Cationic Copolymerization of Isobutylene with 4-Vinylbenzenecyclobutylene: Characteristics and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization
2.3. Measurements
3. Results and Discussion
3.1. Random Copolymers of IB and 4-VBCB
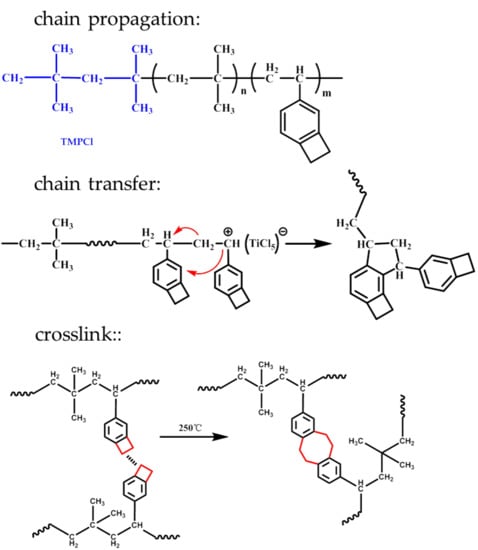
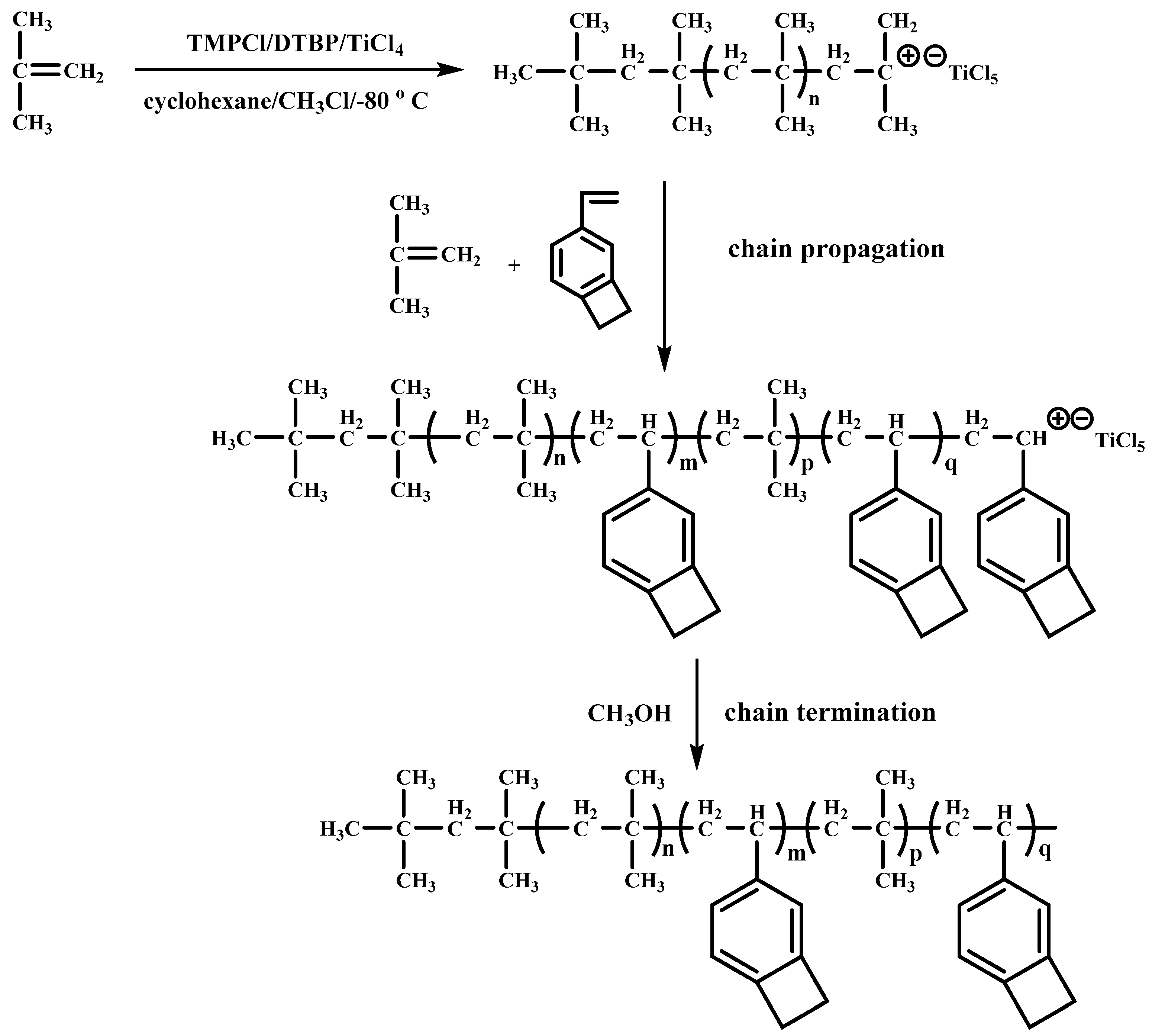
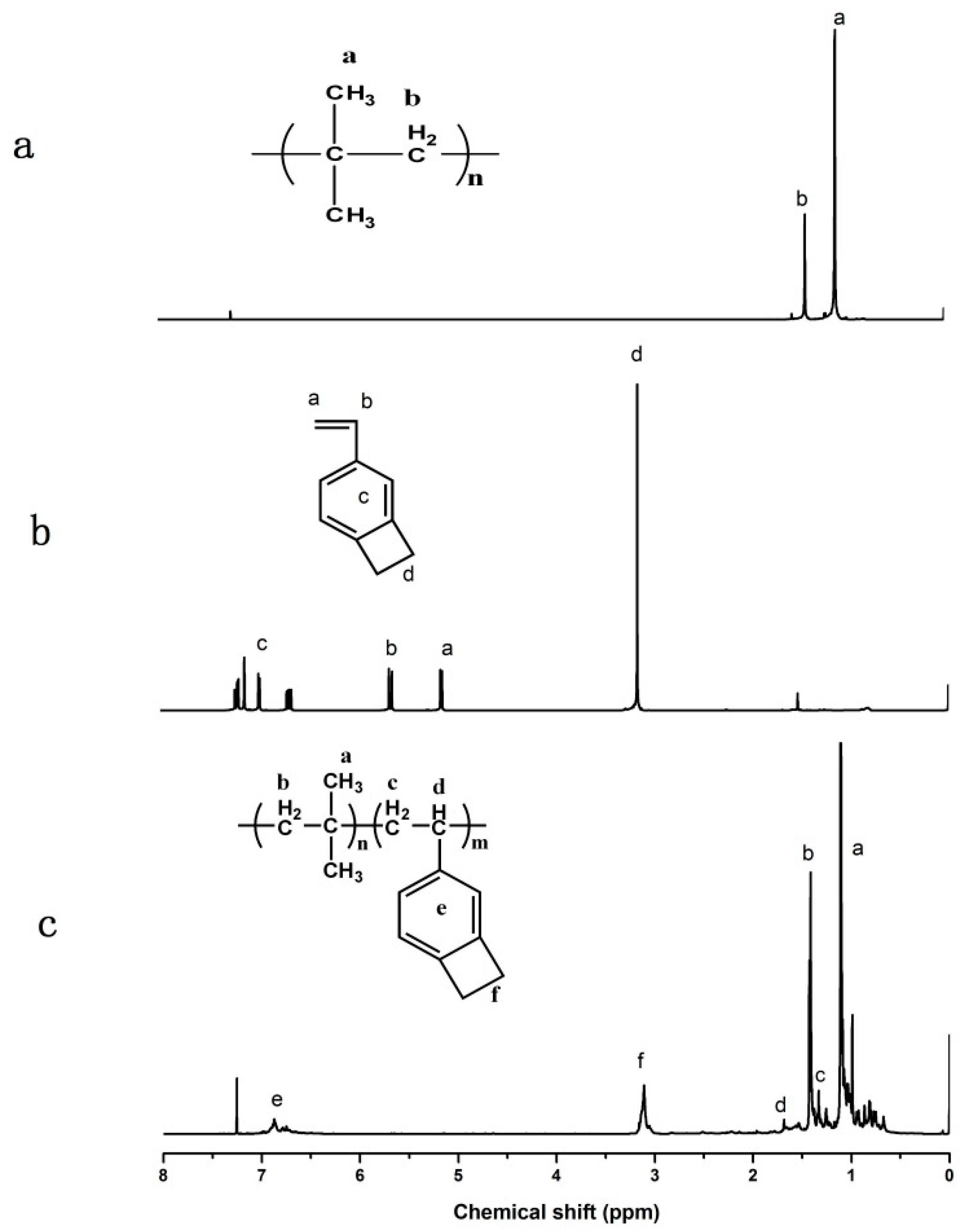
3.1.1. Copolymerization of IB and 4-VBCB
3.1.2. Reactivity Ratio of IB and 4-VBCB
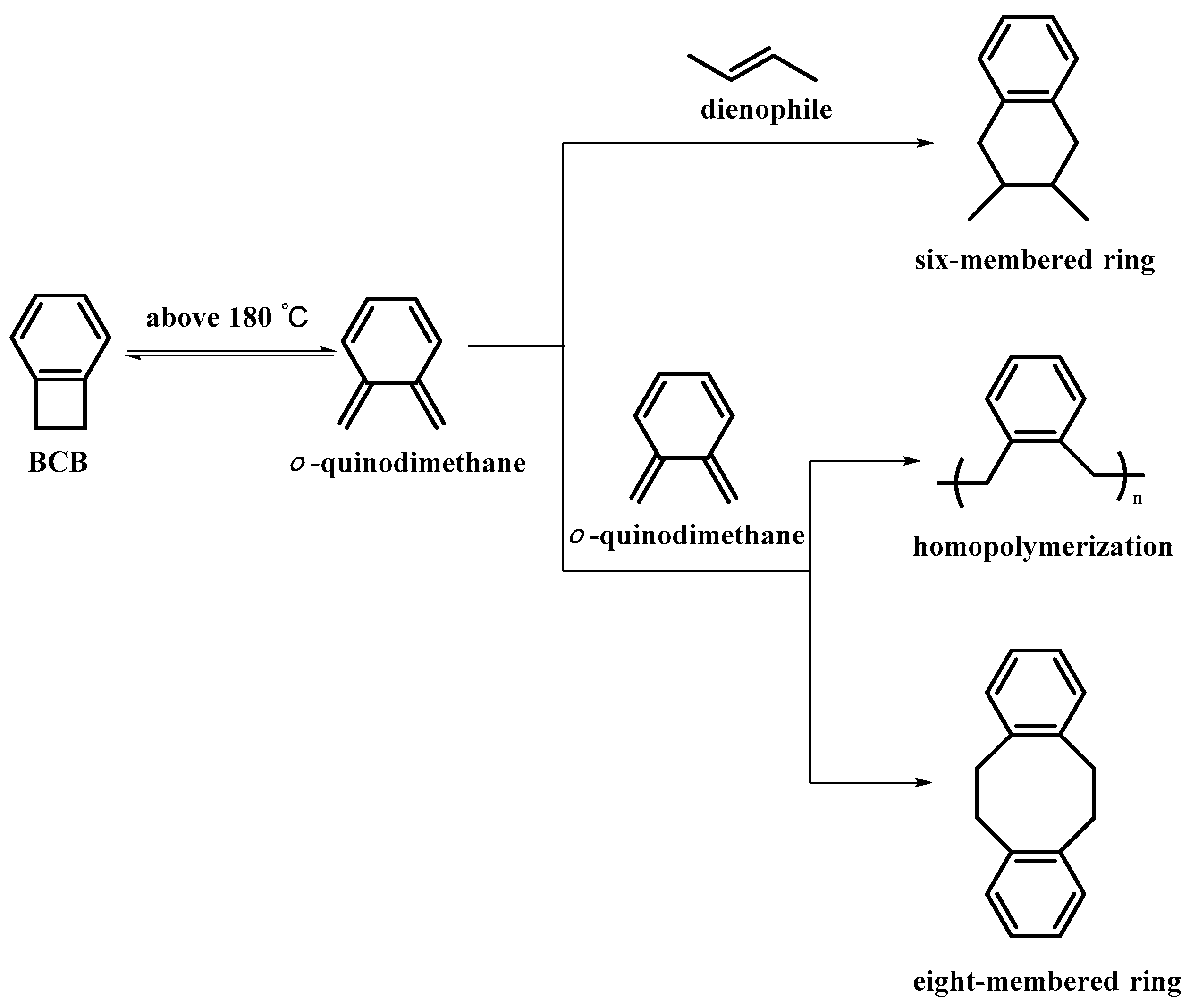
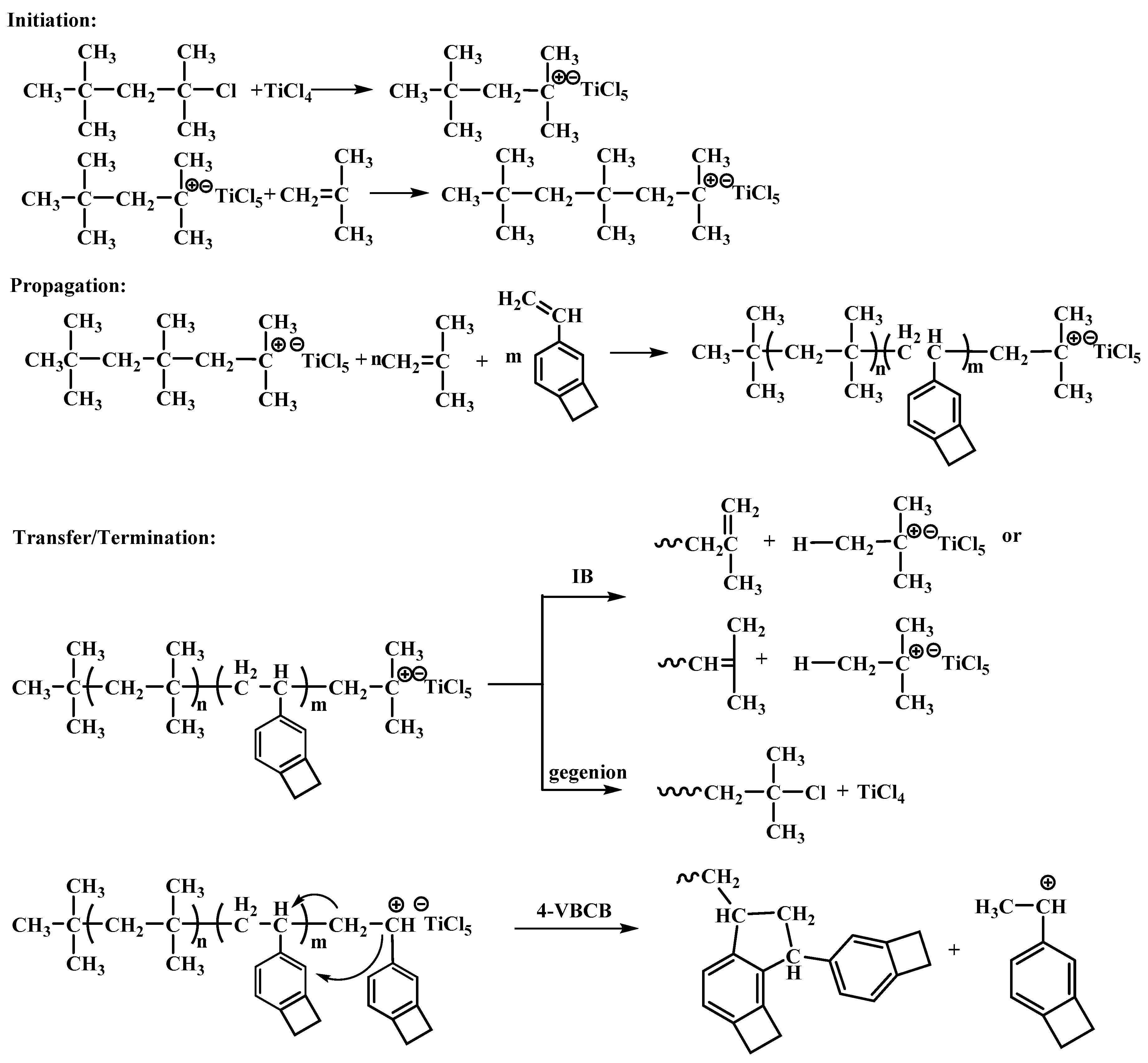
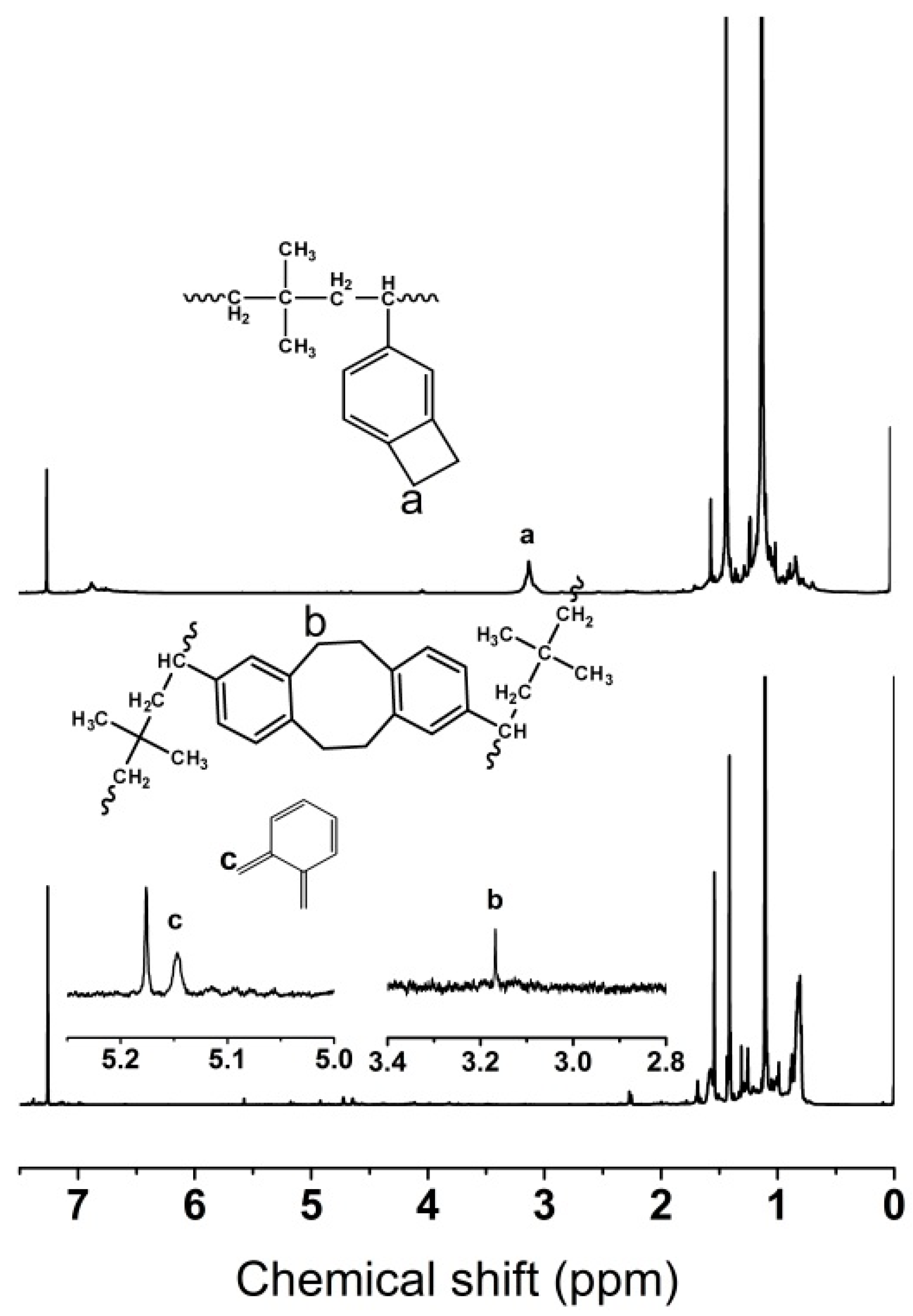
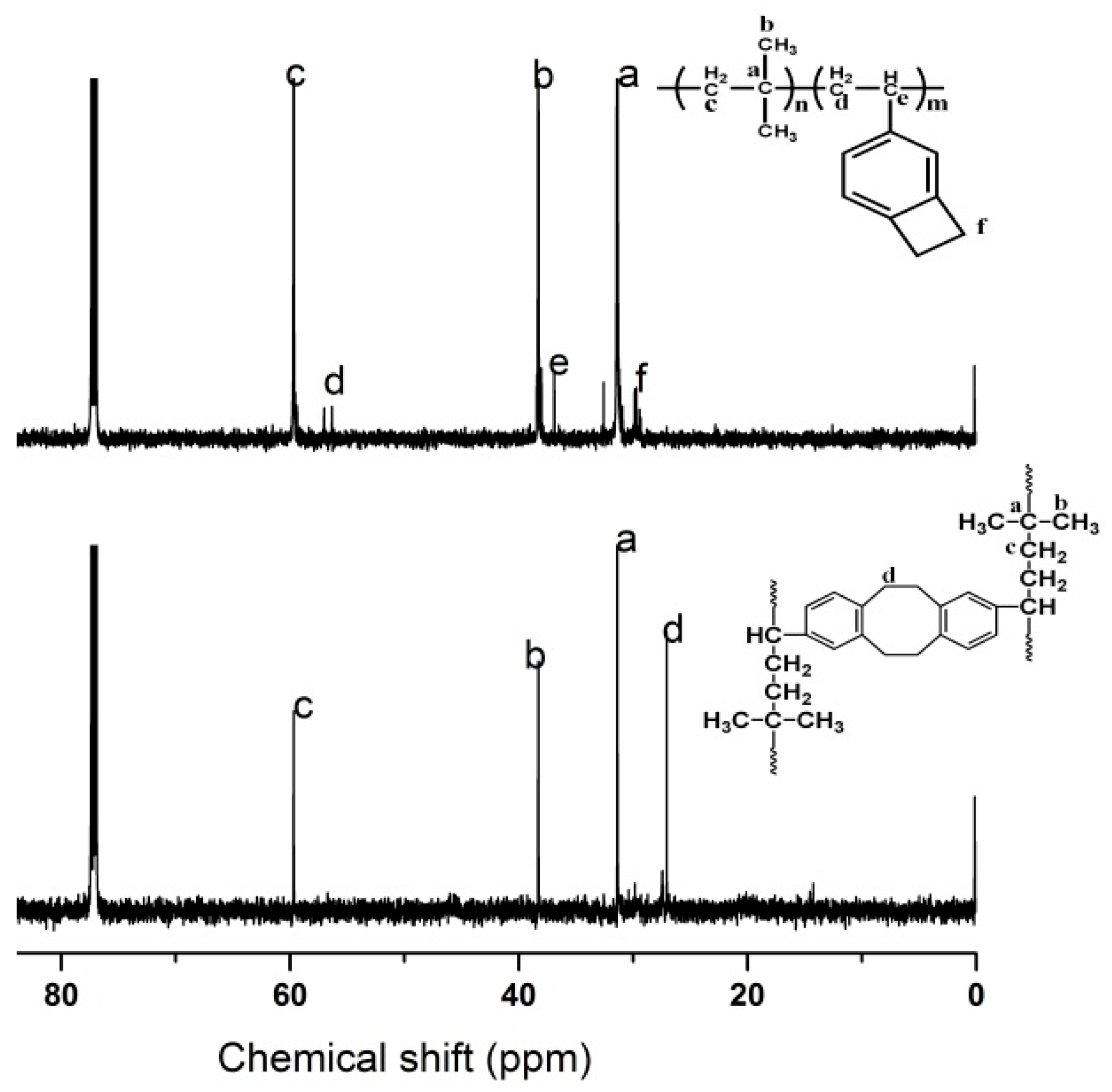
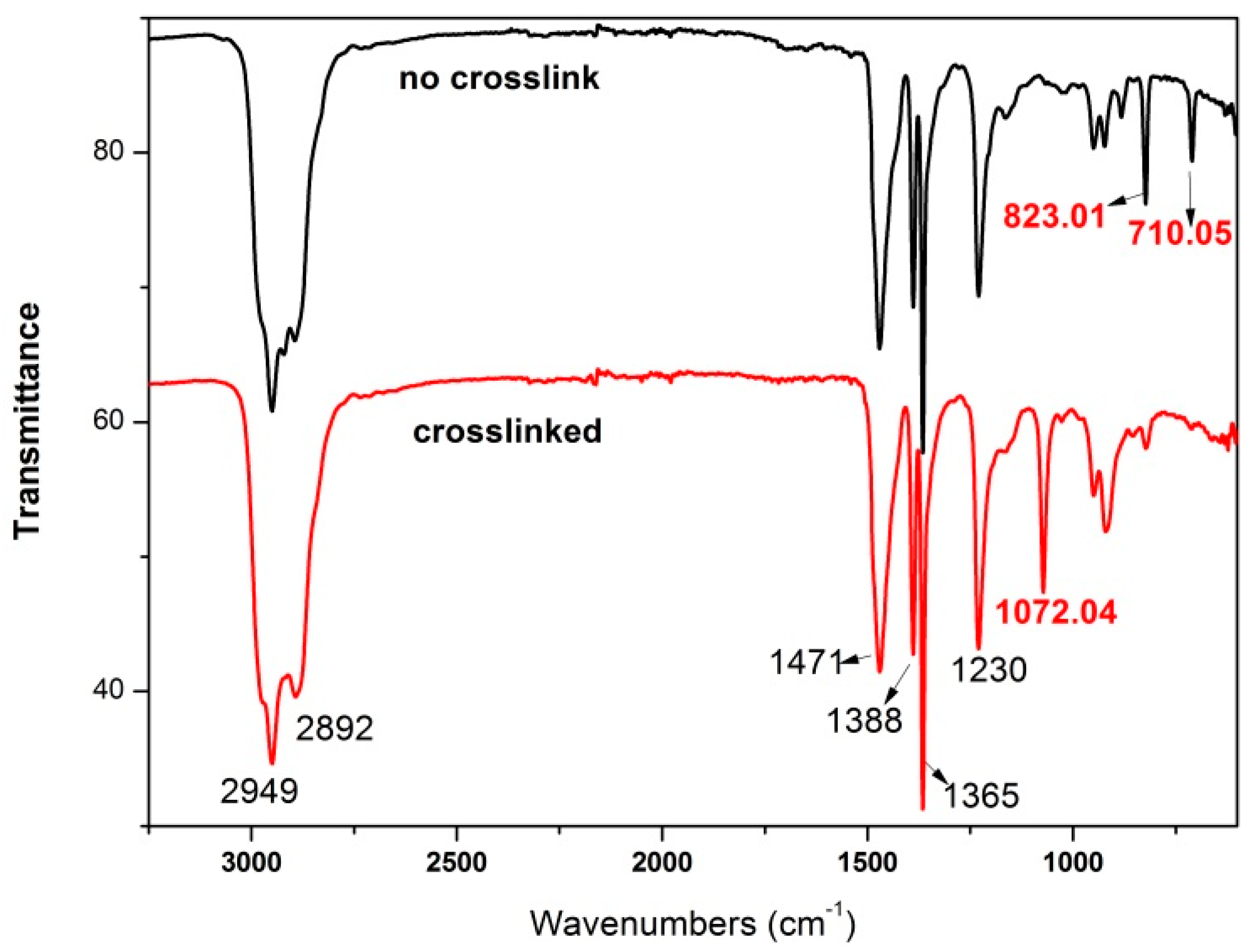
3.1.3. Characteristics and Mechanisms
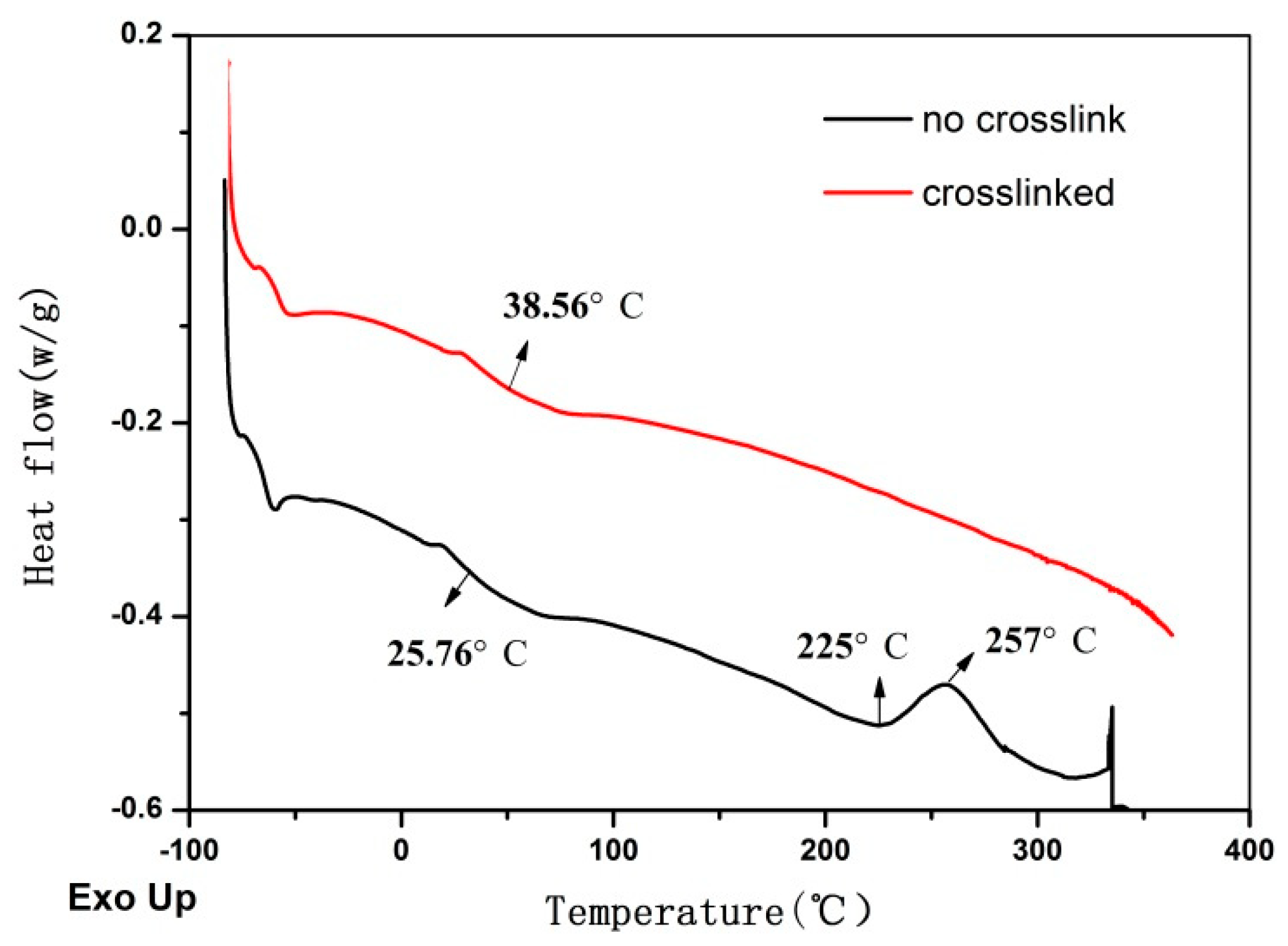
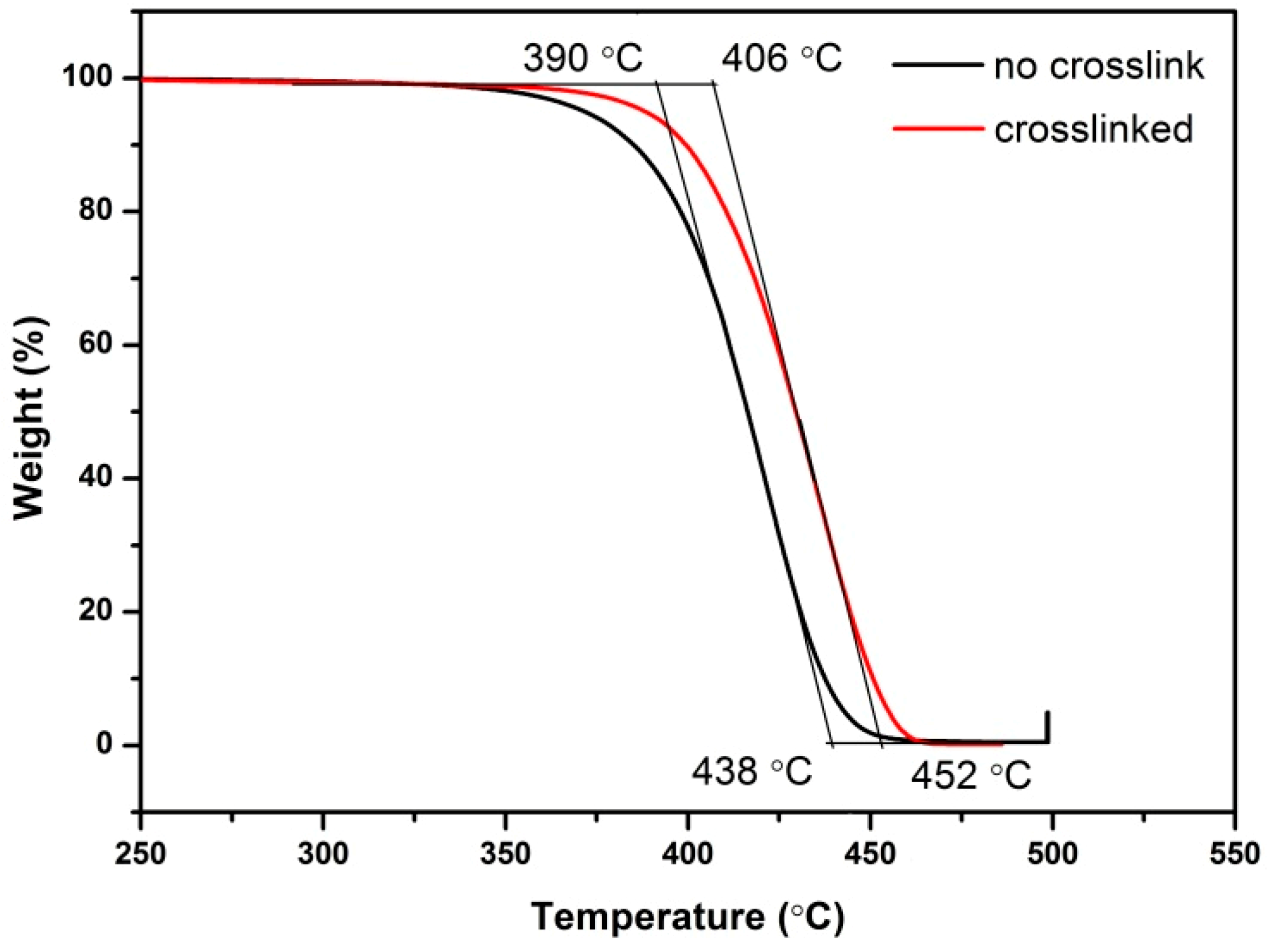
3.2. Thermal Properties of Poly(IB-co-4-VBCB) Random Copolymer
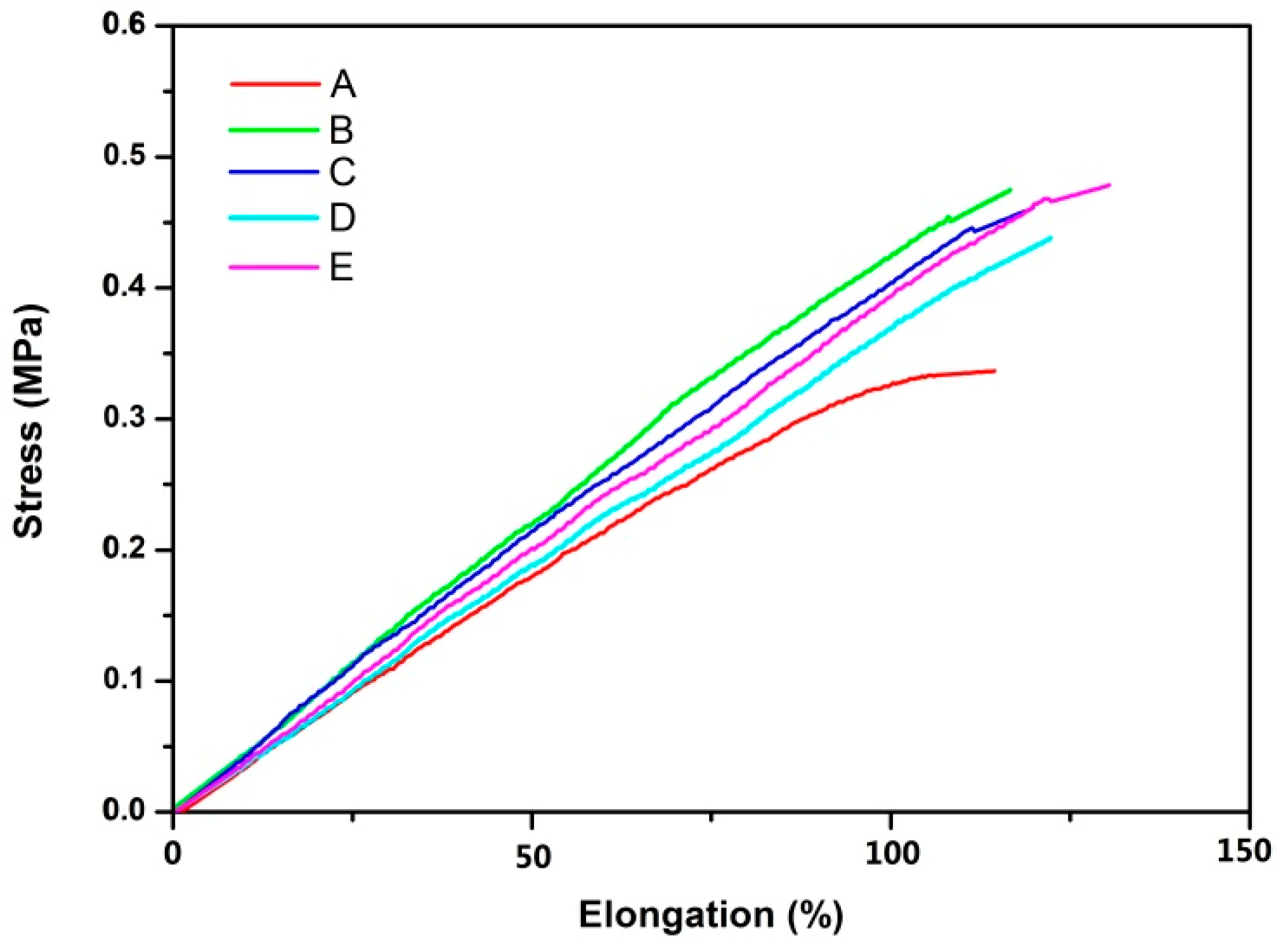
3.3. Mechanical Properties of Poly(IB-co-4-VBCB) Random Copolymer.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Faust, R.; Kennedy, J.P. Living carbocationic polymerization. IV. Living polymerization of isobutylene. Polym. Sci. Part A Polym. Chem 1987, 25, 1847–1869. [Google Scholar] [CrossRef]
- Faust, R.; Kennedy, J.P. Living carbocationic polymerization. Polym. Bull. 1986, 15, 317–323. [Google Scholar] [CrossRef]
- Roth, M.; Patz, M.; Freter, H.; Mayr, H. Living oligomerization of isobutylene using di- and triisobutylene hydrochlorides as Initiators. Macromolecules 1997, 30, 722–725. [Google Scholar] [CrossRef]
