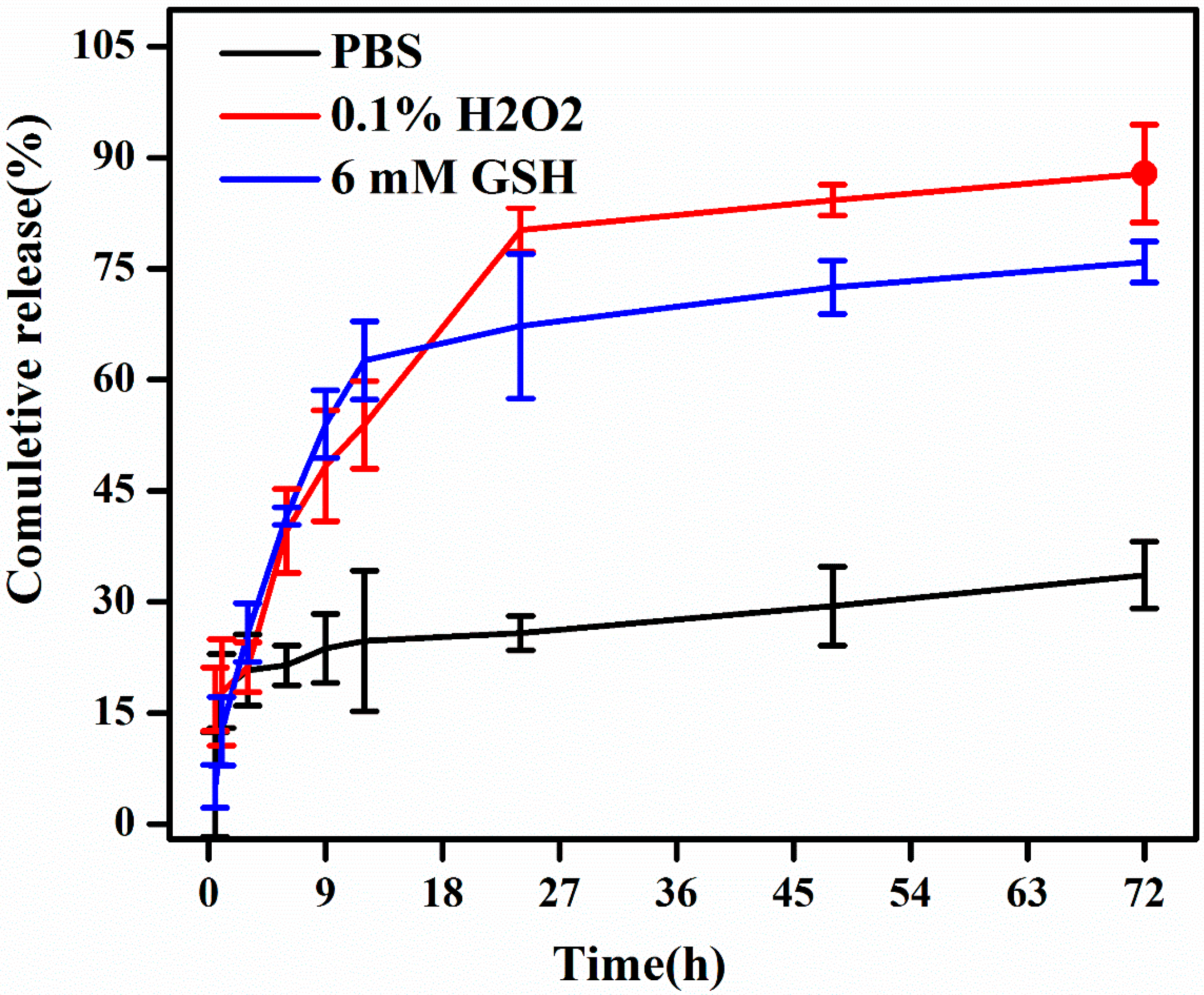
3.3. In Vitro Drug Release Studies
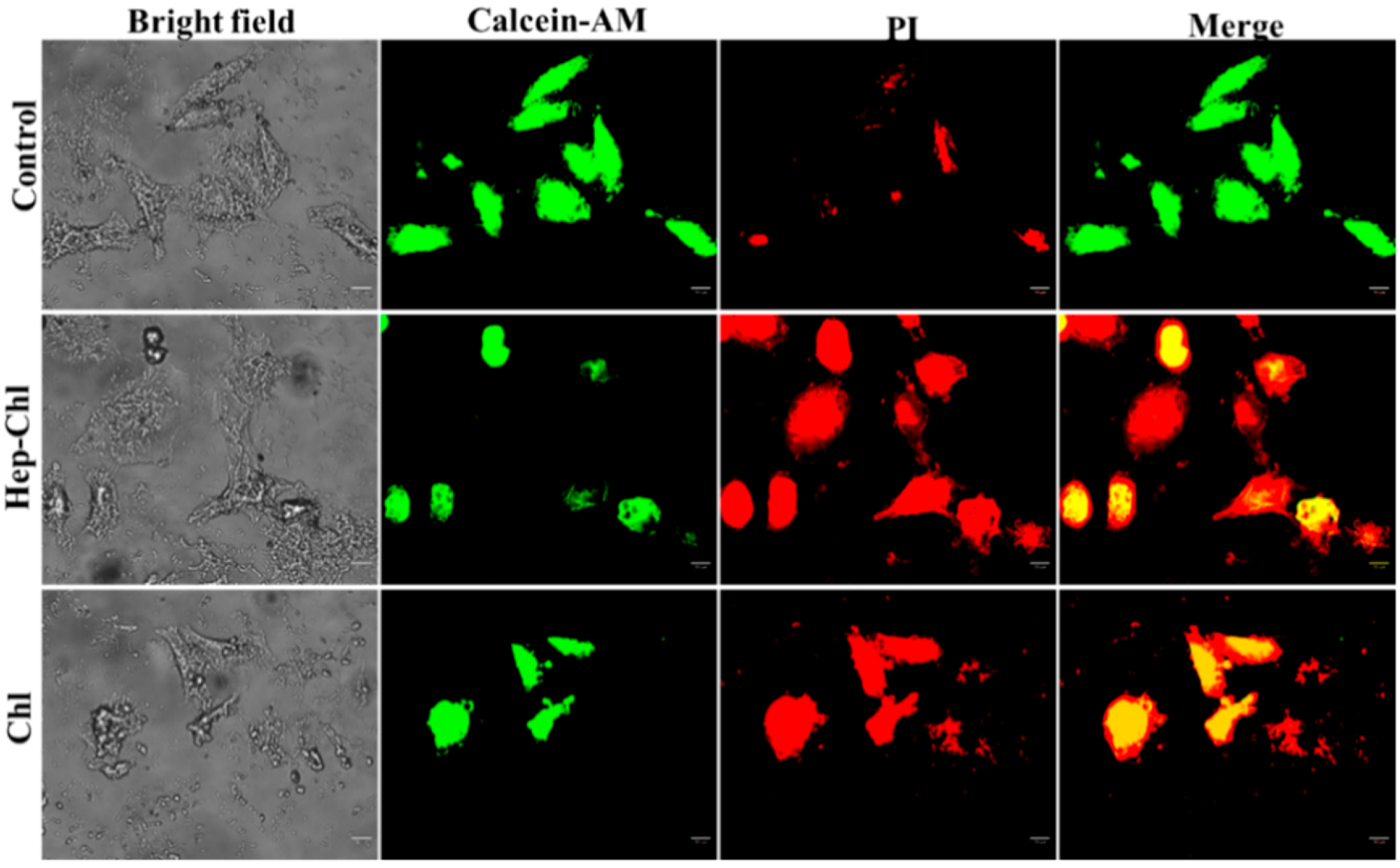
3.4. In Vitro Cytotoxicity Study
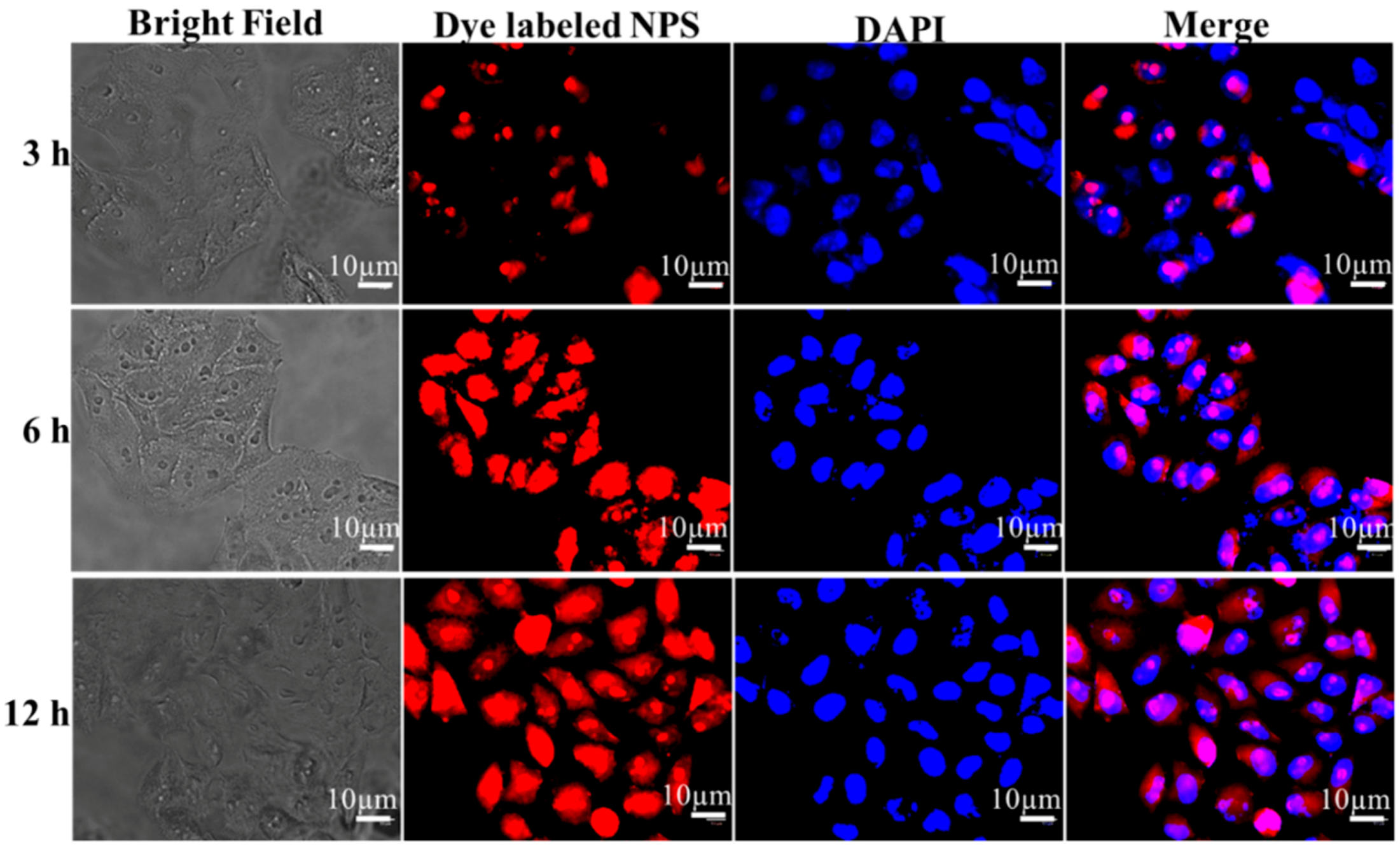
3.5. In Vitro Cellular Uptake of Hep–Chl Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Street, W. Cancer Facts & Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019; pp. 29–70. [Google Scholar]
- Szent-Györgyi, A. Cell division and cancer. Science 1965, 149, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Joshi, A.; Toor, A.P.; Verma, G. Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery, 1st ed.; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. [Google Scholar]
- Pattni, B.S.; Torchilin, V.P. Targeted drug delivery systems: Strategies and challenges. In Targeted Drug Delivery: Concepts and Design; Springer: Berlin, Germany, 2015; pp. 3–38. [Google Scholar]
- Orive, G.; Hernandez, R.M.; Gascón, A.R.; Domínguez-Gil, A.; Pedraz, J.L. Drug delivery in biotechnology: Present and future. Curr. Opin. Biotechnol. 2003, 14, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.T.; Nicolas, J.; Maksimenko, A.; Desmaële, D.; Couvreur, P. Multifunctional squalene-based prodrug nanoparticles for targeted cancer therapy. Chem. Commun. 2014, 50, 533–5338. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, B.; Huang, Z.; Xu, P.; Zheng, H.; Yin, Y.; Xu, H.; Liu, X.; Chen, L.; Lou, Y. A novel melphalan polymeric prodrug: Preparation and property study. Carbohydr. Polym. 2014, 111, 928–935. [Google Scholar] [CrossRef]
- Li, N.-N.; Lin, J.; Gao, D.; Zhang, L.-M. A macromolecular prodrug strategy for combinatorial drug delivery. J. Colloid Interface Sci. 2014, 417, 301–309. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Cook, B.W. Anticoagulation Management. In Seminars in Interventional Radiology; Thieme Medical Publishers: New York, NY, USA, 2010; pp. 360–369. [Google Scholar]