- Storey, R.F.; Chisholm, B.J.; Curry, C.L. Carbocation rearrangement in controlled/living isobutylene polymerization. Macromolecules 1995, 28, 4055–4061. [Google Scholar] [CrossRef]
- Storey, R.F.; Choate, K.R., Jr. Kinetic investigation of the living cationic polymerization of isobutylene using a t-Bu-m-DCC/TiCl4/2,4-DMP initiating system. Macromolecules 1997, 30, 4799–4806. [Google Scholar] [CrossRef]
- Tawada, M.; Faust, R. Living cationic polymerization of isobutylene with mixtures of titanium tetrachloride/titanium tetrabromide. Macromolecules 2005, 38, 4989–4995. [Google Scholar] [CrossRef]
- Puskas, J.E.; Brister, L.B.; Michel, A.J.; Lanzendorfer, M.G.; Jamieson, D.; Pattern, W.G. Novel substituted epoxide initiators for the carbocationic polymerization of isobutylene. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 444–452. [Google Scholar] [CrossRef]
- Bahadur, M.; Shaffer, T.D.; Ashbaugh, J.R. Dimethylaluminum chloride catalyzed living isobutylene polymerization. Macromolecules 2000, 33, 9548–9552. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, P.; Guo, W.; Li, S.; Yang, X.; Shang, Y. Living cationic sequential block copolymerization: Synthesis and characterization of poly(4-(2-hydroxyethyl)styrene-b-isobutylene-b-4-(2-hydroxyethyl)styrene) triblock copolymers. Polym. J. 2010, 42, 268–272. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, W.; Li, S.; Gong, H. Synthesis of poly(isobutylene-b-alpha-methylstyrene) copolymers by living cationic polymerization. Acta Polym. Sin. 2008, 8, 574–580. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, W.; Li, S.; Gong, H. Kinetic studies on homopolymerization of alpha-methylstyrene and sequential block copolymerization of isobutylene with alpha-methylstyrene by living/controlled cationic polymerization. Polym. Korea 2008, 32, 366–371. [Google Scholar]
- Zhang, X.; Guo, W.; Wu, Y.; Shang, Y.; Li, S.; Xiong, W. Synthesis of random copolymer of isobutylene with p-methylstyrene by cationic polymerization in ionic liquids. E-Polymers 2018, 18, 423–431. [Google Scholar] [CrossRef]
- Yang, S.; Fan, Z.; Zhang, F.; Li, S.; Wu, Y. Functionalized Copolymers of Isobutylene with Vinyl Phenol: Synthesis, Characterization, and Property. Chin. J. Polym. Sci. 2019, 37, 919–929. [Google Scholar] [CrossRef]
- Xie, Y.; Chang, J.; Wu, Y.; Yang, D.; Wang, H.; Zhang, T.; Li, S.; Guo, W. Synthesis and properties of bromide- functionalized poly(isobutylene-co-p-methylstyrene) random copolymer. Polym. Int. 2017, 66, 468–476. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Midha, S.; Tsunogae, Y. Tsunogae Living carbocationic polymerization. 56. Polyisobutylene-containing block polymers by sequential monomer addition. 8. Synthesis, characterization, and physical properties of poly(indene-b-isobutylene-b-indene) thermoplastic elastomers. Macromolecules 1993, 26, 429–435. [Google Scholar] [CrossRef]