- Goh, M.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Andrgie, A.T.; Mekuria, S.L.; Addisu, K.D.; Hailemeskel, B.Z.; Hsu, W.H.; Tsai, H.C.; Lai, J.Y. Non-Anticoagulant Heparin Prodrug Loaded Biodegradable and Injectable Thermoresponsive Hydrogels for Enhanced Anti-Metastasis Therapy. Macromol. Biosci. 2019, 19, 1800409. [Google Scholar] [CrossRef]
- Kohn, K.W.; Hartley, J.A.; Mattes, W.B. Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res. 1987, 15, 10531–10549. [Google Scholar] [CrossRef]
- Hu, X.; Liu, R.; Zhang, D.; Zhang, J.; Li, Z.; Luan, Y. Rational design of an amphiphilic chlorambucil prodrug realizing self-assembled micelles for efficient anticancer therapy. ACS Biomater. Sci. Eng. 2018, 4, 973–980. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, W.; He, R.; Fang, S.; Zhang, Y.; Yao, C.; Ismail, M.; Li, X. Improvement of Stability and Anticancer Activity of Chlorambucil-Tetrapeptide Conjugate Vesicles. Chin. J. Chem. 2016, 34, 609–616. [Google Scholar] [CrossRef]
- Tadros, M.I.; Al-mahallawi, A.M. Long-circulating lipoprotein-mimic nanoparticles for smart intravenous delivery of a practically-insoluble antineoplastic drug: Development, preliminary safety evaluations and preclinical pharmacokinetic studies. Int. J. Pharm. 2015, 493, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Campos, B.; Barata, C.; Lacorte, S. Degradation and toxicity of mitoxantrone and chlorambucil in water. Int. J. Environ. Sci. Technol. 2015, 12, 633–640. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, C.; Ren, C.; Liu, J.; Zhang, Y.; Wang, J.; Huang, F.; Zhang, L.; Liu, J. Supramolecular Hydrogel Based on Chlorambucil and Peptide Drug for Cancer Combination Therapy. ACS Appl. Mater. Interfaces 2018, 11, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, D.; Su, Y.; Huang, W.; Zhou, Y.; Cui, D.; Zhu, X.; Yan, D. Combination of small molecule prodrug and nanodrug delivery: Amphiphilic drug–drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756. [Google Scholar] [CrossRef]
- Birhan, Y.S.; Hailemeskel, B.Z.; Mekonnen, T.W.; Hanurry, E.Y.; Darge, H.F.; Andrgie, A.T.; Chou, H.-Y.; Lai, J.-Y.; Hsiue, G.-H.; Tsai, H.-C. Fabrication of redox-responsive Bi (mPEG-PLGA)-Se2 micelles for doxorubicin delivery. Int. J. Pharm. 2019, 567, 118486. [Google Scholar] [CrossRef]
- Hailemeskel, B.Z.; Hsu, W.-H.; Addisu, K.D.; Andrgie, A.T.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Diselenide linkage containing triblock copolymer nanoparticles based on Bi (methoxyl poly (ethylene glycol))-poly (ε-carprolactone): Selective intracellular drug delivery in cancer cells. Mater. Sci. Eng. C 2019, 103, 109803. [Google Scholar] [CrossRef]
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Lale, S.V.; Koul, V. Stimuli-Responsive Polymeric Nanoparticles for Cancer Therapy. In Polymer Gels; Springer: Berlin, Germany, 2018; pp. 27–54. [Google Scholar]
- Wen, H.; Li, Y.; Zhao, X. Redox-Sensitive Polymeric Nanoparticles for Intracellular Drug Delivery. In Bio-Inspired Nanomaterials and Applications: Nano Detection, Drug/Gene Delivery, Medical Diagnosis and Therapy; World Scientific: Baltimore, MD, USA, 2015; pp. 21–48. [Google Scholar]
- Wen, H.; Li, Y. Redox sensitive nanoparticles with disulfide bond linked sheddable shell for intracellular drug delivery. Med. Chem. 2014, 4, 748–755. [Google Scholar] [CrossRef]
- Chang, S.; Feng, B. Redox-responsive disulfide bond-bridged mPEG-PBLA prodrug micelles for enhanced paclitaxel biosafety, targeting and antitumor efficacy. Front. Oncol. 2019, 9, 823. [Google Scholar] [CrossRef]
- Han, X.; Chen, J.; Jiang, M.; Zhang, N.; Na, K.; Luo, C.; Zhang, R.; Sun, M.; Lin, G.; Zhang, R. Paclitaxel–paclitaxel prodrug nanoassembly as a versatile nanoplatform for combinational cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 33506–33513. [Google Scholar] [CrossRef] [PubMed]