- Li, J.; Wu, K.; Huang, S.; Zhang, J.; Zhao, M.; Gong, G.; Guo, W.; Wu, Y. Synthesis and Properties of Hydroxytelechelic Polyisobutylenes by End Capping with tert-Butyl-dimethyl-(4-methyl-pent-4-enyloxy)-silane. Chin. J. Polym. Sci. 2019, 37, 936–942. [Google Scholar] [CrossRef]
- Hahn, S.F.; Martin, S.J.; McKelvy, M.L.; Patrick, D.W. Thermal polymerization of bis(benzocyclobutene) monomers containing. alpha.,.beta.-disubstituted ethenes. Macromolecules 1993, 26, 3870–3877. [Google Scholar] [CrossRef]
- Kirchhoff, R.A.; Bruza, K.J. Benzocyclobutenes in polymer synthesis. Prog. Polym. Sci. 1993, 18, 85–185. [Google Scholar] [CrossRef]
- John, M.W.; William, C.P.; Robert, A.D. Benzocyclobutenes as styrene monomer scavengers and molecular weight “stabilizers” in atactic and syndiotactic polystyrenes. J. Appl. Polym. Sci. 2000, 78, 2008–2015. [Google Scholar]
- So, Y.H.; Foster, P.; Im, J.H.; Garrou, P.; Hetzner, J.; Stark, E. Divinylsiloxane-bisbenzocyclobutene-based polymer modified with polystyrene polybutadiene–polystyrene triblock copolymers. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1591–1599. [Google Scholar] [CrossRef]
- Okay, O.; Erman, B.; Durmaz, S. Solution cross-linked poly(isobutylene) gels: Synthesis and swelling behavior. Macromolecules 2000, 33, 4822–4827. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, Y.; Xiao, F. Synthesis, thermal and mechanical properties of benzocyclobutene-functionalized siloxane thermosets with different geometric structures. Eur. Polym. J. 2012, 48, 751–760. [Google Scholar] [CrossRef]
- So, Y.H.; Hahn, S.F.; Li, Y.; Reinhard, M.T. Styrene 4-vinylbenzocyclobutene copolymer for microelectronic applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2799–2806. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Hu, H.; Wei, X.; Yu, H.; Yang, J. Photoactive polymers with benzocyclobutene/silacyclobutane dual crosslinked structure and low dielectric constant. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1920–1928. [Google Scholar] [CrossRef]
- Sakellariou, G.; Avgeropoulos, A.; Hadjichristidis, H.; Mays, J.W.; Baskaran, D. Functionalized organic nanoparticles from core-crosslinked poly(4-vinylbenzocyclobutene-b-butadiene) diblock copolymer micelles. Polymer 2009, 10, 038. [Google Scholar] [CrossRef]
- Harth, V.; Horn, B.V.; Lee, V.Y.; Germack, D.S.; Gonzales, C.P.; Miller, R.D.; Hawker, C.J. A facile approach to architecturally defined nanoparticles via intramolecular chain collapse. J. Am. Chem. Soc. 2002, 124, 8653–8660. [Google Scholar] [CrossRef]