- Ashwinkumar, N.; Maya, S.; Jayakumar, R. Redox-responsive cystamine conjugated chitin–hyaluronic acid composite nanogels. RSC Adv. 2014, 4, 49547–49555. [Google Scholar] [CrossRef]
- Sim, H.J.; Thambi, T.; Lee, D.S. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J. Mater. Chem. B 2015, 3, 8892–8901. [Google Scholar] [CrossRef]
- Addisu, K.D.; Hailemeskel, B.Z.; Mekuria, S.L.; Andrgie, A.T.; Lin, Y.-C.; Tsai, H.-C. Bioinspired, Manganese-Chelated Alginate–Polydopamine Nanomaterials for Efficient in Vivo T 1-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2018, 10, 5147–5160. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Pan, M.; Su, C.-Y. Synthesis, photophysical properties and in vitro evaluation of a chlorambucil conjugated ruthenium (ii) complex for combined chemo-photodynamic therapy against HeLa cells. J. Mater. Chem. B 2017, 5, 4623–4632. [Google Scholar] [CrossRef]
- Vijayashree, I.; Niranjana, P.; Prabhu, G.; Sureshbabu, V.; Manjanna, J. Conjugation of Au nanoparticles with chlorambucil for improved anticancer activity. J. Clust. Sci. 2017, 28, 133–148. [Google Scholar] [CrossRef]
- Li, N.-N.; Zheng, B.-N.; Lin, J.-T.; Zhang, L.-M. New heparin–indomethacin conjugate with an ester linkage: Synthesis, self aggregation and drug delivery behavior. Mater. Sci. Eng. C 2014, 34, 229–235. [Google Scholar] [CrossRef]
- Shaker, S.; Gardouh, A.R.; Ghorab, M.M. Factors affecting liposomes particle size prepared by ethanol injection method. Res. Pharm. Sci. 2017, 12, 346. [Google Scholar] [CrossRef]
- Hanurry, E.Y.; Hsu, W.-H.; Darge, H.F.; Birhan, Y.S.; Mekonnen, T.W.; Andrgie, A.T.; Chou, H.-Y.; Cheng, C.-C.; Lai, J.-Y.; Tsai, H.-C. In vitro siRNA delivery via diethylenetriamine-and tetraethylenepentamine-modified carboxyl group-terminated Poly (amido) amine generation 4.5 dendrimers. Mater. Sci. Eng. C 2019, 106, 110245. [Google Scholar] [CrossRef]