- Takeshi, E.; Toshio, K.; Toshikazu, T.; Keisuke, C. Synthesis of poly(4-vinylbenzocyclobutene) and its reaction with dienophiles. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 707–715. [Google Scholar]
- Xu, Y.; Zhu, F.; Xie, L.; Yang, J.; Zhang, L.; Xie, R. Synthesis and property studies of oligomer obtained from the reaction of 4-vinylbenzocyclobutene and styrene with divinyl tetramethyl disiloxane-bisbenzocyclobutene. E-Polymers 2010, 10, 117. [Google Scholar] [CrossRef]
- Sakellariou, G.; Baskaran, D.; Hadjichristidis, N.; Mays, J.W. Well-defined poly(4-vinylbenzocyclobutene): #160 synthesis by living anionic polymerization and characterization. Macromolecules 2006, 39, 2525–3530. [Google Scholar]
- Sheriff, J.; Thomas, E.C.; Phat, L.; Kothadia, R.; George, S.; Kato, Y.; Pinchuk, L.; Slepian, M.J.; Bluestein, D. Physical characterization and platelet interactions under shear flows of a novel thermoset polyisobutylene-based co-polymer. ACS Appl. Mater. Interfaces 2015, 7, 22058–22066. [Google Scholar] [CrossRef] [PubMed]
- Hadjikyriacou, S.; Acar, M.; Faust, R. Living and controlled polymerization of isobutylene with alkylaluminum halides as coinitiators. Macromolecules 2004, 37, 7543–7547. [Google Scholar] [CrossRef]
- Gronowski, A.; Wojtczak, Z. Copolymerizations of styrene with magnesium, calcium, strontium and barium acrylates in dimethyl sulfoxide. Macromol. Chem. Phys. 1989, 190, 2063–2069. [Google Scholar] [CrossRef]
- Yan, P.F.; Guo, A.R.; Liu, Q. Living cationic polymerization of isobutylene coinitiated by FeCl3 in the presence of isopropanol. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3383–3392. [Google Scholar] [CrossRef]
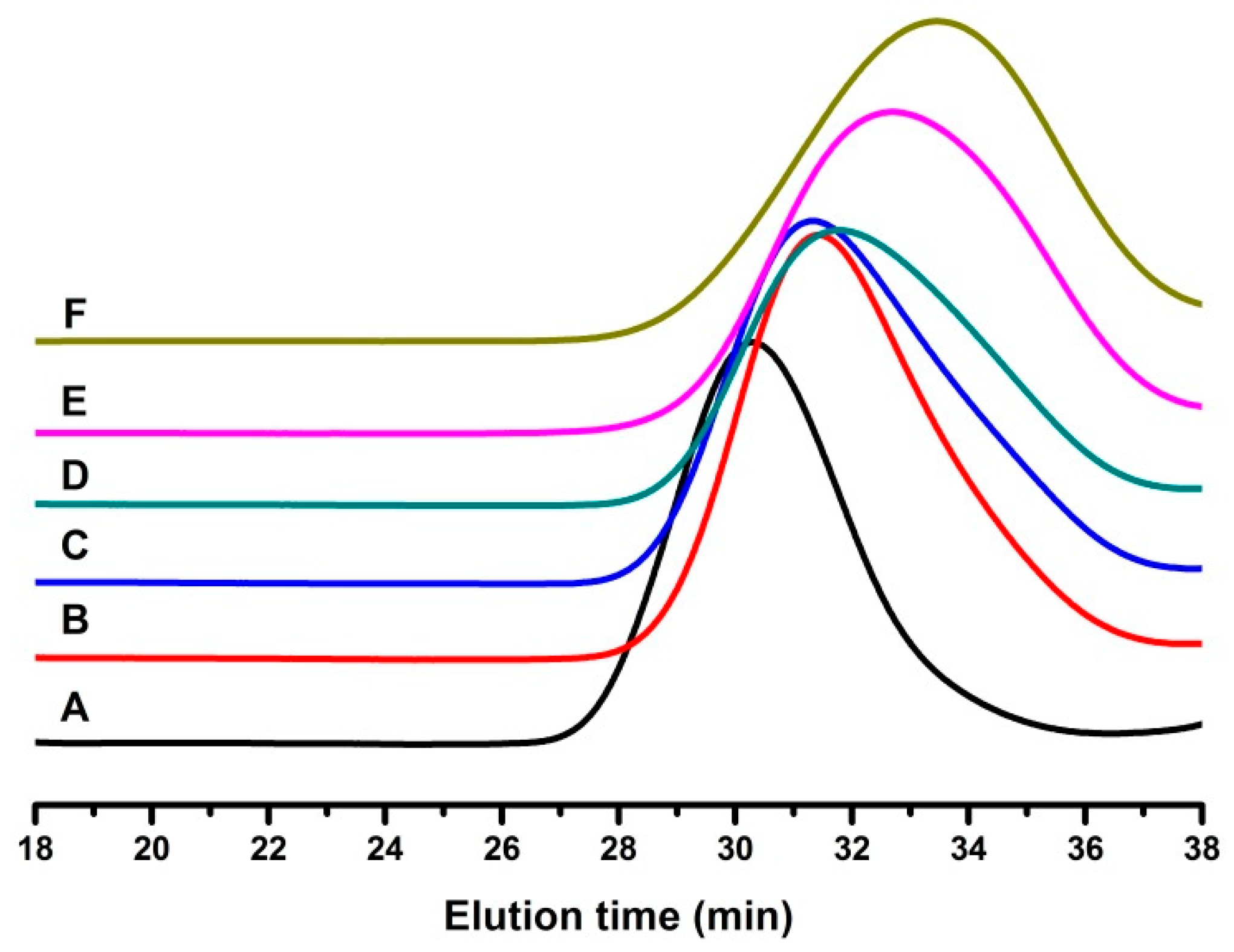
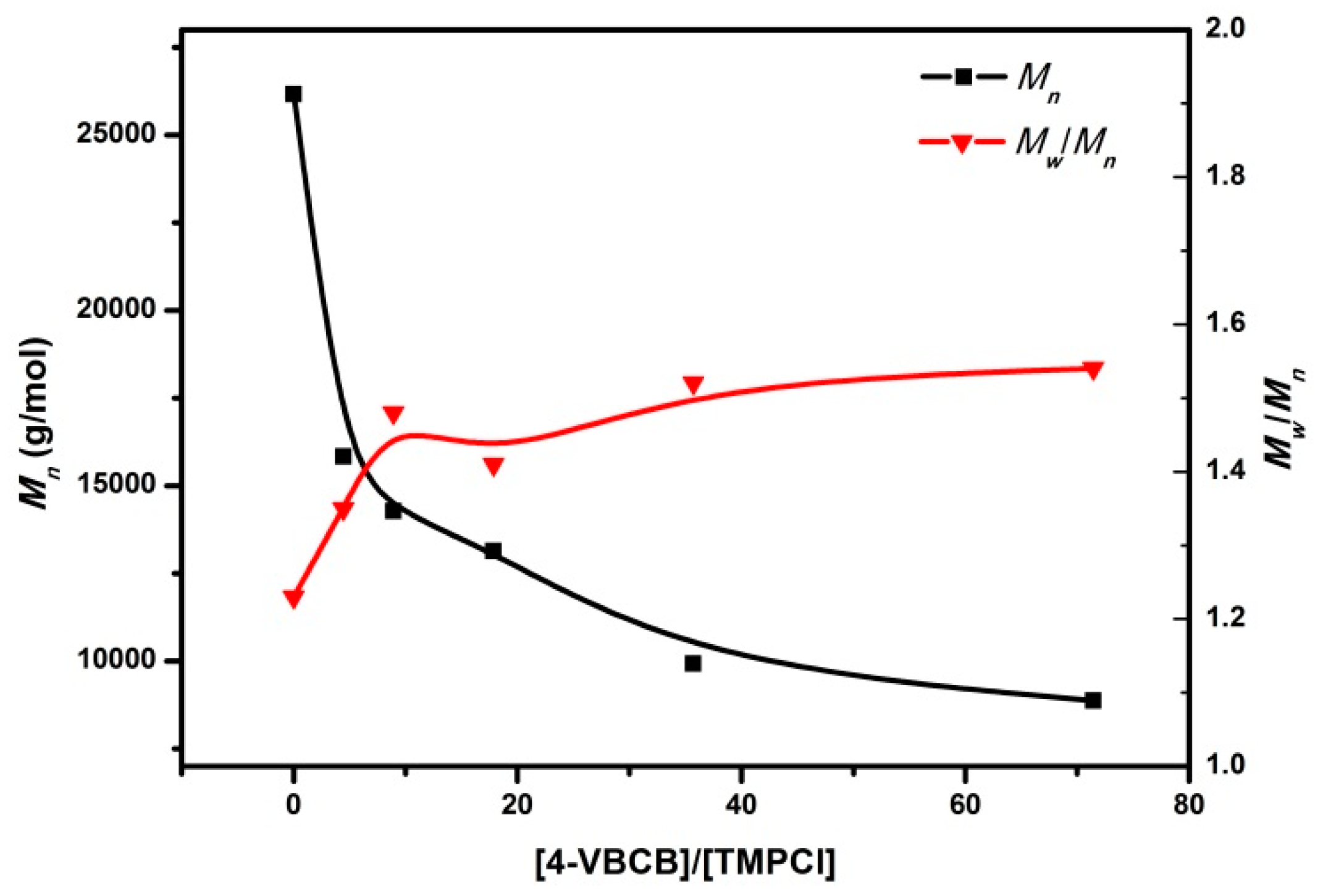
| Run | Conversion (%) | Monomer Feed | Compositions of Copolymer | ||
|---|---|---|---|---|---|
| IB (%) | 4-VBCB (%) | IB (%) | 4-VBCB (%) | ||
| 1 | 14.05 | 90.91 | 9.09 | 84.33 | 15.67 |
| 2 | 12.88 | 95.24 | 4.76 | 88.28 | 11.72 |
| 3 | 12.15 | 97.56 | 2.44 | 94.25 | 5.75 |
| 4 | 14.42 | 98.36 | 1.64 | 96.97 | 3.03 |
| 5 | 14.23 | 98.77 | 1.23 | 97.47 | 2.53 |
| Run | [4-VBCB]/[TMPCl] | Mn (g·mol−1) | Mw/Mn | Yield (%) |
|---|---|---|---|---|
| A | 0 | 26,100 | 1.23 | 97.80 |
| B | 4.45 | 15,800 | 1.35 | 98.00 |
| C | 8.91 | 14,300 | 1.48 | 93.96 |
| D | 17.86 | 13,100 | 1.41 | 96.67 |
| E | 35.71 | 9920 | 1.52 | 97.30 |
| F | 71.43 | 8860 | 1.54 | 96.95 |
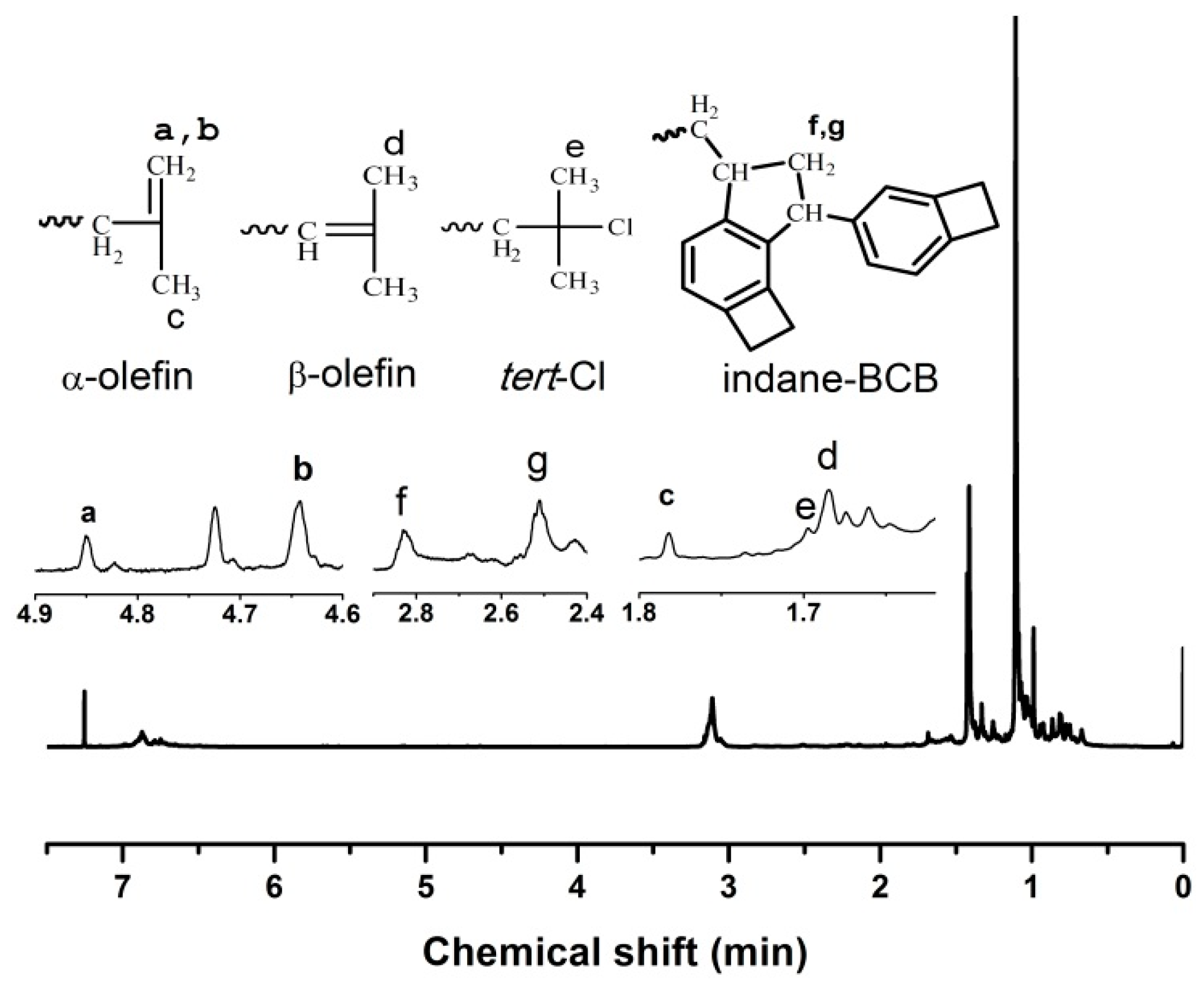
| [4-VBCB]/[IB] | 4-VBCB Components in Copolymer (mol %) | Mn (g·mol−1) | Mw/Mn | End-Group Composition (mol %) | |||
|---|---|---|---|---|---|---|---|
| α-olefin | β-olefin | tert-Cl | Indane-BCB | ||||
| 0 | 0 | 26,100 | 1.23 | 63.49 | 33.31 | 3.20 | 0 |
| 1/10 | 0.61 | 15,800 | 1.35 | 36.52 | 27.64 | 12.49 | 23.35 |
| 1/20 | 1.32 | 14,300 | 1.48 | 28.60 | 21.17 | 9.96 | 40.28 |
| 1/40 | 2.68 | 13,100 | 1.41 | 20.76 | 17.26 | 9.13 | 52.85 |