- Ruan, Z.; Yuan, P.; Li, T.; Tian, Y.; Cheng, Q.; Yan, L. Redox-responsive prodrug-like PEGylated macrophotosensitizer nanoparticles for enhanced near-infrared imaging-guided photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 135, 25–35. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Xu, Y.; Chen, Z.; Zhang, J.; Ping, Q.; Xiao, Y. Tween 80-modified hyaluronic acid-ss-curcumin micelles for targeting glioma: Synthesis, characterization and their in vitro evaluation. Int. J. Biol. Macromol. 2018, 120, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, N.; Mo, L.; Wu, M.; Yang, R.; Xu, X.; Huang, Y.; Lin, J.; Zhang, L.-M.; Jiang, X. Reduction sensitive hyaluronan-SS-poly (ε-caprolactone) block copolymers as theranostic nanocarriers for tumor diagnosis and treatment. Mater. Sci. Eng. C 2019, 98, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhao, D.; Zhang, Q.; Xu, J.; Yuan, G.; Zhuo, R.; Li, F. A co-delivery system based on a reduction-sensitive polymeric prodrug capable of loading hydrophilic and hydrophobic drugs for combination chemotherapy. Polym. Chem. 2016, 7, 5966–5977. [Google Scholar] [CrossRef]
- Xia, Y.; He, H.; Liu, X.; Hu, D.; Yin, L.; Lu, Y.; Xu, W. Redox-responsive, core-crosslinked degradable micelles for controlled drug release. Polym. Chem. 2016, 7, 6330–6339. [Google Scholar] [CrossRef]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in cancer cell death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef]
- Doskey, C.M.; Buranasudja, V.; Wagner, B.A.; Wilkes, J.G.; Du, J.; Cullen, J.J.; Buettner, G.R. Tumor cells have decreased ability to metabolize H2O2: Implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016, 10, 274–284. [Google Scholar] [CrossRef]
- Song, N.; Liu, W.; Tu, Q.; Liu, R.; Zhang, Y.; Wang, J. Preparation and in vitro properties of redox-responsive polymeric nanoparticles for paclitaxel delivery. Colloids Surf. B Biointerfaces 2011, 87, 454–463. [Google Scholar] [CrossRef]
- Zeng, X.; Morgenstern, R.; Nyström, A.M. Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-mediated drug resistance. Biomaterials 2014, 35, 1227–1239. [Google Scholar] [CrossRef]
- Ye, W.-L.; Du, J.-B.; Na, R.; Song, Y.-F.; Mei, Q.-B.; Zhao, M.-G.; Zhou, S.-Y. Cellular uptake and antitumor activity of DOX-hyd-PEG-FA nanoparticles. PLoS ONE 2014, 9, e97358. [Google Scholar] [CrossRef]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 2016, 6, 38541. [Google Scholar] [CrossRef] [PubMed]



| Conjugate Prodrug | Feed Chl (mM) | Conjugated Chl (mM) | Yield (%) |
|---|---|---|---|
| Hep–Chl-1 | 0.36 | 0.27 | 75 |
| Hep–Chl-2 | 0.53 | 0.31 | 60 |
| Hep–Chl-3 | 0.65 | 0.32 | 49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrgie, A.T.; Birhan, Y.S.; Mekonnen, T.W.; Hanurry, E.Y.; Darge, H.F.; Lee, R.-H.; Chou, H.-Y.; Tsai, H.-C. Redox-Responsive Heparin–Chlorambucil Conjugate Polymeric Prodrug for Improved Anti-Tumor Activity. Polymers 2020, 12, 43. https://doi.org/10.3390/polym12010043
Andrgie AT, Birhan YS, Mekonnen TW, Hanurry EY, Darge HF, Lee R-H, Chou H-Y, Tsai H-C. Redox-Responsive Heparin–Chlorambucil Conjugate Polymeric Prodrug for Improved Anti-Tumor Activity. Polymers. 2020; 12(1):43. https://doi.org/10.3390/polym12010043
Chicago/Turabian StyleAndrgie, Abegaz Tizazu, Yihenew Simegniew Birhan, Tefera Worku Mekonnen, Endiries Yibru Hanurry, Haile Fentahun Darge, Rong-Ho Lee, Hsiao-Ying Chou, and Hsieh-Chih Tsai. 2020. "Redox-Responsive Heparin–Chlorambucil Conjugate Polymeric Prodrug for Improved Anti-Tumor Activity" Polymers 12, no. 1: 43. https://doi.org/10.3390/polym12010043
APA StyleAndrgie, A. T., Birhan, Y. S., Mekonnen, T. W., Hanurry, E. Y., Darge, H. F., Lee, R.-H., Chou, H.-Y., & Tsai, H.-C. (2020). Redox-Responsive Heparin–Chlorambucil Conjugate Polymeric Prodrug for Improved Anti-Tumor Activity. Polymers, 12(1), 43. https://doi.org/10.3390/polym12010043