| 1/60 | 5.25 | 9920 | 1.52 | 14.89 | 16.35 | 8.94 | 59.81 |
| 1/80 | 10.64 | 8860 | 1.54 | 13.21 | 16.72 | 7.71 | 62.36 |
| Num | 4-VBCB Components in Copolymer (mol %) | Maximum Tensile Stress (MPa) | Elongation at Break (%) |
|---|---|---|---|
| A | 0.72 | 0.37 | 114 |
| B | 1.47 | 0.43 | 117 |
| C | 3.29 | 0.47 | 120 |
| D | 6.86 | 0.47 | 122 |
| E | 11.67 | 0.53 | 130 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, S.; Shang, Y.; Huang, S.; Wu, K.; Guo, W.; Wu, Y. Cationic Copolymerization of Isobutylene with 4-Vinylbenzenecyclobutylene: Characteristics and Mechanisms. Polymers 2020, 12, 201. https://doi.org/10.3390/polym12010201
Chen Z, Li S, Shang Y, Huang S, Wu K, Guo W, Wu Y. Cationic Copolymerization of Isobutylene with 4-Vinylbenzenecyclobutylene: Characteristics and Mechanisms. Polymers. 2020; 12(1):201. https://doi.org/10.3390/polym12010201
Chicago/Turabian StyleChen, Zhifei, Shuxin Li, Yuwei Shang, Shan Huang, Kangda Wu, Wenli Guo, and Yibo Wu. 2020. "Cationic Copolymerization of Isobutylene with 4-Vinylbenzenecyclobutylene: Characteristics and Mechanisms" Polymers 12, no. 1: 201. https://doi.org/10.3390/polym12010201
APA StyleChen, Z., Li, S., Shang, Y., Huang, S., Wu, K., Guo, W., & Wu, Y. (2020). Cationic Copolymerization of Isobutylene with 4-Vinylbenzenecyclobutylene: Characteristics and Mechanisms. Polymers, 12(1), 201. https://doi.org/10.3390/polym12010201